The Extraction, Functionalities and Applications of Plant Polysaccharides in Fermented Foods: A Review
Abstract
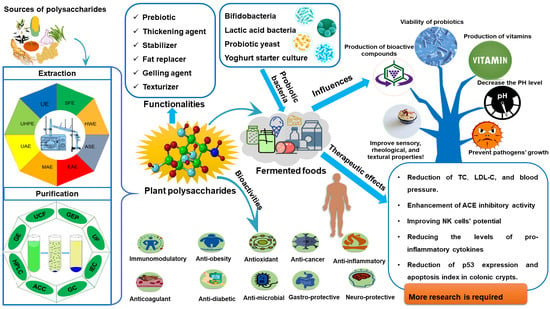
:1. Introduction
| Polysaccharide | Main Sources | EM | Molecular Structure | FM | Functions | References |
|---|---|---|---|---|---|---|
| Cellulose | Coconut fiber Grains, fruit, vegetable | AHE, APOE, UA | β-(1→4)-d-glucopyranose, homopolysaccharides, and linear | Ice cream, sausage, cheese | Thickening agent, stabilizer, fat replacer | [23] |
| Pectin | Plant cell wall, vegetable, fruit | HWE, MAE, UE | α-(1→4)-d-Methoxylated galacturonic acids, Branched/coiled | Yogurt, sausage | Gelling, antimicrobial agent | [24] |
| β-glucan | Barley, Oat, Wheat bran | AE, ALE, EE | β-(1→4)-d-Glucose and β-(1→3)-d-glucose | Cheese | Prebiotic, fat replacer | [25,26] |
| Inulin | Chicory root, Jerusalem artichoke | HWE, UE | β-(1→2)-d-Fructose, linear | Yogurt, cheese | Fat replacer, texturizer, gel-forming | [27] |
| OP | Okra fruit | HWE, MAE, PWE | l-rhamnose (l-Rha), d-galacturonic acid (d-GalA), and (1→4)-α-GalAp-(1→2)-α-Rhap-(1→d-galactose (d-Gal) | Yogurt | Stabilizer | [28,29] |
| KP | Oil palm tree | AE, AE, HWE | β-glycosidic bonds | Yogurt | Prebiotic | [30,31] |
| DOP | Dendrobium Officinale | HWE, ETE | Ribose, glucose, xylose, rhamnose, arabinose, mannose, and galactolipid | Yogurt | Prebiotic | [32] |
| TG | Tragacanth | ESM | d-galactose, d-galacturonic acid, l-arabinose, l-fucose, l-rhamnose, d-xylose and d-glucose | Sausage, yogurt | Fat replacer, stabilizer, prebiotic | [14,33] |
2. Plant Polysaccharides
2.1. Polysaccharides: Classification and Chemical Structure
2.2. Plant Polysaccharides Extraction and Purification
2.2.1. Extraction
2.2.2. Purification
3. Functionalities and Applications of Plant Polysaccharides in Fermented Foods
3.1. Rheological Properties and Applications as Thickener, Stabilizer and Fat Replacer
3.2. Biological Activities and Applications
3.2.1. The Health Benefits of Fermented Foods Containing Plant Polysaccharides and Probiotics
Plant Polysaccharides as Prebiotics
Effects of Plant Polysaccharides on the Growth of Probiotics
| Product | Polysaccharide | Probiotics | VC | Effects | References |
|---|---|---|---|---|---|
| Dairy Products | |||||
| Yogurt | inulin | BB, LB, SS, LA | 6.40–7.78 | Increased the organoleptic properties of low-fat synthetic yogurt and was comparable to full-fat probiotic yogurt in its performance characteristics. | [122] |
| BL, LA, ST, LB | >6.0 | There were appropriate sensory quality attributes and had identical ratings to the control yogurt study. | [123] | ||
| TG, inulin | LC, BB | 6.0–7.8 | The texture of the yogurt was degraded, the syneresis was increased, and the sensory score was low. | [14,124] | |
| β-glucan | BA | >7.0 | Sensory characteristics of probiotic yogurts enhanced hastened acidification and increased viscosity. | [111] | |
| SGP | ST, LB, LPL, LRH | >6.0 | There was an enhancement of both the proliferation of LAB and acidifying activity. | [12] | |
| Inulin, modified starch | LC | >6.0 | There was a detrimental effect on product acceptability (overall impression, flavor, appearance, and texture). | [125] | |
| Probiotic powder milk | Hi-maize starch | LPL | >8.0 | There were consistently maintained viable cell counts in refrigerated conditions, simulated gastric and intestinal transit. | [126] |
| Creamy goat cheese | Inulin | LA, BA | >6.0 | Better consistency and were less firm; increased fatty acids, lactic acid and essential amino acids lead to higher acidity and lower pH. | [127] |
| Ice cream | Inulin | SB | >6.0 | Enhance the physicochemical properties. | [107] |
| Inulin, resistant starch | LPL | >7.0 | There was a substantially improved probiotic viability; microcapsules containing inulin outperformed those containing starch in terms of probiotic life. | [128] | |
| Non-dairy products | |||||
| Soymilk | Inulin | BA, LA, ST | >6.0 | Fermentation reduced the amount of raffinose and stachyose. Therefore, there is no impact on the rate of acidification. | [129] |
| Fruit and vegetable Juices | Inulin | LB | >6.0 | Fermented fig juices increased polyphenols’ bioavailability and were rich in antioxidants. | [130] |
| LPL, LA | >6.0 | The quality of juices increased; monosaccharide concentration remained high, and the best survival of L. plantarum at 30 days. | [115,116] | ||
| LRH | >7.0 | Overall acceptability due to flavor, texture and seemed to favor apple cider juice with long-chain inulin fiber. | [121] | ||
