Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review
Abstract
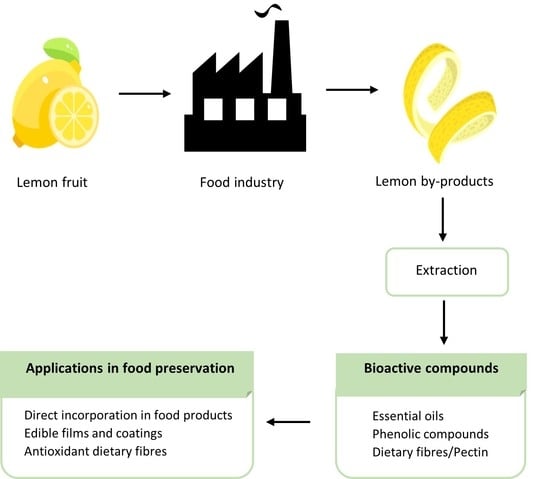
:1. Introduction
2. Studies Published in the Last Two Decades—An Overview
2.1. Research Methodology
2.2. Results
3. Lemon by-Products: Value-Added Compounds
3.1. Volatile Compounds/Essential Oils
3.1.1. Composition
3.1.2. Extraction Technologies and Preservative-Related Properties
3.2. Phenolic Compounds
3.2.1. Composition
3.2.2. Extraction Technologies and Preservative-Related Properties
3.3. Dietary Fibre/Pectin
3.3.1. Composition
3.3.2. Extraction Technologies and Preservative-Related Properties
4. Applications of Lemon Bioactive Fractions in Food Preservation
4.1. Direct Incorporation in Food Products
4.2. Edible Films and Coatings
4.3. Antioxidant Dietary Fibres
| By-Products/ Bioactive Fractions | Food | Property | Packaging | Results | References |
|---|---|---|---|---|---|
| Direct incorporation in food products | |||||
| Lemon EO | Minced beef meat | Antimicrobial and antioxidant activity | Lemon EO with preservative effect against Listeria monocytogenes inoculated in minced beef meat. | [18] | |
| Lemon peel powder | ABT synbiotic yoghurt and milk | Antioxidant, antibacterial and probiotic activity | LP improved the antioxidant property of the ABT synbiotic yoghurt and milk; 0.5% LP addition showed higher inhibition against S. aureus, B. subtilis, and E. coli compared to the control; Enhanced the viabilities of probiotic starter cultures with the incorporation of LPs in yoghurt during cold storage. | [55] | |
| Lemon peel and lemon pomace extract | Biscuits | Antioxidant activity | The enriched biscuits with LP and pomace showed higher phenolic content, higher antioxidant activity, longer induction period, and higher intrinsic resistance to lipid oxidation than the control. | [95] | |
| Edible films and coatings | |||||
| Lemon EO | Strawberries | Antifungal activity | Chitosan | Delayed ripening with a lower respiration rate was observed in strawberries coated with lemon EO-based chitosan coatings. | [20] |
| Lemon EO | Vegetable products | Antimicrobial activity | Novel edible coating with modified chitosan and nano emulsified lemon EO | Increases antimicrobial activity and prolongs the shelf life of vegetable products. | [107] |
| Lemon EO | Strawberries | Antimicrobial activity | Incorporated lemon EO on the modified chitosan edible coating | Protects the storage-keeping quality of strawberries. | [114] |
| Lemon EO | Biscuit | Antimicrobial activity | Low density polyethylene (LDPE) films | Acts as flavouring films for packaging biscuit, prevents changes in water-vapor permeability and mechanical properties. | [115] |
| Lemon EO (+ thyme and cinnamon) | Antimicrobial activity | Chitosan | Chitosan film combined with lemon, thyme and cinnamon essential oils provide a new formulation for antimicrobial films. | [116] | |
| Lemon extract | Carrots | Antimicrobial and antioxidant activity | Pectin coating with lemon by-product extract | Improvement microbiological stability of fresh-cut carrots, showing the lowest value of total bacterial (2.58 log CFU g−1) Antioxidant activity level (289.49 µM Trolox/100 g). | [117] |
| Antioxidant dietary fibres | |||||
| Lemon fibre/pectin | Cookie | Fat replacer | Incorporating lemon pectin (2.5%, 7% and 10%) reduce 10% of fat material without significant texture differences; the addition of pectin increases water content. | [66] | |
| Antioxidant fibre from lemon albedo | Cooked bologna sausages | Fat replacer | Incorporating two types of lemon albedo: raw and cooked, at different concentrations (2.5%, 5%, 7.5% and 10%) in cooked bologna sausages, decreases the fat content and residual nitrite content. | [112] | |
| Lemon fibre | Low-fat beef hamburgers | Fat replacer | Incorporating lemon fibre (2%, 4% and 6%) to produce low fat beef hamburgers. | [113] | |
| Lemon fibre | Frankfurters | Fat replacer | Lemon fibre led to a faster relaxation time in low-fat frankfurters, and a significant increase in the proportion of immobilized water. | [118] | |
| Lemon fibre/Pectin | Gluten-Free Biscuit | Nutrition fortifier | Replacing 2.5 wt% of the rice flour with lemon pectin obtained from lemon processing waste. | [119] | |
