Detection of Carrageenan in Meat Products Using Lectin Histochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Model Samples
2.2. Preparation of Samples from the Market Network
2.3. Buffer Preparation
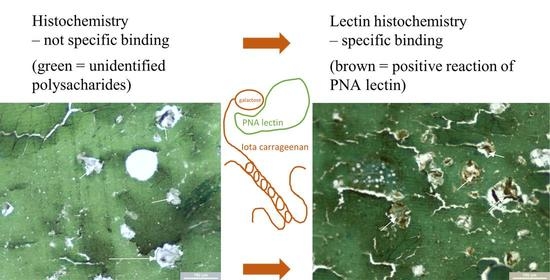
2.4. Lectin Histochemistry
2.5. Statistical Processing
2.6. Specificity and Sensitivity
3. Results and Discussion
3.1. Determination of Lectin Concentration
3.2. Method Repeatability
3.3. Limit of Determination (LoD)
3.4. Cross-Reactivity
3.5. Detection of Carrageenan in Meat Products from the Market Network
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.T.N.T.; Nguyen, B.T.; Nicolai, T.; Renou, F. Mobility of carrageenan chains in iota- and kappa carrageenan gels. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 113–118. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A review of property enhancement techniques for carrageenan-based films and coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Kozlowska, J.; Pauter, K.; Sionkowska, A. Carrageenan-based hydrogels: Effect of sorbitol and glycerin on the stability, swelling and mechanical properties. Polym. Test. 2018, 67, 7–11. [Google Scholar] [CrossRef]
- Tarté, R. Ingredients in Meat Products: Properties, Functionality and Applications; Springer Science+Business Media: New York, NY, USA, 2009; ISBN 9780387713267. [Google Scholar]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Cui, B.; Liu, Y. Influence of cations on texture, compressive elastic modulus, sol-gel transition and freeze-thaw properties of kappa-carrageenan gel. Carbohydr. Polym. 2018, 202, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Hassan, R.A.; Heng, L.Y.; Tan, L.L. Novel DNA biosensor for direct determination of carrageenan. Sci. Rep. 2019, 9, 19. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 1129/2011 EUR-Lex-32011R1129-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1129 (accessed on 19 January 2021).
- Bixler, H.J. The carrageenan controversy. J. Appl. Phycol. 2017, 29, 2201–2207. [Google Scholar] [CrossRef]
- Tobacman, J.K. Reply to comments regarding “The Carrageenan Controversy”. J. Appl. Phycol. 2017, 29, 2209–2211. [Google Scholar] [CrossRef]
- Quemener, B.; Marot, C.; Mouillet, L.; Da Riz, V.; Diris, J. Quantitative analysis of hydrocolloids in food systems by methanolysis coupled to reverse HPLC. Part 1. Gelling carrageenans. Food Hydrocoll. 2000, 14, 19–28. [Google Scholar] [CrossRef]
- Ziółkowska, D.; Kaniewska, A.; Lamkiewicz, J.; Shyichuk, A. Determination of carrageenan by means of photometric titration with Methylene Blue and Toluidine Blue dyes. Carbohydr. Polym. 2017, 165, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Meyerhoff, M.E.; Badr, I.H.A.; Abd-Rabboh, H.S.M. Determination of carrageenan in food products using potentiometric polyion sensors. Electroanalysis 2002, 14, 439–444. [Google Scholar] [CrossRef]
- Flint, F.O. Micro-technique for the identification of food hydrocolloids. Analyst 1990, 115, 61. [Google Scholar] [CrossRef]
- Bednářová, M.; Pospiech, M.; Jandásek, J.; Tremlová, B. Carrageenans in the meat industry: Detection using microscopic methods. Maso Int. 2014, 1, 11–14. [Google Scholar]
- Brooks, S.; Leathem, A.; Schumacher, U. Lectin Histochemistry: A Concise Practical Handbook, 1st ed.; Gerland Science: Oxford, UK, 1996; ISBN 1 85996 100 2. [Google Scholar]
- Pihíková, D.; Kasák, P.; Tkac, J. Glycoprofiling of cancer biomarkers: Label-free electrochemical lectin-based biosensors. Open Chem. 2015, 13, 636–655. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 827. [Google Scholar] [CrossRef]