| Cereal beverages | Inulin | LC, LPL | >6.0 | There were good sensory qualities and viability of over 55% for all the strains under gastric conditions. | [4] |
The Health Benefits of Fermented Foods Containing Plant Polysaccharides and Probiotics
| Products | Polysaccharide | Health Effect | Condition | References |
|---|---|---|---|---|
| Yogurt | Dietary fiber | Fortified yogurts substantially lowered TC, LDL-C, and blood pressure in hyper cholesterol patients. | In vivo | [131,135] |
| Inulin | Synbiotic yogurt’s intake strengthened hepatic characteristics in nonalcoholic fatty liver disease patients. | In vivo | [136] | |
| Inulin and wheat fiber | There was a rise in proteolysis levels resulting in antioxidant and ACE-inhibitory properties | In vitro | [137] | |
| PR | It intensified the levels of pro-inflammatory cytokines and improved the activity of NK cells. | In vitro | [134] | |
| Sheep milk ice cream | Inulin | L. casei had a strong adhesive capacity for Caco-2 cells, ensuring a therapeutic impact on the host; significant antioxidant and anti-hypertensive properties increased the product’s bioactivity. | In vitro | [138] |
| Synbiotic group significantly reduced p53 expression and apoptosis index in colonic crypts and significantly reduced micronucleated colon cells. | In vivo | [139] | ||
| Soy yogurt | Inulin | There was a protective effect on milk cultures’ viability, with decreased pH, total phenolic content and increased acidity had higher antioxidant activity during storage. | In vitro | [112] |
| Salami | Dietary fibers | There was an increase in antioxidant capacity, production of SCFAs, the change in gut microbiota structure, and reduction of intestinal pathogens. | In vitro | [140] |
| Citrus fiber | In four weeks, there were improved inflammatory, immunological, antioxidant plasmatic markers, and butyrate production. | In vivo | [141] | |
| Sausages | Inulin | There was an influence on the intestinal microbiota activity, elevated levels of SCFAs in fecal and plasma metabolome, and increased Bifidobacterium. | In vivo | [142] |
| Oat-Banana Fermented Beverage | β -glucan | The relative gene expression levels in the selected strains were related to the L-lactic acid produced in the two media. The plant matrix promoted greater ldhL gene expression in the first 4 h of the experiment. | In vitro | [143] |
3.2.2. Plant Polysaccharides and Microbial Fermentation Products as Sources of Bioactive Compounds
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Acronyms Used
| LAB | Lactic acid bacteria | UHPE | Ultra-high pressure extraction |
| MW | Molecular weight | TC | Total cholesterol |
| FOS | Fructooligosaccharide | LDL-C | Low-density lipids cholesterol |
| SCFAs | Short-chain fatty acids | ACE | Angiotensin-converting enzyme |
| UAE | Ultrasonic-assisted extraction | PR | Polysaccharide rhamnogalacturonan |
| ASE | Alkaline solvent extraction | OP | Okra polysaccharide |
| EAE | Enzymatic-assisted extraction | KP | Kernel polysaccharide |
| UAEE | Ultrasonic-assisted enzymatic extraction | DOP | Dendrobium Officinale polysaccharide |
| MAE | Microwave-assisted extraction | TG | Tragacanth gum |
| UMAE | Ultrasound-microwave assisted extraction | ldhL | L-lactate dehydrogenase gene |
| HPLC | High-performance liquid chromatography | PBMCs | peripheral blood mononuclear cells |
| T2DM | type 2 diabetes mellitus |
References
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef]
- Figueroa, C.; Echeverría, G.; Villarreal, G.; Martínez, X.; Ferreccio, C.; Rigotti, A. Introducing Plant-Based Mediterranean Diet as a Lifestyle Medicine Approach in Latin America: Opportunities Within the Chilean Context. Front. Nutr. 2021, 8, 680452. [Google Scholar] [CrossRef]
- Li, H.; Jia, P.; Fei, T. Associations between taste preferences and chronic diseases: A population-based exploratory study in China. Public Health Nutr. 2021, 24, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Nouri, L.; Mortazavian, A.M. Development of a functional synbiotic beverage fortified with different cereal sprouts and prebiotics. J. Food Sci. Technol. 2021, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.; Bedani, R.; LeBlanc, J.G.; Saad, S.M.I. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int. J. Food Microbiol. 2017, 261, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, N.N.; Jaafar, S.N.S.; Mohamed, M.A. Patents on Polysaccharide Applications. Polysacch. Prop. Appl. 2021, 591–606. [Google Scholar]