| Lemon fibre from pomace | Dough andMantou (steamed bread) | Nutrition fortifier | The substitution of 3 or 6 g lemon fibre per 100 g flour can produce healthy and acceptable mantou with higher free total phenolic content and antioxidant capacity. | [120] | |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque De Castro, M.D. Characterization of Lemon (Citrus limon) Polar Extract by Liquid Chromatography-Tandem Mass Spectrometry in High Resolution Mode. J. Mass Spectrom. 2015, 50, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- FAO Citrus Fruit. Fresh and Processed- Statistical Bulletin 2016. Trade Mark. Div. 2017, 47. [Google Scholar]
- Kim, S.Y. Chemical Composition and Antioxidant Activity of Crude Polysaccharide from Citron (Citrus Junos Sieb. Ex Tanaka) Seed. Prev. Nutr. Food Sci. 2018, 23, 335–340. [Google Scholar] [CrossRef]
- Sayinci, B.; Ercisli, S.; Ozturk, I.; Eryilmaz, Z.; Demir, B. Determination of Size and Shape in the “Moro” Blood Orange and “Valencia” Sweet Orange Cultivar and Its Mutants Using Image Processing. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Farhat, A.; Fabiano-Tixier, A.S.; El Maataoui, M.; Maingonnat, J.F.; Romdhane, M.; Chemat, F. Microwave Steam Diffusion for Extraction of Essential Oil from Orange Peel: Kinetic Data, Extract’s Global Yield and Mechanism. Food Chem. 2011, 125, 255–261. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros, G.; Viuda-Martos, M. Valorization of Citrus Co-Products: Recovery of Bioactive Compounds and Application in Meat and Meat Products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Tinh, N.T.T.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods 2021, 10, 240. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of Phenolic Profile and Antioxidant Capacity of Different Fruit Part from Lemon (Citrus limon Burm.) Cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Klimek-szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Garcýa, R.; Campos, D.A.; Vilas-Boas, A.; Madureira, A.R.; Pintado, M. Natural Antimicrobials from Vegetable By-Products: Extraction, Bioactivity, and Stability. Food Microbiol. Biotechnol. 2020, 249–286. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of Active Films Utilizing Antioxidant Compounds Obtained from Tomato and Lemon By-Products for Use in Food Packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus Spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar] [CrossRef] [Green Version]
- FDA. FDA Substances Generally Recognized as Safe. Fed. Regist. 2016, 81, 54960–55055. [Google Scholar]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical Composition of Commercial Citrus Fruit Essential Oils and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential Antimicrobial Uses of Essential Oils in Food: Is Citrus the Answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria Monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef] [Green Version]
- Di Rauso Simeone, G.; Di Matteo, A.; Rao, M.A.; Di Vaio, C. Variations of Peel Essential Oils during Fruit Ripening in Four Lemon (Citrus limon (L.) Burm. F.) Cultivars. J. Sci. Food Agric. 2020, 100, 193–200. [Google Scholar] [CrossRef]
- Perdones, A.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of Chitosan-Lemon Essential Oil Coatings on Volatile Profile of Strawberries during Storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Xu, Y.; Chen, L.; Cao, J.; Jiang, W. An Advance on Nutritional Profile, Phytochemical Profile, Nutraceutical Properties, and Potential Industrial Applications of Lemon Peels: A Comprehensive Review. Trends Food Sci. Technol. 2022, 124, 219–236. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus Peel as a Source of Functional Ingredient: A Review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Julaeha, E.; Nurzaman, M.; Wahyudi, T.; Nurjanah, S.; Permadi, N.; Anshori, J. Al The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review. Molecules 2022, 27, 8090. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, H.W.; Wu, Y.; Zhou, F.; Hua, C.; Ba, L.; Shamim, S.; Zhang, W. Characterization of Volatile Compounds and Microstructure in Different Tissues of ‘Eureka’ Lemon (Citrus limon). Int. J. Food Prop. 2022, 25, 404–421. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on Flavouring Group Evaluation 25, Revision 3 (FGE.25Rev3): Aliphatic Hydrocarbons from Chemical Group 31. Available online: https://0-efsa-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/epdf/10.2903/j.efsa.2015.4069 (accessed on 7 February 2023).