- Mubarak, H.J.; Al-Taee, A.A.; Al-Araji, A.S. Lectin histochemistry of tracheo-esophageal region in chick embryos. Iraqi J. Med. Sci. 2013, 11, 4–11. [Google Scholar]
- Díaz, M.; González, N.; Zanuzzi, C.; Najle, R.; Barbeito, C.G. Lectin histochemistry for detecting cadmium-induced changes in the glycosylation pattern of rat placenta. Biotech. Histochem. 2017, 92, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, M.; Tremlová, B.; Renčová, E.; Randulová, Z.; Lukášková, R.Z.; Pokorná, J. Comparison of the results of the ELISA, histochemical, and immunohistochemical detection of soya proteins in meat products. Czech. J. Food Sci. 2011, 29, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Trullols, E.; Ruisánchez, I.; Rius, F.X. Validation of qualitative analytical methods. TrAC Trends Anal. Chem. 2004, 23, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Řezáčová-Lukášková, Z.; Tremlová, B.; Pospiech, M.; Renčová, E.; Randulová, Z. Immunohistochemical detection of wheat protein in model samples. Czech. J. Food Sci. 2010, 28, 516–519. [Google Scholar] [CrossRef]
- McCabe, A.; Dolled-Filhart, M.; Camp, R.L.; Rimm, D.L. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J. Natl. Cancer Inst. 2005, 97, 1808–1815. [Google Scholar] [CrossRef]
- Hashim, O.H.; Jayapalan, J.J.; Lee, C.S. Lectins: An effective tool for screening of potential cancer biomarkers. PeerJ 2017, 2017, e3784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef]
- Bednářová, M.; Pospiech, M.; Tremlová, B.; Řezáčová-Lukášková, Z.; Bednář, J. Antigen retrieval and fixation of sections on slides for immunohistochemical detection of soya protein in meat products. J. Food Nutr. Res. 2015, 54, 1–8. [Google Scholar]
- Ling, Y.P.; Heng, L.Y. Reflectance based sensor for carrageenan utilizing methylene blue embedded acrylic microspheres. Sens. Actuators B Chem. 2014, 192, 247–252. [Google Scholar] [CrossRef]
- Taylor, S.L.; Nordlee, J.A.; Niemann, L.M.; Lambrecht, D.M. Allergen immunoassays-considerations for use of naturally incurred standards. Anal. Bioanal. Chem. 2009, 395, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kaltner, H.; Dong, X.; Gabius, H.J.; Brewer, C.F. Comparative cross-linking activities of lactose-specific plant and animal lectins and a natural lactose-binding immunoglobulin G fraction from human serum with asialofetuin. Glycobiology 1996, 6, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigh, J.; Buchmann, K. Comparative analysis of cross-reactivity between Ichthyophthirius and Tetrahymena. Bull. Eur. Assoc. Fish. Pathol. 2002, 22, 37–44. [Google Scholar]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Applications of natural polymer gum Arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Ong-Abdullah, J.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, K.A.; Mohammadi, A.H. Effects of Tragacanth Gum as a natural polymeric surfactant and soluble ions on chemical smart water injection into oil reservoirs. J. Mol. Struct. 2020, 1200, 78. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Xie, H.; Yan, S.; Yin, H. A simple method to calculate the degree of polymerization of alginate oligosaccharides and low molecular weight alginates. Carbohydr. Res. 2019, 486. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Wang, Q.; Tang, Y.; Li, F.; Zhao, J.; Zhang, Y.; Ming, J. Combined effect of carboxymethylcellulose and salt on structural properties of wheat gluten proteins. Food Hydrocoll. 2019, 97, 89. [Google Scholar] [CrossRef]