- Hassan, L.K.; Haggag, H.F.; ElKalyoubi, M.H.; Abd El-Aziz, M.; El-Sayed, M.M.; Sayed, A.F. Physico-chemical properties of yoghurt containing cress seed mucilage or guar gum. Ann. Agric. Sci. 2015, 60, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Ye, Z.; Liu, S.; Yang, Y.; Dong, J.; Wang, K.; Zhang, S.; Shen, Q.; Li, X.; Liu, D. Effects of crude Sphallerocarpus gracilis polysaccharides as potential prebiotics on acidifying activity and growth of probiotics in fermented milk. LWT 2021, 149, 111882. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Marikkar, M.N.; Ariff, A.; Amid, M.; Lamasudin, D.U.; Manap, M.Y.A.; Mustafa, S. Defatted coconut residue crude polysaccharides as potential prebiotics: Study of their effects on proliferation and acidifying activity of probiotics in vitro. J. Food Sci. Technol. 2017, 54, 164–173. [Google Scholar]
- Ghaderi-Ghahfarokhi, M.; Yousefvand, A.; Ahmadi Gavlighi, H.; Zarei, M. The effect of hydrolysed tragacanth gum and inulin on the probiotic viability and quality characteristics of low-fat yoghurt. Int. J. Dairy Technol. 2021, 74, 161–169. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Ma, Y.; Jia, X.; Wang, G.; Zhang, M. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.-R.; Wang, S.-P.; Gan, R.-Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M. Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional colloids from proteins and polysaccharides for food applications. Trends Food Sci. Technol. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Tesfaye, W.; Suarez-Lepe, J.A.; Loira, I.; Palomero, F.; Morata, A. Dairy and Nondairy-Based Beverages as a Vehicle for Probiotics, Prebiotics, and Symbiotics: Alternatives to Health Versus Disease Binomial Approach Through Food. In Milk-Based Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 473–520. [Google Scholar]
- Rosa, M.C.; Carmo, M.R.S.; Balthazar, C.F.; Guimarães, J.T.; Esmerino, E.A.; Freitas, M.Q.; Silva, M.C.; Pimentel, T.C.; Cruz, A.G. Dairy products with prebiotics: An overview of the health benefits, technological and sensory properties. Int. Dairy J. 2021, 117, 105009. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of pectin-rich orange fibre on gel characteristics and sensory properties in lactic acid fermented yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. Extraction and Isolation of β-Glucan from Grain Sources—A Review. J. Food Sci. 2017, 82, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydinol, P.; Ozcan, T. Production of reduced-fat Labneh cheese with inulin and β-glucan fibre-based fat replacer. Int. J. Dairy Technol. 2018, 71, 362–371. [Google Scholar] [CrossRef]
- Rubel, I.A.; Iraporda, C.; Novosad, R.; Cabrera, F.A.; Genovese, D.B.; Manrique, G.D. Inulin rich carbohydrates extraction from Jerusalem artichoke (Helianthus tuberosus L.) tubers and application of different drying methods. Food Res. Int. 2018, 103, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef]
- Bello, B.; Mustafa, S.; Tan, J.S.; Ibrahim, T.A.T.; Tam, Y.J.; Ariff, A.B.; Manap, M.Y.; Abbasiliasi, S. Evaluation of the effect of soluble polysaccharides of palm kernel cake as a potential prebiotic on the growth of probiotics. 3 Biotech 2018, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Abbasiliasi, S.; Tan, J.S.; Bello, B.; Ibrahim, T.A.T.; Tam, Y.J.; Ariff, A.; Mustafa, S. Prebiotic efficacy of coconut kernel cake’s soluble crude polysaccharides on growth rates and acidifying property of probiotic lactic acid bacteria in vitro. Biotechnol. Biotechnol. Equip. 2019, 33, 1216–1227. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Dai, L.; Lu, S.; Luo, Z.; Qiu, Z.; Li, J.; Li, P.; Du, B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide: Structure and immunoregulatory activities. Int. J. Biol. Macromol. 2019, 135, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Sarteshnizi, R.A.; Gavlighi, H.A.; Nikoo, M.; Azizi, M.H.; Sadeghinejad, N. Effect of partial replacement of fat with added water and tragacanth gum (Astragalus gossypinus and Astragalus compactus) on the physicochemical, texture, oxidative stability, and sensory property of reduced fat emulsion type sausage. Meat Sci. 2019, 147, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, E.; Hosseini, A.; Sobhani Nasab, A.; Adib, K.; Ganjali, M.R.; Pourmortazavi, S.M.; Ahmadi, F.; Marzi Khosrowshahi, E.; Mirsadeghi, S.; Rahimi-Nasrabadi, M. Application of polysaccharide biopolymers as natural adsorbent in sample preparation. Crit. Rev. Food Sci. Nutr. 2021, 1–28. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 1–13. [Google Scholar] [CrossRef]
