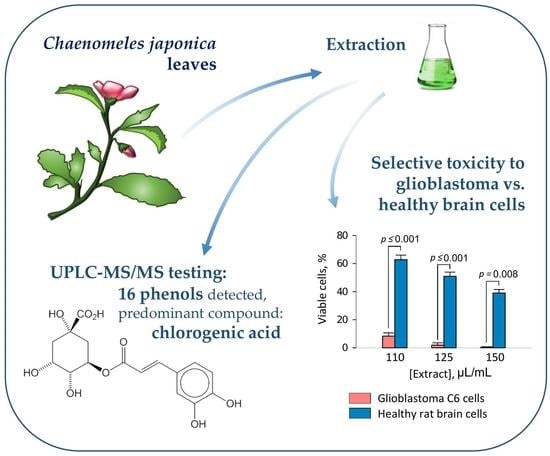
Investigation of Phenolic Composition and Anticancer Properties of Ethanolic Extracts of Japanese Quince Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extract Preparation
2.3. Evaluation of Phenolic Compound Composition (UPLC-ESI-MS/MS Conditions)
2.4. C6 and HROG36 Cell Culture
2.5. Primary Neuronal-Glial Cell Culture
2.6. Treatments of the Cells with Quince Leaf Extracts
2.7. Evaluation of Cellular Viability
2.8. Statistical Analysis
3. Results
3.1. Phenolic Compound Composition of Japanese Quince Leaves
3.2. The Effect of Quince Leaf Extracts on Viability of C6 and HROG36 Glioblastoma Cells
3.3. The Effect of Quince Leaf Extracts on Viability of Primary Non-Cancerous Brain Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bieniasz, M.; Dziedzic, E.; Kaczmarczyk, E. The effect of storage and processing on Vitamin C content in Japanese quince fruit. Folia Hortic. 2017, 29, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Wu, J.; Li, H.; Zhong, P.-X.; Xu, Y.-J.; Li, C.-H.; Ji, K.-X.; Wang, L.-S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Urbanavičiūtė, I.; Liaudanskas, M.; Bobinas, Č.; Šarkinas, A.; Rezgienė, A.; Viskelis, P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods 2020, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Su, X.L. Anticancer effect of ursolic acid via mitochondria-dependent pathways (Review). Oncol. Lett. 2019, 17, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Du, R.; Yu, X. Ursolic acid exhibits potent anticancer effects in human metastatic melanoma cancer cells (SK-MEL-24) via apoptosis induction, inhibition of cell migration and invasion, cell cycle arrest, and inhibition of mitogen-activated protein kinase (MAPK)/ERK signaling pathway. Med. Sci. Monit. 2019, 25, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food Chem. 2010, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Huang, H.Y.; Wu, Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 5012–5018. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Domingues, A.L.; Seabra, R.M.; Ferreira, M.A. Phenolic profile of quince fruit (Cydonia oblonga Miller) (pulp and peel). J. Agric. Food Chem. 2002, 50, 4615–4618. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Gorlach, S.; Wagner, W.; Podsdek, A.; Szewczyk, K.; Koziokiewicz, M.; Dastych, J. Procyanidins from Japanese quince (Chaenomeles japonica) fruit induce apoptosis in human colon cancer caco-2 cells in a degree of polymerization—Dependent manner. Nutr. Cancer 2011, 63, 1348–1360. [Google Scholar] [CrossRef]
- Lewandowska, U.; Szewczyk, K.; Owczarek, K.; Hrabec, Z.; Podsȩdek, A.; Koziołkiewicz, M.; Hrabec, E. Flavanols from Japanese quince (Chaenomeles Japonica) fruit inhibit human prostate and breast cancer cell line invasiveness and cause favorable changes in Bax/Bcl-2 mRNA Ratio. Nutr. Cancer 2013, 65, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Hrabec, E.; Fichna, J.; Sosnowska, D.; Koziołkiewicz, M.; Szymański, J.; Lewandowska, U. Flavanols from Japanese quince (Chaenomeles japonica) fruit suppress expression of cyclooxygenase-2, metalloproteinase-9, and nuclear factor-kappaB in human colon cancer cells. Acta Biochim. Pol. 2017, 64, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Sosnowska, D.; Polka, D.; Owczarek, K.; Gorlach-Lira, K.; Oliveira De Verasa, B.; Lewandowska, U. Comparison of phenolic compounds, antioxidant and cytotoxic activity of extracts prepared from Japanese quince (Chaenomeles japonica L.) leaves. J. Physiol. Pharmacol. 2020, 71, 1–10. [Google Scholar] [CrossRef]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current challenges and opportunities in treating glioblastomas. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22199–22224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanaviciute, I.; Liaudanskas, M.; Seglina, D.; Viskelis, P. Japanese Quince Chaenomeles Japonica (Thunb.) Lindl. ex Spach Leaves a New Source of Antioxidants for Food. Int. J. Food Prop. 2019, 22, 795–803. [Google Scholar] [CrossRef] [Green Version]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Balion, Z.; Cėpla, V.; Svirskiene, N.; Svirskis, G.; Druceikaitė, K.; Inokaitis, H.; Rusteikaitė, J.; Masilionis, I.; Stankevičienė, G.; Jelinskas, T.; et al. Cerebellar cells self-assemble into functional organoids on synthetic, chemically crosslinked ECM-mimicking peptide hydrogels. Biomolecules 2020, 10, 754. [Google Scholar] [CrossRef]