- Da Silva, H.M.; Mageste, A.B.; Silva, S.J.B.E.; Ferreira, D.G.M. Anthocyanin immobilization in carboxymethylcellulose/starch films: A sustainable sensor for the detection of Al(III) ions in aqueous matrices. Carbohydr. Polym. 2020, 230, 79. [Google Scholar] [CrossRef] [PubMed]
- Paradee, N.; Sirivat, A.; Niamlang, S.; Prissanaroon-Ouajai, W. Effects of crosslinking ratio, model drugs, and electric field strength on electrically controlled release for alginate-based hydrogel. J. Mater. Sci. Mater. Med. 2012, 118, 4571–4577. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.H. Synthesis, equilibrium swelling, kinetics, permeability and applications of environmentally responsive gels. Adv. Polym. Sci. 1993, 110, 80–144. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Bagchi, G.D.; Srivastava, G.N. Spices and flavoring (flavouring) crops/Fruits and seeds. Encycl. Food Sci. Nutr. 2003, 67, 5465–5477. [Google Scholar]
- Thomas, M.A.; Lemmer, B. HistoGreen: A new alternative to 3,3′-diaminobenzidine- tetrahydrochloride-dihydrate (DAB) as a peroxidase substrate in immunohistochemistry? Brain Res. Protoc. 2005, 14, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hopf, H.; Kandler, O. Characterization of the “reserve cellulose” of the endosperm of Carum carvi as a β(1-4)-mannan. Phytochemistry 1977, 16, 1715–1717. [Google Scholar] [CrossRef]
- Besusparis, J.; Plancoulaine, B.; Rasmusson, A.; Augulis, R.; Green, A.R.; Ellis, I.O.; Laurinaviciene, A.; Herlin, P.; Laurinavicius, A. Impact of tissue sampling on accuracy of Ki67 immunohistochemistry evaluation in breast cancer. Diagn. Pathol. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goethals, L.; Perneel, C.; Debucquoy, A.; de Schutter, H.; Borghys, D.; Ectors, N.; Geboes, K.; McBride, W.H.; Haustermans, K.M. A new approach to the validation of tissue microarrays. J. Pathol. 2006, 208, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Řezáčová-Lukášková, Z.; Pospiech, M.; Tremlová, B.; Renčová, E.; Petrášová, M. Quantitative immunohistochemical method for detection of wheat protein in model sausage. Acta Vet. Brno 2014. [Google Scholar] [CrossRef] [Green Version]
- Trevethan, R. Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Knulst, A.C.; Koers, W.J.; Penninks, A.H.; Peppelman, H.; Vlooswijk, R.; Pigmans, I.; van Duijn, G.; Hessing, M. Comparison of different immunochemical methods for the detection and quantification of hazelnut proteins in food products. J. Immunol. Methods 1999, 229, 107–120. [Google Scholar] [CrossRef]
- Fæste, C.K.; Jonscher, K.R.; Louis, S.I.T.; Klawitter, J.; Løvberg, K.E.; Moen, L.H. Differentiating cross-reacting allergens in the immunological analysis of celery (Apium graveolens) by mass spectrometry. J. AOAC Int. 2010, 93, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartůňková, J.; Paulík, M. Examination Methods in Immunology, 2nd ed.; Grada: Prague, Czech Republic, 2011; ISBN 978-80-247-3533-7. [Google Scholar]
- Heick, J.; Fischer, M.; Kerbach, S.; Tamm, U.; Popping, B. Application of a liquid chromatography tandem mass spectrometry method for the simultaneous detection of seven allergenic foods in flour and bread and comparison of the method with commercially available ELISA test kits. J. AOAC Int. 2011, 94, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Lukášková, Z.Ř.; Tremlová, B.; Pospiech, M.; Renčová, E.; Randulová, Z.; Steinhauser, L.; Reichová, A.; Bednář, J. Comparison of immunohistochemical, histochemical and immunochemical methods for the detection of wheat protein allergens in meat samples and cooked, dry, raw and fermented sausage samples. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Amigo, C.; Popping, B. Accuracy of ELISA detection methods for gluten and reference materials: A realistic assessment. J. Agric. Food Chem. 2013. [Google Scholar] [CrossRef]