- Maji, B. Introduction to natural polysaccharides. In Functional Polysaccharides for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–31. [Google Scholar]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y. A Review of Isolation, Chemical Properties, and Bioactivities of Polysaccharides from Bletilla striata. BioMed Res. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Mishra, S.K.; Yadav, H. Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obes. Control Ther. Open Access 2017, 4, PMC6249025. [Google Scholar]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and solubility. Solubility Polysacch. 2017, 7–21. [Google Scholar] [CrossRef] [Green Version]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S. Plant Polysaccharides-Based Multiple-Unit Systems for Oral Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Dhara, A.K.; Pal, D. Plant Polysaccharides in Pharmaceutical Applications. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 93–125. [Google Scholar]
- Wang, F.; Wang, W.; Niu, X.; Huang, Y.; Zhang, J. Isolation and Structural Characterization of a Second Polysaccharide from Bulbs of Lanzhou Lily. Appl. Biochem. Biotechnol. 2018, 186, 535–546. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.-Y.; Xiang, X.-R.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.-Y. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int. J. Biol. Macromol. 2020, 146, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.-H.; Liu, H.; Wu, C.-Y.; Zhao, L.; Zhang, Q.; Lin, D.-R. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow chrysanthemum (Coreopsis Tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Chen, K.; Zhang, R.; Chen, X.; Hu, P.; Kan, J. Polysaccharides from bamboo shoots processing by-products: New insight into extraction and characterization. Food Chem. 2018, 245, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Jia, D.; Yao, K.; Liu, W. The synergy of Box-Behnken designs on the optimization of polysaccharide extraction from mulberry leaves. Ind. Crops Prod. 2017, 99, 70–78. [Google Scholar] [CrossRef]
- Chen, G.; Fang, C.; Ran, C.; Tan, Y.; Yu, Q.; Kan, J. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, C.; Ni, Y.; Yao, L.; Jiang, H.; Ren, X.; Fu, Y.; Zhao, C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019, 206, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Liu, Y.; Tao, C.; Liu, Y.; Liu, M.; Wu, J.; Lv, Z. CO2 supercritical fluid extraction and characterization of polysaccharide from bamboo (Phyllostachys heterocycla) leaves. J. Food Meas. Charact. 2018, 12, 35–44. [Google Scholar] [CrossRef]
- Wu, H.; Shang, H.; Guo, Y.; Zhang, H.; Wu, H. Comparison of different extraction methods of polysaccharides from cup plant (Silphium perfoliatum L.). Process Biochem. 2020, 90, 241–248. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Rozi, P.; Mutailifu, P.; Gao, Y.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Effects of different extraction techniques on physicochemical properties and biological activities of polysaccharides from Fritillaria pallidiflora Schrenk. Process Biochem. 2019, 83, 189–197. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, X.; Huang, G. Preparation, structure, and properties of tea polysaccharide. Chem. Biol. Drug Des. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N.; Ramu Ganesan, A.; Velmurugan, K.; Sathishkumar, P.; Jayakumar, R.; Seedevi, P. Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits—A comprehensive review. Carbohydr. Polym. 2020, 238, 116185. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Wei, H.; Zhang, Y.; Guo, Z.; Qiu, Y.; Wen, L.; Xie, Z. Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agric. 2020, 100, 5603–5616. [Google Scholar]
- Wang, Y.; Li, X.; Zhao, P.; Qu, Z.; Bai, D.; Gao, X.; Zhao, C.; Chen, J.; Gao, W. Physicochemical characterizations of polysaccharides from Angelica Sinensis Radix under different drying methods for various applications. Int. J. Biol. Macromol. 2019, 121, 381–389. [Google Scholar] [CrossRef]
