Synergistic Effect of Static Magnetic Field and Modified Atmosphere Packaging in Controlling Blown Pack Spoilage in Meatballs
Abstract
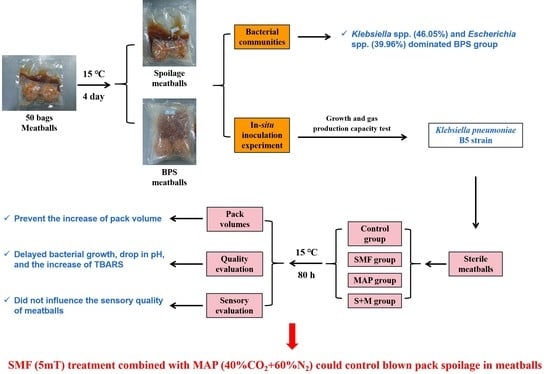
:1. Introduction
2. Materials and Methods
2.1. Meatballs Samples
2.2. Experimental Design
2.2.1. Microbial Comparison in Meatballs with or without BPS
2.2.2. Screening and Characteristics of Gas-Forming Bacterial Strains in BPS Meatballs
2.2.3. In Situ Inoculation Experiment, Growth and Gas Production Capacity of Gas-Producing Bacteria
2.2.4. Effects of SMF Combined with MAP on BPS of Meatballs
2.3. Test Methods
2.3.1. Determining Packs Volumes and Gas Compositions
2.3.2. Enumeration of Microorganisms
2.3.3. Bacterial Diversity
2.3.4. Growth Curves of Gas-Forming Bacterial Strains
2.3.5. pH
2.3.6. Total Volatile Basic Nitrogen (TVB-N)
2.3.7. TBARS
2.3.8. Sensory Evaluation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Pack Volumes and Gas Compositions
3.2. Microbiological Analysis
3.3. Isolation and Verification of Package-Swelling Ability of Bacteria Strains in Meatballs
3.4. Effects of SMF Combined with MAP on Volume and CO2 Content in Packaging
3.5. Effects of SMF Combined with MAP on Bacterial Growth in Meatballs
3.6. Effects of SMF Combined with MAP on pH, TBARS, TVB-N and Sensory
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, R.; Fanning, S.; Whyte, P.; Kerry, J.; Bolton, D. An investigation of the effect of rapid slurry chilling on blown pack spoilage of vacuum-packaged beef primals. Lett. Appl. Microbiol. 2017, 64, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.R.; Paulo, É.N.; Sant’Ana, A.S.; Chaves, R.D.; Massaguer, P.R. Involvement of Clostridium gasigenes and C. algidicarnis in “blown pack” spoilage of Brazilian vacuum-packed beef. Int. J. Food Microbiol. 2011, 148, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Broda, D.M.; Saul, D.J.; Lawson, P.A.; Bell, R.G.; Musgrave, D.R. Clostridium gasigenes sp. nov., a psychrophile causing spoilage of vacuum-packed meat. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 1, 107. [Google Scholar] [CrossRef] [Green Version]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M. Spoilage of vacuum-packed beef by a clostridium sp. J. Sci. Food Agric. 1989, 49, 473–486. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Ray, B.; Field, R.A.; Johnson, M.C. Spoilage of vacuum-packaged refrigerated beef by Clostridium. J. Food Protect. 1989, 52, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ward, P.; Mcmullen, L.M.; Yang, X. A case of “blown pack” spoilage of vacuum-packaged pork likely associated with Clostridium estertheticum in Canada. Lett. Appl. Microbiol. 2020, 70, 13–20. [Google Scholar] [CrossRef]
- Húngaro, H.M.; Caturla, M.Y.R.; Horita, C.N.; Furtado, M.M.; Sant’Ana, A.S. Blown pack spoilage in vacuum-packaged meat: A review on clostridia as causative agents, sources, detection methods, contributing factors and mitigation strategies. Trends Food Sci. Technol. 2016, 52, 123–138. [Google Scholar] [CrossRef]