- Petrášová, M.; Pospiech, M.; Tremlová, B.; Tauferová, A.; Marcinčák, S. Comparison of immunofluorescence method with histochemical and ELISA methods focusing on wheat protein detection in meat products. Food Agric. Immunol. 2017, 28, 1094–1104. [Google Scholar] [CrossRef]
- Høyer, P.E.; Kirkeby, S. The impact of fixatives on the binding of lectins to N-acetyl-glucosamine residues of human syncytiotrophoblast: A quantitative histochemical study. J. Histochem. Cytochem. 1996, 44, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Bartlová, M.; Javůrková, Z.; Pospiech, M.; Bartl, P.; Luňáková, L.; Běhalová, H.; Tremlová, B. Identification of κ, ι, λ-carrageenan by image analysis. Maso 2018, 29, 51–53. [Google Scholar]



| Concentration (μgmL−1) | Dilution | Lectin PNA | Lectin BSA |
|---|---|---|---|
| 10.00 | 1:100 | +++ | +++ |
| 1.00 | 1:1000 | +++ | +++ |
| 0.40 | 1:2500 | +++ | +++ |
| 0.20 | 1:5000 | +++ | +++ |
| 0.13 | 1:7500 | +++ | +++ |
| 0.10 | 1:10,000 | +++ | +++ |
| Sample | Evaluators | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Lectin PNA | Lectin BSA | Lectin PNA | Lectin BSA | Lectin PNA | Lectin BSA | |
| κ-carrageenan (10g kg−1) | 20/0 | 17/0 | 20/0 | 16/0 | 20/0 | 16/0 |
| ι-carrageenan (10g kg−1) | 20/0 | 20/0 | 20/0 | 20/0 | 20/0 | 20/0 |
| λ-carrageenan (10g kg−1) | 20/0 | 20/0 | 19/± | 20/0 | 20/0 | 20/0 |
| M21 | 20/0 | 19/0 | 20/0 | 19/0 | 20/0 | 19/0 |
| Carrageenan Concentration [g kg−1] | Lectin PNA | Lectin BSA | ||||
|---|---|---|---|---|---|---|
| κ | ι | λ | κ | ι | λ | |
| 0.01 | P | P | P | P | P | P |
| 0.1 | P | P | P | P | P | P |
| 1 | P | P | P | P | P | P |
| 10 | P | P | P | P | P | P |
| 100 | P | P | P | P | P | P |
| Identification or Number of Samples | Composition of Model Sample | Lectin PNA | Lectin BSA |
|---|---|---|---|
| CR1 | native potato starch | N | N |
| CR2 | native tapioca starch | N | N |
| CR3 | native corn starch | N | N |
| CR4 | waxy corn | N | N |
| CR5 | meat + 1% lupine | N | N |
| CR6 | meat + 1% amaranth | N | N |
| CR7 | meat + fiber | N | N |
| CR8 | meat + 1% wheat protein | N | N |
| CR9 | meat + emulac cc | N | N |
| CR10 | meat + whey powder | N | N |
| CR11 | meat + tonsils + brain + caraway | FP | FP |
| CR12 | meat + 1% pea flour | N | N |
| CR13 | meat + 1% pea protein 80 | N | N |
| CR14 | meat + 1% agar-agar | ± | ± |
| CR15 | meat + 1% guar gum | N | N |
| CR16 | meat + 1% sodium alginate | ± | ± |
| CR17 | meat + 1% carboxymethylcellulose | ± | ± |
| CR18 | meat + 1% carob | FP | FP |
| CR19 | meat + 1% gum arabic | N | N |
| CR20 | meat + 0.9% E407 + 0.3% polyphosphate | P | P |
| CR21 | meat + 0.9% E407 + 2.1% nitrite salt | P | P |
| CR22 | meat + 0.9% E407 + 0.05% ascorbic acid | P | P |
| CR23 | meat + 0.9% E407 + 2.1% table salt | P | P |
| CR24 | meat + 0.9% E407 + 1% emulac cc | N | ± |
| CR25 | meat + 0.9% E407 + 5% collagen | N | N |
| CR26 | meat + 0.9% E407 + 1% soy | P | P |
| CR27 | meat + 0.9% E407 + 1% whey | P | P |
| CR28 | meat + 0.9% E407 + 0.3% polyphosphates + 2.1% nitrite salt | P | P |
| CR29 | meat + 0.9% E407 + 0.3% polyphosphates + 2.1% nitrite salt + 0.05% ascorbic acid | P | P |
| CR30 | meat + 1% black pepper crushed | FP | FP |
| CR31 | meat + 1% white pepper ground | FP | FP |