- Simsek, M.; Asiyanbi-Hammed, T.T.; Rasaq, N.; Hammed, A.M. Progress in Bioactive Polysaccharide-Derivatives: A Review. Food Rev. Int. 2021, 1–16. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Du, W.; Qian, L.; Xu, Y.; Huang, Y.; Xiong, Q.; Li, H.; Yuan, J. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge. Int. J. Biol. Macromol. 2020, 150, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Khan, B.M.; Cheong, K.L.; Liu, Y. Pumpkin polysaccharides: Purification, characterization and hypoglycemic potential. Int. J. Biol. Macromol. 2019, 139, 842–849. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Zhao, Y.; Liu, Y. Separation of polysaccharides from Spirulina platensis by HSCCC with ethanol-ammonium sulfate ATPS and their antioxidant activities. Carbohydr. Polym. 2017, 173, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, X.; Huang, Y.; Liao, J.; Liu, Y.; Pan, Y. Rapid quality control of medicine and food dual purpose plant polysaccharides by matrix assisted laser desorption/ionization mass spectrometry. Analyst 2020, 145, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Niu, F.-J.; Xie, Y.-X.; Xie, S.-M.; Liu, Y.-N.; Yang, Y.-Y.; Zhou, C.-Z.; Wan, X.-H. A review: Natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 2020, 129, 110469. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; de Oliveira, W.F.; dos Santos Silva, P.M.; dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Qin, N.; Yan, C.; Wang, S. Isolation, purification, characterization and bioactivities of a glucan from the root of Pueraria lobata. Food Funct. 2018, 9, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694–701. [Google Scholar] [CrossRef]
- Venugopal, V. Marine Polysaccharides: Food Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Khubber, S.; Chaturvedi, K.; Thakur, N.; Sharma, N.; Yadav, S.K. Low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Food Hydrocoll. 2021, 111, 106240. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Xu, R.; Wang, H.; Chen, J.-Y.; Tu, Z.-C. Structural Properties, Bioactivities, and Applications of Polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A Review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef] [PubMed]
- Arango, O.; Trujillo, A.J.; Castillo, M. Influence of fat substitution by inulin on fermentation process and physical properties of set yoghurt evaluated by an optical sensor. Food Bioprod. Process. 2020, 124, 24–32. [Google Scholar] [CrossRef]
- Ivanova, M.; Petkova, N.; Todorova, M.; Dobreva, V.; Vlaseva, R.; Denev, P.; Hadjikinov, D.; Bouvard, V. Influence of citrus and celery pectins on physicochemical and sensory characteristics of fermented dairy products. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 533–545. [Google Scholar]
- Yu, D.; Kwon, G.; An, J.; Lim, Y.-S.; Jhoo, J.-W.; Chung, D. Influence of prebiotic biopolymers on physicochemical and sensory characteristics of yoghurt. Int. Dairy J. 2021, 115, 104915. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Alburihi, S.; Nasser, Y.H.; Nagi, Y.M. Effects of Polysaccharides from Mango’Peel on Physiochemical and Sensory Properties of Non-Fat Yoghurts. J. Adv. Dairy Res. 2017, 5, 2. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. The Functional Aspects of Beta Glucan for Dairy Industry. Sci. Food Up-to-Date Adv. Res. Educ. Ideas 2017, 14, 264. [Google Scholar]
- Say, D.; Soltani, M.; Güzeler, N. Texture, colour and sensory properties of non-fat yoghurt as influenced by tara gum or combinations of tara gum with buttermilk powder. Mljekarstvo/Dairy 2020, 70, 313–324. [Google Scholar] [CrossRef]
- Bhaskar, D.; Khatkar, S.K.; Chawla, R.; Panwar, H.; Kapoor, S. Effect of β-glucan fortification on physico-chemical, rheological, textural, colour and organoleptic characteristics of low fat dahi. J. Food Sci. Technol. 2017, 54, 2684–2693. [Google Scholar] [CrossRef]
- Dai, S.; Jiang, F.; Corke, H.; Shah, N.P. Physicochemical and textural properties of mozzarella cheese made with konjac glucomannan as a fat replacer. Food Res. Int. 2018, 107, 691–699. [Google Scholar] [CrossRef]
- Borges, J.V.; de Souza, J.A.; Fagnani, R.; Costa, G.N.; Dos Santos, J.S. Reduced-fat Frescal sheep milk cheese with inulin: A first report about technological aspects and sensory evaluation. J. Dairy Res. 2019, 86, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Kilcawley, K.N.; Miao, S.; Fenelon, M.; Kelly, A.; Sheehan, J.J. Exploring the potential of polysaccharides or plant proteins as structuring agents to design cheeses with sensory properties focused toward consumers in East and Southeast Asia: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Câmara, A.K.F.I.; de Souza Paglarini, C.; Vidal, V.A.S.; Dos Santos, M.; Pollonio, M.A.R. Meat products as prebiotic food carrier. Probiotic Prebiotics Foods Chall. Innov. Adv. 2020, 94, 223. [Google Scholar]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in foods and its physiological functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Menegas, L.Z.; Pimentel, T.C.; Garcia, S.; Prudencio, S.H. Dry-fermented chicken sausage produced with inulin and corn oil: Physicochemical, microbiological, and textural characteristics and acceptability during storage. Meat Sci. 2013, 93, 501–506. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Ignácio, E.O.; Bis-Souza, C.V.; da Silva-Barretto, A.C. Performance of reduced fat-reduced salt fermented sausage with added microcrystalline cellulose, resistant starch and oat fiber using the simplex design. Meat Sci. 2021, 175, 108433. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, B.; Zhang, Y. Protective effects of Acanthopanax polysaccharides on cerebral ischemia–reperfusion injury and its mechanisms. Int. J. Biol. Macromol. 2015, 72, 946–950. [Google Scholar] [CrossRef]
- Misaki, R.; Fujiyama, K.; Kajiura, H. Structure and Biological Functions of Plant Glycans and Polysaccharides; Elsevier: Oxford, UK, 2021. [Google Scholar]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60–S84. [Google Scholar] [CrossRef]
- Patel, M.K.; Tanna, B.; Gupta, H.; Mishra, A.; Jha, B. Physicochemical, scavenging and anti-proliferative analyses of polysaccharides extracted from psyllium (Plantago ovata Forssk) husk and seeds. Int. J. Biol. Macromol. 2019, 133, 190–201. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Wang, Y.; Chen, S.-Y. Astragalus polysaccharide alleviates type 2 diabetic rats by reversing the glucose transporters and sweet taste receptors/GLP-1/GLP-1 receptor signaling pathways in the intestine-pancreatic axis. J. Funct. Foods 2021, 76, 104310. [Google Scholar] [CrossRef]
- Bai, R.; Li, W.; Li, Y.; Ma, M.; Wang, Y.; Zhang, J.; Hu, F. Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T.Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. Int. J. Biol. Macromol. 2018, 120, 1544–1550. [Google Scholar] [CrossRef]
- Shoukat, M.; Sorrentino, A. Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 2021, 56, 2088–2097. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Misra, S.; Mohapatra, S.; Sahu, P.S. Prebiotics and synbiotics: Recent concepts in nutrition. Food Biosci. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Kehinde, B.A.; Panghal, A.; Garg, M.K.; Sharma, P.; Chhikara, N. Vegetable milk as probiotic and prebiotic foods. Probiotic Prebiotics Foods Chall. Innov. Adv. 2020, 94, 115. [Google Scholar]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Júnior, M.R.M.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [Green Version]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, A.; Aziz, T.; Al-Dalali, S.; Zhang, J.; ud Din, J.; Chen, C.; Cao, Y.; Fatima, H.; Yang, Z. Characterization of synbiotic ice cream made with probiotic yeast Saccharomyces boulardii CNCM I-745 in combination with inulin. LWT 2021, 141, 1110910. [Google Scholar] [CrossRef]
- Florowska, A.; Krygier, K.; Florowski, T.; Dłużewska, E. Prebiotics as functional food ingredients preventing diet-related diseases. Food Funct. 2016, 7, 2147–2155. [Google Scholar] [CrossRef]
- Wong, S.-S.; Wicklund, R.; Bridges, J.; Whaley, J.; Koh, Y.B. Starch swelling behavior and texture development in stirred yogurt. Food Hydrocoll. 2020, 98, 105274. [Google Scholar] [CrossRef]
- Ozcan, O.; Ozcan, T.; Yilmaz-Ersan, L.; Akpinar-Bayizit, A.; Delikanli, B. The use of prebiotics of plant origin in functional milk products. Food Sci. Technol. 2016, 4, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Kurtuldu, O.; Ozcan, T. Effect of β-glucan on the properties of probiotic set yoghurt with Bifidobacterium animalis subsp. lactis strain Bb-12. Int. J. Dairy Technol. 2018, 71, 157–166. [Google Scholar] [CrossRef]
- Choudhary, S.; Singh, M.; Sharma, D.; Attri, S.; Sharma, K.; Goel, G. Principal component analysis of stimulatory effect of synbiotic combination of indigenous probiotic and inulin on antioxidant activity of soymilk. Probiotics Antimicrob. Proteins 2019, 11, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Effect of symbiotic interaction between a fructooligosaccharide and probiotic on the kinetic fermentation and chemical profile of maize blended rice beverages. Food Res. Int. 2017, 100, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.F.; Ramos, C.L. Functional Beverages from Cereals. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–379. [Google Scholar]