- Schuster, J.A.; Klingl, A.; Vogel, R.F.; Ehrmann, M.A. Polyphasic characterization of two novel Lactobacillus spp. isolated from blown salami packages: Description of Lactobacillus halodurans sp. nov. and Lactobacillus salsicarnum sp. nov. Syst. Appl. Microbiol. 2019, 42, 126023. [Google Scholar] [CrossRef]
- Pinheiro, R.S.B.; Jorge, A.M.; Yokoo, M.J. Correlações entre medidas determinadas in vivo por ultrassom e na carcaça de ovelhas de descarte. Rev. Bras. Zootec. 2010, 39, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Broda, D.M. The effect of peroxyacetic acid-based sanitizer, heat and ultrasonic waves on the survival of Clostridium estertheticum spores in vitro. Lett. Appl. Microbiol. 2007, 45, 336–341. [Google Scholar] [CrossRef]
- Boerema, J.A.; Broda, D.M.; Penney, N.; Brightwell, G. Influence of peroxyacetic acid-based carcass rinse on the onset of “blown pack” spoilage in artificially inoculated vacuum-packed chilled beef. J. Food Prot. 2007, 70, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Adam, K.H.; Flint, S.H.; Brightwell, G. Reduction of spoilage of chilled vacuum-packed lamb by psychrotolerant clostridia. Meat Sci. 2013, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hashish, A.H.; El-Missiry, M.A.; Abdelkader, H.I.; Abou-Saleh, R.H. Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol. Environ. Saf. 2008, 71, 895–902. [Google Scholar] [CrossRef]
- Albuquerque, W.; Costa, R.; Fernandes, T.; Porto, A. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 2016, 121, 16–28. [Google Scholar] [CrossRef]
- Filipič, J.; Kraigher, B.; Tepuš, B.; Kokol, V.; Mandic-Mulec, I. Effects of low-density static magnetic fields on the growth and activities of wastewater bacteria Escherichia coli and Pseudomonas putida. Bioresour. Technol. 2012, 120, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Huang, H.; Deng, A.; Pan, C. Effects of static magnetic fields on Escherichia coli. Micron 2009, 40, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, S.; Klausmann, S.; Kreyenschmidt, J. Effect of high-oxygen and oxygen-free modified atmosphere packaging on the spoilage process of poultry breast fillets. Poult. Sci. 2015, 94, 96–103. [Google Scholar] [CrossRef]
- Li, R.; Cai, L.; Gao, T.; Li, C.; Zhou, G.; Ye, K. Comparing the quality characteristics and bacterial communities in meatballs with or without blown pack spoilage. LWT 2020, 130, 109529. [Google Scholar] [CrossRef]
- Huang, L. IPMP 2013—A comprehensive data analysis tool for predictive microbiology. Int. J. Food Microbiol. 2014, 171, 100–107. [Google Scholar] [CrossRef]
- Broda, D.M.; Delacy, K.M.; Bell, R.G.; Braggins, T.J.; Cook, R.L. Psychrotrophic Clostridium spp. associated with “blown pack” spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 1996, 29, 335–352. [Google Scholar] [CrossRef]