| CR32 | meat + 1% caraway ground | FP | FP |
| CR33 | meat + 1% konjac gum | N | N |
| CR34 | meat + 1% xanthan gum | N | N |
| CR35 | tragacanth gum | N | N |
| Sample | Lectin PNA | Lectin BSA | Declaration | Notes |
|---|---|---|---|---|
| CR-MP-1 | N | N | N | pâté without E407 |
| CR-MP-2 | P | P | P | pâté with E407 |
| CR-MP-3 | N | FP | N | ham—injection 20% nitrite salt 2.5%, without carrageenan |
| CR-MP-4 | P | P | P | ham—injection 20% nitrite salt 2.5%, κ- carrageenan 1% |
| CR-MP-5 | P | P | P | ham—injection 20% nitrite salt 2.5%, λ-carrageenan 1% |
| CR-MP-6 | P | P | P | ham—injection 20% nitrite salt 2.5%, ι-carrageenan 1% |
| CR-MP-7 | N | N | N | MP—without additive |
| CR-MP-8 | P | FN | P | MP—E407 0.3% |
| CR-MP-9 | P | P | P | MP—E407 0.6% |
| CR-MP-10 | P | P | P | MP—E407 0.9% |
| CR-MP-11 | P | P | P | MP—E407 1.2% |
| CR-MP-12 | P | P | P | MP—E407a 0.3% |
| CR-MP-13 | P | P | P | MP—E407a 0.6% |
| CR-MP-14 | P | P | P | MP—E407a 0.9% |
| CR-MP-15 | P | P | P | MP—E407a 1.2% |
| CR-MP-16 | P | P | P | MP—κ-carrageenan standard 0.3% |
| CR-MP-17 | P | P | P | MP—κ-carrageenan standard 0.6% |
| CR-MP-18 | P | P | P | MP—κ-carrageenan standard 0.9% |
| CR-MP-19 | P | FN | P | MP—κ-carrageenan standard 1.2% |
| CR-MP-20 | P | P | P | MP—λ-carrageenan 0.3% |
| CR-MP-21 | P | P | P | MP—λ-carrageenan 0.6% |
| CR-MP-22 | P | P | P | MP—λ-carrageenan 0.9% |
| CR-MP-23 | P | P | P | MP—λ-carrageenan 1.2% |
| CR-MP-24 | P | P | P | MP—κ-carrageenan 0.3% |
| CR-MP-25 | P | P | P | MP—κ-carrageenan 0.6% |
| CR-MP-26 | P | P | P | MP—κ-carrageenan 0.9% |
| CR-MP-27 | P | P | P | MP—κ-carrageenan 1.2% |
| Sample | Lectin PNA | Lectin BSA | Additive Used |
|---|---|---|---|
| Positive/Negative * | Positive/Negative * | ||
| M1 | 8/0 | 8/0 | P (E407) |
| M2 | 8/0 | 8/0 | P (E407a) |
| M3 | 6/2 | 5/3 | P (E407) |
| M4 | 8/0 | 8/0 | P (E407) |
| M5 | 8/0 | 7/1 | P (E407) |
| M6 | 8/0 | 8/0 | P (E407) |
| M7 | 8/0 | 8/0 | P (E407a) |
| M8 | 8/0 | 8/0 | P (E407) |
| M9 | 7/1 | 5/3 | P (E407) |
| M10 | 8/0 | 7/1 | P (E407) |
| M11 | 8/0 | 8/0 | P (E407a) |
| M12 | 8/0 | 8/0 | N |
| M13 | 8/0 | 5/3 | P (E407) |
| M14 | 8/0 | 8/0 | P (E407) |
| M15 | 7/0 | 8/0 | P (E407) |
| M16 | 8/0 | 8/0 | P (E407) |
| M17 | 2/6 | 2/6 | N |
| M18 | 8/0 | 8/0 | N |
| M19 | 6/0 | 8/0 | P (E407) |
| M20 | 7/1 | 8/0 | N |
| M21 | 8/0 | 8/0 | P (E407) |
| M22 | 8/0 | 8/0 | P (E407) |
| M23 | 0/7 | 0/7 | P (E407) |
| M24 | 8/0 | 8/0 | P (E407a) |
| M25 | 3/5 | 3/5 | N |
| M26 | 8/0 | 5/3 | N |
| M27 | 0/8 | 0/8 | N |
| M28 | 0/8 | 0/7 | N |
| M39 | 0/8 | 0/8 | N |
| M30 | 0/8 | 0/8 | N |
| M31 | 2/6 | 3/5 | N |
| M32 | 2/6 | 2/4 | N |
| M33 | 0/8 | 0/8 | N |
| M34 | 5/3 | 5/1 | N |
| M35 | 0/8 | 1/7 | N |
| M36 | 0/7 | 0/5 | N |
| M37 | 6/2 | 4/2 | N |
| M38 | 0/7 | 0/5 | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlová, M.; Tremlová, B.; Marcinčák, S.; Pospiech, M. Detection of Carrageenan in Meat Products Using Lectin Histochemistry. Foods 2021, 10, 764. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040764
Bartlová M, Tremlová B, Marcinčák S, Pospiech M. Detection of Carrageenan in Meat Products Using Lectin Histochemistry. Foods. 2021; 10(4):764. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040764
Chicago/Turabian StyleBartlová, Marie, Bohuslava Tremlová, Slavomír Marcinčák, and Matej Pospiech. 2021. "Detection of Carrageenan in Meat Products Using Lectin Histochemistry" Foods 10, no. 4: 764. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040764