- Sartori Tamburlin, I.; Roux, E.; Feuillée, M.; Labbé, J.; Aussaguès, Y.; El Fadle, F.E.; Fraboul, F.; Bouvier, G. Toxicological Safety Assessment of Essential Oils Used as Food Supplements to Establish Safe Oral Recommended Doses. Food Chem. Toxicol. 2021, 157, 112603. [Google Scholar] [CrossRef]
- Caputo, L.; Cornara, L.; Bazzicalupo, M.; De Francesco, C.; De Feo, V.; Trombetta, D.; Smeriglio, A. Chemical Composition and Biological Activities of Essential Oils from Peels of Three Citrus Species. Molecules 2020, 25, 1890. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Hernández, M.G.; Sánchez-Bravo, P.; Hernández, F.; Carbonell-Barrachina, Á.A.; Pastor-Pérez, J.J.; Legua, P. Determination of the Volatile Profile of Lemon Peel Oils as Affected by Rootstock. Foods 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, S.; Zhao, C.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. The Chemical Composition and Antibacterial and Antioxidant Activities of Five Citrus Essential Oils. Molecules 2022, 27, 7044. [Google Scholar] [CrossRef] [PubMed]
- Marsol-Vall, A.; Sgorbini, B.; Cagliero, C.; Bicchi, C.; Eras, J.; Balcells, M. Volatile Composition and Enantioselective Analysis of Chiral Terpenoids of Nine Fruit and Vegetable Fibres Resulting from Juice Industry By-Products. J. Chem. 2017, 2017, 8675014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive Comparative Analysis of Volatile Compounds in Citrus Fruits of Different Species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina Pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [Green Version]
- Pieracci, Y.; Pistelli, L.; Cecchi, M.; Pistelli, L.; De Leo, M. Phytochemical Characterization of Citrus-Based Products Supporting Their Antioxidant Effect and Sensory Quality. Foods 2022, 11, 1550. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Ramos, M.; Hamzaoui, M.; Kohnen, S.; Jiménez, A.; Garrigós, M.C. Optimisation of Sequential Microwave-Assisted Extraction of Essential Oil and Pigment from Lemon Peels Waste. Foods 2020, 9, 1493. [Google Scholar] [CrossRef]
- Di Vaio, C.; Graziani, G.; Gaspari, A.; Scaglione, G.; Nocerino, S.; Ritieni, A. Essential Oils Content and Antioxidant Properties of Peel Ethanol Extract in 18 Lemon Cultivars. Sci. Hortic. 2010, 126, 50–55. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 10334. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P. Essential Oils, Phytoncides, Aromachology, and Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-Products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of Different Isolation Methods of Essential Oil from Citrus Fruits: Cold Pressing, Hydrodistillation and Microwave “dry” Distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Moosavy, M.H.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.A.; Mardani, K. Antioxidant and Antimicrobial Activities of Essential Oil of Lemon (Citrus limon) Peel in Vitro and in a Food Model. J. Food Qual. Hazards Control 2017, 4, 42–48. [Google Scholar]
- Asker, M.; El-Gengaihi, S.E.; Hassan, E.M.; Mohammed, M.A.; Abdelhamid, S.A. Phytochemical Constituents and Antibacterial Activity of Citrus Lemon Leaves. Bull. Natl. Res. Cent. 2020, 44, 194. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Moayyedi, M. Comparison of Heat and Mass Transfer of Different Microwave-Assisted Extraction Methods of Essential Oil from Citrus limon (Lisbon Variety) Peel. Food Sci. Nutr. 2015, 3, 506–518. [Google Scholar] [CrossRef]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Inhibition of Enzymes Linked to Type-2 Diabetes and Hypertension by Essential Oils from Peels of Orange and Lemon. Int. J. Food Prop. 2017, 20, S586–S594. [Google Scholar] [CrossRef] [Green Version]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Tangerine (Citrus Reticulata L.), Grapefruit (Citrus Paradisi L.), Lemon (Citrus Lemon L.) and Cinnamon (Cinnamomum Zeylanicum Blume). Z. Nat. C 2021, 76, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.; El-Readi, M.Z.; Nibret, E.; Sporer, F.