- Chaves, R.D.; Silva, A.R.; SantAna, A.S.; Campana, F.B.; Massaguer, P.R. Gas-producing and spoilage potential of Enterobacteriaceae and lactic acid bacteria isolated from chilled vacuum-packaged beef. Int. J. Food Sci. Technol. 2012, 47, 1750–1756. [Google Scholar] [CrossRef]
- Massa, S.; Gardini, F.; Sinigaglia, M.; Guerzoni, M.E. Klebsiella pneumoniae as a spoilage organism in mozzarella Cheese. J. Dairy Sci. 1992, 75, 1411–1414. [Google Scholar] [CrossRef]
- De Vos, P. Bergey’s Manual of Systematic Bacteriology; GGMJ: Bekasi Kota, Indonesia, 2009. [Google Scholar]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, H.; Qin, L.; Pang, Z.; Qin, T.; Ren, H.; Pan, Z.; Zhou, J. Frequency, antimicrobial resistance and genetic diversity of Klebsiella pneumoniae in food samples. PLoS ONE 2016, 11, e153561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Yang, G.; Ye, Q.; Wu, Q.; Zhang, J.; Huang, Y. Phenotypic and genotypic characterization of Klebsiella pneumoniae isolated from retail foods in China. Front. Microbiol. 2018, 9, 289. [Google Scholar] [CrossRef]
- Abdel-Rhman, S.H. Characterization of β-lactam resistance in K. pneumoniae associated with ready-to-eat processed meat in Egypt. PLoS ONE 2020, 15, e238747. [Google Scholar] [CrossRef]
- Calbo, E.; Freixas, N.; Xercavins, M.; Riera, M.; Nicolas, C.; Monistrol, O. Foodborne nosocomial outbreak of SHV1 and CTX-M-15-producing Klebsiella pneumoniae: Epidemiology and control. Clin. Infect. Dis. 2011, 52, 743–749. [Google Scholar] [CrossRef]
- Srinivasan, S.; Feng, S.; Lin, Y. Dissolved carbon dioxide concentration profiles during very-high-gravity ethanol fermentation. Biochem. Eng. J. 2012, 69, 41–47. [Google Scholar] [CrossRef]
- Mills, J.; Horváth, K.M.; Brightwell, G. Antimicrobial effect of different peroxyacetic acid and hydrogen peroxide formats against spores of Clostridium estertheticum. Meat Sci. 2018, 143, 69–73. [Google Scholar] [CrossRef]
- Dixon Nm, K.D. A Review:The inhibition by CO2 of the growth and metabolism of microorganisms. J. Appl. Bacteriol. 1989, 67, 109–136. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of modified atmosphere packaging and active/smart technologies to red meat and poultry: A Review. Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Bubanja, I.N.; Lončarević, B.; Lješević, M.; Beškoski, V.; Gojgić-Cvijović, G.; Velikić, Z.; Stanisavljev, D. The influence of low-frequency magnetic field regions on the Saccharomyces cerevisiae respiration and growth. Chem. Eng. Process.—Process Intensif. 2019, 143, 107593. [Google Scholar] [CrossRef]
- Cao, J.; Liu, W.; Mei, J.; Xie, J. Effect of locust bean gum-sodium alginate coatings combined with high CO2 modified atmosphere packaging on the quality of turbot (Scophthalmus maximus) during refrigerated storage. Polymers 2021, 13, 4376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Q.; Xu, J.; Sun, F.; Liu, H.; Kong, B. Effects of modified atmosphere packaging with various CO2 concentrations on the bacterial community and shelf-life of smoked chicken Legs. Foods 2022, 11, 559. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Sun, X.; Lu, Q.; Huang, M.; Zhou, G. Effect of normal and modified atmosphere packaging on shelf life of roast chicken meat. J. Food Saf. 2018, 38, e12493. [Google Scholar] [CrossRef]