- Valero-Cases, E.; Frutos, M.J. Effect of inulin on the viability of L. plantarum during storage and in vitro digestion and on composition parameters of vegetable fermented juices. Plant Foods Hum. Nutr. 2017, 72, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Frutos, M.J. Development of prebiotic nectars and juices as potential substrates for Lactobacillus acidophilus: Special reference to physicochemical characterization and consumer acceptability during storage. LWT-Food Sci. Technol. 2017, 81, 136–143. [Google Scholar] [CrossRef]
- Da Costa, G.M.; de Carvalho Silva, J.V.; Mingotti, J.D.; Barão, C.E.; Klososki, S.J.; Pimentel, T.C. Effect of ascorbic acid or oligofructose supplementation on L. paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT 2017, 75, 195–201. [Google Scholar] [CrossRef]
- Miranda, R.F.; da Silva, J.P.; Machado, A.R.F.; da Silva, E.C.; de Souza, R.C.; Marcolino, V.A.; Klososki, S.J.; Pimentel, T.C.; Barão, C.E. Impact of the addition of Lactobacillus casei and oligofructose on the quality parameters of orange juice and hibiscus tea mixed beverage. J. Food Process. Preserv. 2019, 43, e14249. [Google Scholar] [CrossRef]
- Bernal-Castro, C.A.; Díaz-Moreno, C.; Gutiérrez-Cortés, C. Inclusion of prebiotics on the viability of a commercial Lactobacillus casei subsp. rhamnosus culture in a tropical fruit beverage. J. Food Sci. Technol. 2019, 56, 987–994. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- White, J.; Hekmat, S. Development of probiotic fruit juices using Lactobacillus rhamnosus GR-1 fortified with short chain and long chain inulin fiber. Fermentation 2018, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- El-Kholy, W.M.; Aamer, R.A.; Ali, A.N.A. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020, 65, 59–67. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Ramadan, M.F. Changes in the physicochemical and microbiological properties of probiotic-fermented low-fat yoghurt enriched with barley β-glucan during cold storage. Mljekarstvo Časopis Unaprjeđenje Proizvodnje Prerade Mlijeka 2018, 68, 295–309. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Yousefvand, A.; Ahmadi Gavlighi, H.; Zarei, M.; Farhangnia, P. Developing novel synbiotic low-fat yogurt with fucoxylogalacturonan from tragacanth gum: Investigation of quality parameters and Lactobacillus casei survival. Food Sci. Nutr. 2020, 8, 4491–4504. [Google Scholar] [CrossRef]
- Dias, S.S.; de Souza Vergílio, D.; Pereira, A.M.; Klososki, S.J.; Marcolino, V.A.; da Cruz, R.M.S.; Costa, G.N.; Barão, C.E.; Pimentel, T.C. Probiotic Greek yogurt: Effect of the addition of prebiotic fat substitutes on the physicochemical characteristics, probiotic survival, and sensory acceptance. J. Dairy Res. 2021, 88, 98–104. [Google Scholar] [CrossRef]
- Bradford, R.; Reyes, V.; Bonilla, F.; Bueno, F.; Dzandu, B.; Liu, C.; Chouljenko, A.; Sathivel, S. Development of milk powder containing Lactobacillus plantarum NCIMB 8826 immobilized with prebiotic hi-maize starch and survival under simulated gastric and intestinal conditions. Food Prod. Process. Nutr. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Barbosa, I.C.; Oliveira, M.E.G.; Madruga, M.S.; Gullón, B.; Pacheco, M.T.B.; Gomes, A.M.P.; Batista, A.S.M.; Pintado, M.M.E.; Souza, E.L.; Queiroga, R.C.R.E. Influence of the addition of Lactobacillus acidophilus La-05, Bifidobacterium animalis subsp. lactis Bb-12 and inulin on the technological, physicochemical, microbiological and sensory features of creamy goat cheese. Food Funct. 2016, 7, 4356–4371. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of probiotics in multi-polysaccharide microcapsules by electro-hydrodynamic atomization and incorporation into ice-cream formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Battistini, C.; Gullón, B.; Ichimura, E.S.; Gomes, A.M.P.; Ribeiro, E.P.; Kunigk, L.; Moreira, J.U.V.; Jurkiewicz, C. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz. J. Microbiol. 2018, 49, 303–309. [Google Scholar] [CrossRef]
- Khezri, S.; Mahmoudi, R.; Dehghan, P. Fig juice fortified with inulin and Lactobacillus Delbrueckii: A promising functional food. Appl. Food Biotechnol. 2018, 5, 97–106. [Google Scholar]
- Ahmad, N.; Shabbir, U.; Sameen, A.; Manzoor, M.F.; Ahmad, M.H.; Ismail, T.; Ahmed, S.; Siddique, R. Hypocholesterolemic effect of designer yogurts fortified with omega fatty acids and dietary fibers in hypercholesterolemic subjects. Food Sci. Technol. 2021, 2061. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Klososki, S.J.; Silva, R.; Raices, R.S.L.; Silva, M.C.; Freitas, M.Q.; Barão, C.E.; Pimentel, T.C. Passion fruit-flavored ice cream processed with water-soluble extract of rice by-product: What is the impact of the addition of different prebiotic components? LWT 2020, 128, 109472. [Google Scholar] [CrossRef]