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical Composition of the Essential Oils of Two Citrus Species and Their Biological Activities. Pharmazie 2010, 65, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Himed, L.; Merniz, S.; Monteagudo-Olivan, R.; Barkat, M.; Coronas, J. Antioxidant Activity of the Essential Oil of Citrus limon before and after Its Encapsulation in Amorphous SiO2. Sci. Afr. 2019, 6, e00181. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Gargouri, B.; Ammar, S.; Verardo, V.; Besbes, S.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC–DAD-ESI-TOF–MS Based Strategy for New Insights into the Qualitative and Quantitative Phenolic Profile in Tunisian Industrial Citrus limon by-Product and Their Antioxidant Activity. Eur. Food Res. Technol. 2017, 243, 2011–2024. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef]
- Sanches, V.L.; Cunha, T.A.; Viganó, J.; de Souza Mesquita, L.M.; Faccioli, L.H.; Breitkreitz, M.C.; Rostagno, M.A. Comprehensive Analysis of Phenolics Compounds in Citrus Fruits Peels by UPLC-PDA and UPLC-Q/TOF MS Using a Fused-Core Column. Food Chem. X 2022, 14, 100262. [Google Scholar] [CrossRef]
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in Vitro. Front. Nutr. 2022, 9, 1075. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque de Castro, M.D. Comparative Study of the Effect of Sample Pretreatment and Extraction on the Determination of Flavonoids from Lemon (Citrus limon). PLoS ONE 2016, 11, e0148056. [Google Scholar] [CrossRef] [PubMed]
- Fathy, H.M.; Abd El-Maksoud, A.A.; Cheng, W.; Elshaghabee, F.M.F. Value-Added Utilization of Citrus Peels in Improving Functional Properties and Probiotic Viability of Acidophilus-Bifidus-Thermophilus (ABT)-Type Synbiotic Yoghurt during Cold Storage. Foods 2022, 11, 2677. [Google Scholar] [CrossRef]
- Santarelli, V.; Neri, L.; Carbone, K.; Macchioni, V.; Pittia, P. Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity. Plants 2022, 11, 41. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Panesar, P.S.; Bera, M. Comparative Study on the Extraction and Quantification of Polyphenols from Citrus Peels Using Maceration and Ultrasonic Technique. Curr. Res. Nutr. Food Sci. 2019, 7, 678–685. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon Residues for the Recovery of Antioxidants: Evaluation and Optimization of Microwave and Ultrasound Application to Solvent Extraction. Ind. Crops Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Zou, G.S.; Li, S.J.; Zheng, S.L.; Pan, X.; Huang, Z.P. Lemon-Peel Extract Ameliorates Rheumatoid Arthritis by Reducing Xanthine Oxidase and Inflammatory Cytokine Levels. J. Taiwan Inst. Chem. Eng. 2018, 93, 54–62. [Google Scholar] [CrossRef]
- Gao, X.; Xu, D.; Zhang, X.; Zhao, H. Protective Effect of Lemon Peel Polyphenols on Oxidative Stress-Induced Damage to Human Keratinocyte HaCaT Cells Through Activation of the Nrf2/HO-1 Signaling Pathway. Front. Nutr. 2021, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Benohoud, M.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. Green Extraction of Polyphenols from Citrus Peel By-Products and Their Antifungal Activity against Aspergillus Flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Biradar, R. Comparative Evaluation of Soxhlet and Ultrasonics on the Structural Morphology and Extraction of Bioactive Compounds of Lemon (Citrus limon L.) Peel. J. Food Chem. Nanotechnol. 2019, 5, 56–64. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the Effect of High Pressure on Total Phenolic Content, Antioxidant and Antimicrobial Activity of Citrus Peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Ayora-Talavera, T.D.R.; Ramos-Chan, C.A.; Covarrubias-Cárdenas, A.G.; Sánchez-Contreras, A.; García-Cruz, U.; Pacheco, L.N.A. Evaluation of Pectin Extraction Conditions and Polyphenol Profile from Citrus x Lantifoliawaste: Potential Application as Functional Ingredients. Agriculture 2017, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017, 9340427. [Google Scholar] [CrossRef] [Green Version]