- Kikowska, M.; Włodarczyk, A.; Rewers, M.; Sliwinska, E.; Studzińska-Sroka, E.; Witkowska-Banaszczak, E.; Stochmal, A.; Żuchowski, J.; Dlugaszewska, J.; Thiem, B. Micropropagation of Chaenomeles japonica: A step towards production of polyphenol-rich extracts showing antioxidant and antimicrobial activities. Molecules 2019, 24, 1314. [Google Scholar] [CrossRef] [Green Version]
- Di Camillo Orfali, G.; Duarte, A.C.; Bonadio, V.; Martinez, N.P.; De Araújo, M.E.M.B.; Priviero, F.B.M.; Carvalho, P.O.; Priolli, D.G. Review of anticancer mechanisms of isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Apoptotic Effects of Quercitrin on DLD-1 Colon Cancer Cell Line. Pathol. Oncol. Res. 2015, 21, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, A.; Xu, X.; Sun, H.; Tang, J.; Ouyang, Z. Pharmacological activities and pharmacokinetic study of hyperoside: A short review. Trop. J. Pharm. Res. 2017, 16, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hui, L.; Foster, D.A.; Drain, C.M. Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: Targeting and incapacitating cancer cells. Biochemistry 2004, 43, 10918–10929. [Google Scholar] [CrossRef] [PubMed]
- Salucci, M.; Bugianesi, R.; Maiani, G.; Stivala, L.A.; Vannini, V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Br. J. Cancer 2002, 86, 1645–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deka, S.; Gorai, S.; Manna, D.; Trivedi, V. Evidence of PKC Binding and Translocation to Explain the Anticancer Mechanism of Chlorogenic Acid in Breast Cancer Cells. Curr. Mol. Med. 2017, 17, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.D.; Monteiro, M.C.; Teodoro, A.J. Anticancer Properties of Hydroxycinnamic Acids—A Review. Cancer Clin. Oncol. 2012, 1, 109. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Murata, T.; El-Rayes, B.F.; Shoji, M. The flavonoid p-hydroxycinnamic acid exhibits anticancer effects in human pancreatic cancer MIA PaCa-2 cells in vitro: Comparison with gemcitabine. Oncol. Rep. 2015, 34, 3304–3310. [Google Scholar] [CrossRef] [Green Version]
- Ekbatan, S.S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer caco-2 cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Dong, X.; Liu, D.; Hao, S.; He, F. Anti-tumor activity of chlorogenic acid by regulating the mTORC2 signaling pathway and disrupting F-actin organization. Int. J. Clin. Exp. Med. 2019, 12, 4818–4828. [Google Scholar]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Danhier, P.; Bański, P.; Payen, V.L.; Grasso, D.; Ippolito, L.; Sonveaux, P.; Porporato, P.E. Cancer metabolism in space and time: Beyond the Warburg effect. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, J.A.; Brown, J.S.; Anderson, A.R.A. The impact of proliferation-migration tradeoffs on phenotypic evolution in cancer. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.S.; Jang, H.J.; Kim, S.M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012, 689, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Paliwal, P.; Mukherjee, S.; Patnaik, N.; Krishnamurthy, S.; Patnaik, R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica 2019, 49, 339–345. [Google Scholar] [CrossRef] [PubMed]



| Compound | Retention Time, min | Molecular Ion (m/z) | Production (m/z) | Cone Voltage, V | Collision Chamber Energy, eV |
|---|---|---|---|---|---|
| (+)-Catechin | 3.50 | 289 | 123 | 60 | 34 |
| Chlorogenic acid | 3.52 | 353 | 191 | 32 | 14 |