- Balogu, T.V.; Attansey, C.R. Effect of static magnetic field on microbial growth kinetics and physiochemical properties of nono (fermented milk drink). J. Microbiol. Biotechnol. Food Sci. 2017, 7, 75–78. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P.; Uran, H.; Topuz, O.K. The effect of modified atmosphere packaging on the quality and shelf life of frankfurter type-sausages. J. Food Qual. 2010, 33, 367–380. [Google Scholar] [CrossRef]
- Viana, B.I.; Domenici, M.O.; Jorge, E.V.; Vieira, F.J.; Lopes, F.M.; Cangussu, A.S.R.; Sobrinho, E.M. Growth conditions of clostridium perfringens type B for production of toxins used to obtain veterinary vaccines. Bioprocess Biosyst. Eng. 2014, 37, 1737–1742. [Google Scholar] [CrossRef]
- Luong, N.M.; Coroller, L.; Zagorec, M.; Moriceau, N.; Anthoine, V.; Guillou, S.; Membré, J.M. A Bayesian Approach to Describe and Simulate the pH Evolution of Fresh Meat Products Depending on the Preservation Conditions. Foods 2022, 11, 1114. [Google Scholar] [CrossRef]





| Item | Sensory Description | Score |
|---|---|---|
| Color | Shiny and even flesh color | 8–10 |
| Slightly shiny, uneven flesh color | 4–7 | |
| Uneven color, dull | 1–3 | |
| Odor | Rich and pure, and the overall smell is harmonious | 8–10 |
| Strong fragrance, no bad smell | 4–7 | |
| Unpleasant smell, the overall smell is not harmonious | 1–3 | |
| Organizational structure | Smooth cut surface and dense structure | 8–10 |
| Slightly rough-cut surface and loose structure | 4–7 | |
| Rough cut surface, loose structure | 1–3 | |
| Overall acceptability | Appearance is highly acceptable and appetite is strong | 8–10 |
| Appearance is acceptable, and appetite is slightly strong | 4–7 | |
| Unacceptable appearance, weak appetite | 1–3 |
| Index | S | B |
|---|---|---|
| O2 (%) | 9.06 ± 1.98% a | 0.13 ± 0.03% b |
| CO2 (%) | 7.47 ± 1.27% b | 71.85 ± 0.65% a |
| TVC (log CFU/g) | 8.10 ± 0.20 a | 8.28 ± 0.05 a |
| Strains No. | Volume Increase | CO2 Percentage (%) | Lag | Ymax |
|---|---|---|---|---|
| A2 | 2.27 ± 0.08 | 65.90 ± 0.90 | 4.361 ± 0.021 | 3.505 ± 0.005 |
| A3 | 2.17 ± 0.09 | 65.53 ± 0.95 | 4.512 ± 0.139 | 3.539 ± 0.005 |
| A12 | 2.33 ± 0.13 | 66.63 ± 1.13 | 4.074 ± 0.245 | 3.502 ± 0.015 |
| A35 | 2.03 ± 0.17 | 65.17 ± 1.88 | 4.395 ± 0.227 | 3.549 ± 0.011 |
| A40 | 2.36 ± 0.03 | 67.27 ± 2.39 | 4.885 ± 0.320 | 3.531 ± 0.018 |
| A41 | 2.36 ± 0.05 | 64.53 ± 1.56 | 4.310 ± 0.213 | 3.548 ± 0.005 |
| A43 | 2.42 ± 0.06 | 63.87 ± 2.19 | 4.285 ± 0.115 | 3.556 ± 0.010 |
| A45 | 2.03 ± 0.02 | 56.93 ± 1.44 | 4.613 ± 0.288 | 3.539 ± 0.006 |
| A46 | 2.32 ± 0.03 | 64.07 ± 1.79 | 4.703 ± 0.589 | 3.538 ± 0.006 |
| A49 | 2.13 ± 0.05 | 64.03 ± 0.86 | 4.789 ± 0.135 | 3.555 ± 0.002 |