- Sarfraz, F.; Farooq, U.; Shafi, A.; Hayat, Z.; Akram, K.; Rehman, H.U. Hypolipidaemic effects of synbiotic yoghurt in rabbits. Int. J. Dairy Technol. 2019, 72, 545–550. [Google Scholar] [CrossRef]
- Lee, M.-H.; Kim, M.; Kim, M.; Kwak, J.H.; Chang, D.H.; Yu, W.K.; Lee, S.-H.; Lee, J.H. Consumption of dairy yogurt with the polysaccharide rhamnogalacturonan from the peel of the Korean citrus hallabong enhances immune function and attenuates the inflammatory response. Food Funct. 2016, 7, 2833–2839. [Google Scholar] [CrossRef]
- Ahmad, N.; Manzoor, M.F.; Shabbir, U.; Ahmed, S.; Ismail, T.; Saeed, F.; Nisa, M.; Anjum, F.M.; Hussain, S. Health lipid indices and physicochemical properties of dual fortified yogurt with extruded flaxseed omega fatty acids and fibers for hypercholesterolemic subjects. Food Sci. Nutr. 2020, 8, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi, M.B.H.; Fatemizadeh, S.S.; Tavakoli, M. Release of proteolysis products with ACE-inhibitory and antioxidant activities in probiotic yogurt containing different levels of fat and prebiotics. Int. J. Pept. Res. Ther. 2019, 25, 367–377. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Silva, H.L.A.; Esmerino, E.A.; Rocha, R.S.; Moraes, J.; Carmo, M.A.V.; Azevedo, L.; Camps, I.; Abud, Y.K.D.; Sant’Anna, C. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.F.; de Moura, N.A.; Romualdo, G.R.; Rocha, R.S.; Pimentel, T.C.; Esmerino, E.A.; Freitas, M.Q.; Santillo, A.; Silva, M.C.; Barbisan, L.F.; et al. Synbiotic sheep milk ice cream reduces chemically induced mouse colon carcinogenesis. J. Dairy Sci. 2021, 104, 7406–7414. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Mehta, T.; Pastoriza, S.; Kramer, D.L.; Paliy, O.; Rufián-Henares, J.Á. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT 2019, 105, 355–362. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Francino, M.P.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66, 103790. [Google Scholar] [CrossRef]
- Thøgersen, R.; Castro-Mejía, J.L.; Sundekilde, U.K.; Hansen, L.H.; Hansen, A.K.; Nielsen, D.S.; Bertram, H.C. Ingestion of an Inulin-Enriched Pork Sausage Product Positively Modulates the Gut Microbiome and Metabolome of Healthy Rats. Mol. Nutr. Food Res. 2018, 62, e1800608. [Google Scholar] [CrossRef] [PubMed]
- Goncerzewicz, A.; Misiewicz, A.; Owczarek, L.; Jasińska, U.; Skąpska, S. The Effect of a Newly Developed Oat-Banana Fermented Beverage with a Beta-glucan Additive on ldhL Gene Expression in Streptococcus thermophilus T(K)M(3) KKP 2030p. Curr. Microbiol. 2016, 73, 773–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2020, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-chain fatty acids produced by synbiotic mixtures in skim milk differentially regulate proliferation and cytokine production in peripheral blood mononuclear cells. Int. J. Food Sci. Nutr. 2015, 66, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M. Viability and activity of bifidobacteria during refrigerated storage of yoghurt containing Mangifera pajang fibrous polysaccharides. J. Food Sci. 2012, 77, M624–M630. [Google Scholar] [CrossRef] [PubMed]
- Mosso, A.L.; Jimenez, M.E.; Vignolo, G.; LeBlanc, J.G.; Samman, N.C. Increasing the folate content of tuber based foods using potentially probiotic lactic acid bacteria. Food Res. Int. 2018, 109, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.-C. Folic acid and diseases-supplement it or not? Rev. Assoc. Med. Bras. 2016, 62, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyigaba, T.; Liu, D.; Habimana, J.d.D. The Extraction, Functionalities and Applications of Plant Polysaccharides in Fermented Foods: A Review. Foods 2021, 10, 3004. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123004
Niyigaba T, Liu D, Habimana JdD. The Extraction, Functionalities and Applications of Plant Polysaccharides in Fermented Foods: A Review. Foods. 2021; 10(12):3004. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123004
Chicago/Turabian StyleNiyigaba, Theoneste, Diru Liu, and Jean de Dieu Habimana. 2021. "The Extraction, Functionalities and Applications of Plant Polysaccharides in Fermented Foods: A Review" Foods 10, no. 12: 3004. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10123004