| Caffeic acid | 3.89 | 179 | 107 | 36 | 22 |
| Procyanidin B2 | 4.02 | 577 | 407 | 50 | 20 |
| (-)-Epicatechin | 4.13 | 289 | 123 | 60 | 34 |
| Procyanidin C1 | 4.38 | 865 | 125 | 56 | 60 |
| p-Coumaric acid | 4.73 | 163 | 93 | 28 | 22 |
| Rutin | 5.06 | 609 | 300 | 70 | 38 |
| Hyperoside | 5.20 | 463 | 300 | 50 | 26 |
| Isoquercitrin | 5.30 | 463 | 301 | 52 | 28 |
| Luteolin 7-O-glucoside | 5.31 | 447 | 285 | 66 | 26 |
| Avicularin | 5.59 | 433 | 301 | 50 | 20 |
| Kaempferol 3-O-glucoside | 5.64 | 447 | 284 | 54 | 28 |
| Quercitrin | 5.68 | 447 | 300 | 50 | 26 |
| Phloridzin | 5.88 | 435 | 273 | 42 | 14 |
| Quercetin | 6.86 | 301 | 151 | 48 | 20 |
| Compound, µg/g DW | Japanese Quince Cultivars | ||
|---|---|---|---|
| ‘Darius’ | ‘Rondo’ | ‘Rasa’ | |
| Hydroxycinnamic Acids | |||
| Chlorogenic acid | 5373 ± 244 a | 5773 ± 271 a | 5737 ± 269 a |
| p-Coumaric acid | 155.4 ± 10.2 a | 58.9 ± 3.3 c | 102.9 ± 7.5 b |
| Caffeic acid | 4.8 ± 0.2 a | 0.84 ± 0.03 b | ND |
| Total, µg·g−1 | 5533 ± 202 a | 5833 ± 307 a | 5840 ± 188 a |
| Flavonols | |||
| Isoquercitrin | 2131.1 ± 54.4 a | 418.6 ± 11.5 c | 1700.7 ± 49.6 b |
| Hyperoside | 907.7 ± 40.3 b | 1124.5 ± 51.4 a | 1107.8 ± 50.3 a |
| Quercitrin | 1314.3 ± 39.2 a | 248.5 ± 10.1 c | 350.0 ± 20.2 b |
| Rutin | 189.7 ± 9.0 c | 289.1 ± 13.2 b | 511.2 ± 20.0 a |
| Kaempherol 3-O-glucoside | 318.0 ± 14.3 b | 407.9 ± 18.3 a | 226.9 ± 10.3 c |
| Avicularin | 9.34 ± 0.32 b | 14.97 ± 0.63 a | 5.69 ± 0.20 b |
| Quercetin | 2.14 ± 0.09 a | 2.42 ± 0.10 a | 1.23 ± 0.04 b |
| Total, µg·g−1 | 4872 ± 140 a | 2506 ± 56 c | 3903 ± 14.3 b |
| Flavan-3-Ols | |||
| (-)-Epicatechin | 3963 ± 112 a | 314.3 ± 9.4 c | 2038 ± 88 b |
| Procyanidin C1 | 1333.8 ± 53.5 a | 176.1 ± 8.4 c | 792.4 ± 21.1 b |
| Procyanidin B2 | 1129.3 ± 50.3 a | 209.4 ± 15.6 c | 820.9 ± 33.6 b |
| (+)-Catechin | ND | 0.6 ± 0.02 | ND |
| Total, µg·g−1 | 6426 ± 145 a | 700.4 ± 10.7 c | 3652 ± 73.6 b |
| Others Phenols | |||
| Luteolin 7-O-glucoside | 213.7 ± 8.6 b | 277.9 ± 11.3 a | 155.5 ± 6.9 c |
| Phloridzin | 3.65 ± 0.16 b | 5.32 ± 0.23 a | 5.75 ± 0.25 a |
| Sum of all compounds, µg/g | 17048 ± 461 a | 9322 ± 287 c | 13556 ± 375 b |
| Substance, μg/mL | ‘Rondo’ | ‘Rasa’ | ‘Darius’ | Chlorogenic Acid | Epicate Chin | Hypero Side | Querci Trin | Temozo Lomide |
|---|---|---|---|---|---|---|---|---|
| C6 | 1660.2 | 1560.0 | 1738.9 | 85.5 | 252.2 | 143.1 | 59.3 | 2341.5 |
| HROG36 | 373.8 | 378.5 | 373.5 | 7.3 | 12.3 | 3.8 | 3.4 | 535.8 |
| Compound | Hydroxycinnamic Acids | Flavonols | Flavan-3-Ols | Total Phenols | Chlorogenic Acid | Epicate Chin | Hypero Side | Querci Trin |
|---|---|---|---|---|---|---|---|---|
| C6 | −0.99 | 0.46 | 0.21 | 0.39 | −0.99 | 0.33 | −0.75 | −0.56 |
| HROG36 | −0.86 | −0.38 | 0.28 | −0.42 | −0.81 | −0.21 | −0.31 | −0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvikas, V.; Urbanaviciute, I.; Bernotiene, R.; Kulakauskiene, D.; Morkunaite, U.; Balion, Z.; Majiene, D.; Liaudanskas, M.; Viskelis, P.; Jekabsone, A.; et al. Investigation of Phenolic Composition and Anticancer Properties of Ethanolic Extracts of Japanese Quince Leaves. Foods 2021, 10, 18. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010018
Zvikas V, Urbanaviciute I, Bernotiene R, Kulakauskiene D, Morkunaite U, Balion Z, Majiene D, Liaudanskas M, Viskelis P, Jekabsone A, et al. Investigation of Phenolic Composition and Anticancer Properties of Ethanolic Extracts of Japanese Quince Leaves. Foods. 2021; 10(1):18. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010018
Chicago/Turabian StyleZvikas, Vaidotas, Ieva Urbanaviciute, Rasa Bernotiene, Deimante Kulakauskiene, Urte Morkunaite, Zbigniev Balion, Daiva Majiene, Mindaugas Liaudanskas, Pranas Viskelis, Aiste Jekabsone, and et al. 2021. "Investigation of Phenolic Composition and Anticancer Properties of Ethanolic Extracts of Japanese Quince Leaves" Foods 10, no. 1: 18. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010018