| B5 | 2.62 ± 0.09 | 70.80 ± 1.04 | 3.502 ± 0.143 | 3.624 ± 0.009 |
| B8 | 2.15 ± 0.03 | 64.20 ± 0.70 | 4.150 ± 0.103 | 3.226 ± 0.068 |
| B9 | 2.36 ± 0.08 | 65.27 ± 1.60 | 4.538 ± 0.098 | 3.562 ± 0.007 |
| B15 | 2.33 ± 0.06 | 66.57 ± 1.08 | 4.329 ± 0.100 | 3.545 ± 0.006 |
| B16 | 2.35 ± 0.00 | 65.33 ± 0.84 | 3.573 ± 0.290 | 3.380 ± 0.006 |
| B18 | 2.16 ± 0.00 | 67.73 ± 1.20 | 4.363 ± 0.190 | 3.564 ± 0.015 |
| B19 | 2.13 ± 0.03 | 64.43 ± 1.79 | 4.659 ± 0.101 | 3.559 ± 0.016 |
| B21 | 2.39 ± 0.09 | 63.60 ± 0.80 | 4.529 ± 0.019 | 3.556 ± 0.004 |
| B22 | 2.35 ± 0.01 | 63.17 ± 0.43 | 4.935 ± 0.390 | 3.538 ± 0.015 |
| B24 | 2.11 ± 0.03 | 61.83 ± 1.47 | 3.945 ± 0.067 | 3.252 ± 0.027 |
| B25 | 2.18 ± 0.01 | 61.93 ± 0.30 | 4.568 ± 0.159 | 3.505 ± 0.007 |
| B28 | 2.09 ± 0.04 | 62.13 ± 0.60 | 4.841 ± 0.220 | 3.517 ± 0.011 |
| B29 | 2.12 ± 0.01 | 68.87 ± 0.80 | 4.413 ± 0.103 | 3.236 ± 0.007 |
| B33 | 2.33 ± 0.17 | 65.43 ± 1.52 | 4.224 ± 0.523 | 3.513 ± 0.006 |
| C3 | 2.28 ± 0.06 | 65.63 ± 1.45 | 4.223 ± 0.128 | 3.506 ± 0.012 |
| C6 | 2.24 ± 0.10 | 64.87 ± 1.94 | 3.647 ± 0.172 | 3.358 ± 0.013 |
| C18 | 2.59 ± 0.09 | 64.23 ± 0.47 | 4.468 ± 0.184 | 3.490 ± 0.014 |
| C19 | 2.74 ± 0.16 | 63.90 ± 0.075 | 5.157 ± 0.408 | 3.512 ± 0.002 |
| C21 | 2.66 ± 0.13 | 62.20 ± 0.47 | 4.757 ± 0.155 | 3.467 ± 0.020 |
| C23 | 2.10 ± 0.06 | 62.50 ± 1.45 | 3.630 ± 0.092 | 3.291 ± 0.006 |
| C24 | 2.31 ± 0.08 | 61.10 ± 0.21 | 3.633 ± 0.029 | 3.304 ± 0.003 |
| C25 | 2.15 ± 0.15 | 63.17 ± 0.67 | 4.655 ± 0.181 | 3.519 ± 0.028 |
| C36 | 2.38 ± 0.09 | 62.77 ± 0.96 | 4.572 ± 0.323 | 3.505 ± 0.008 |
| C37 | 2.15 ± 0.03 | 64.07 ± 1.90 | 3.695 ± 0.215 | 3.316 ± 0.002 |
| C41 | 2.10 ± 0.05 | 61.57 ± 0.87 | 4.681 ± 0.192 | 3.517 ± 0.005 |
| Property | Results | Property | Results |
|---|---|---|---|
| Gram stain | − | Urease | + |
| Oxidase | − | Citrate utilization | + |
| H2S | − | Malonate utilization | + |
| Ornithine decarboxylase | − | Rhamnose fermentation | + |
| MR test | − | Inositol fermentation | + |
| VP test | + | Glucose fermentation | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Bassey, A.P.; Bai, Y.; Teng, S.; Zhou, G.; Ye, K. Synergistic Effect of Static Magnetic Field and Modified Atmosphere Packaging in Controlling Blown Pack Spoilage in Meatballs. Foods 2022, 11, 1374. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101374
Chen Y, Bassey AP, Bai Y, Teng S, Zhou G, Ye K. Synergistic Effect of Static Magnetic Field and Modified Atmosphere Packaging in Controlling Blown Pack Spoilage in Meatballs. Foods. 2022; 11(10):1374. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101374
Chicago/Turabian StyleChen, Yongfang, Anthony Pius Bassey, Yun Bai, Shuang Teng, Guanghong Zhou, and Keping Ye. 2022. "Synergistic Effect of Static Magnetic Field and Modified Atmosphere Packaging in Controlling Blown Pack Spoilage in Meatballs" Foods 11, no. 10: 1374. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101374