- Fernández-López, J.; Fernández-Ginés, J.M.; Aleson-Carbonell, L.; Sendra, E.; Sayas-Barberá, E.; Pérez-Alvarez, J.A. Application of Functional Citrus By-Products to Meat Products. Trends Food Sci. Technol. 2004, 15, 176–185. [Google Scholar] [CrossRef]
- Vanitha, T.; Khan, M. Role of Pectin in Food Processing and Food Packaging. In Pectins-Extraction, Purification, Characterization and Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičacuteová, J.; Jurasová, M.; Kukurová, K. Application of Citrus Dietary Fibre Preparations in Biscuit Production. J. Food Nutr. Res. 2011, 50, 182–190. [Google Scholar]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-Products from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre Concentrates from Apple Pomace and Citrus Peel as Potential Fibre Sources for Food Enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Fernández-López, J.; Cruz López-Marcos, M.; Bailina, C.; Viuda-Martos, M.; Angel Pérez-Alvarez, J. Properties of Dietary Fibers from Agroindustrial Coproducts as Source for Fiber-Enriched Foods. Food Bioprocess Technol. 2015, 8, 2400–2408. [Google Scholar] [CrossRef]
- Karaman, E.; Yılmaz, E.; Tuncel, N.B. Physicochemical, Microstructural and Functional Characterization of Dietary Fibers Extracted from Lemon, Orange and Grapefruit Seeds Press Meals. Bioact. Carbohydr. Diet. Fibre 2017, 11, 9–17. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Scurria, A.; Ilharco, L.M.; Pagliaro, M. Pectin: New Science and Forthcoming Applications of the Most Valued Hydrocolloid. Food Hydrocoll. 2022, 127, 107483. [Google Scholar] [CrossRef]
- Gracy Nadar, C.; Arora, A.; Shastri, Y. Sustainability Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. Au 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citruswastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [Green Version]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the Pectin Production Process Using Novel Extraction Techniques: A Review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of Microwave-Assisted Extraction and Structural Characterization of Pectin from Sweet Lemon Peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Pérez, F.; Steigerwald, H.; Schülke, S.; Vieths, S.; Toda, M.; Scheurer, S. The Dietary Fiber Pectin: Health Benefits and Potential for the Treatment of Allergies by Modulation of Gut Microbiota. Curr. Allergy Asthma Rep. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Nancy Picot-Allain, M.C.; Amiri-Rigi, A.; Abdoun-Ouallouche, K.; Aberkane, L.; Djefal-Kerrar, A.; Mahomoodally, M.F.; Emmambux, M.N. Assessing the Bioactivity, Cytotoxicity, and Rheological Properties of Pectin Recovered from Citrus Peels. Food Biosci. 2022, 46, 101550. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional Antioxidant, Non-Cytotoxic Activity of Integral Lemon Pectin from Hydrodynamic Cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Devi, E.; Shukla, N.; Bala, L.; Kumar, A. Extraction of Pectin from Citrus Fruit Peel and Its Utilization in Preparation of Jelly. Int. J. Eng. Res. Technol. 2014, 3, 1925–1932. [Google Scholar]
- Kanmani, P. Extraction and Analysis of Pectin from Citrus Peels: Augmenting the Yield from Citrus limon Using Statistical Experimental Design. Iran. J. Energy Environ. 2014, 5, 303–312. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and Ultrasound Assisted Extraction of Pectin from Various Fruits Peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef]
- Karim, R.; Nahar, K.; Zohora, F.T.; Islam, M.; Bhuiyan, R.H.; Jahan, S.; Shaikh, A.A. Pectin from Lemon and Mango Peel: Extraction, Characterisation and Application in Biodegradable Film. Carbohydr. Polym. Technol. Appl. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Salam, M.; Jahan, N.; Islam, M.; Hoque, M. Extraction of Pectin from Lemon Peel: Technology Development. J. Chem. Eng. 2014, 27, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Ilharco, L.M.; Pagliaro, M. Controlling the Degree of Esterification of Citrus Pectin for Demanding Applications by Selection of the Source. ACS Omega 2017, 2, 7991–7995. [Google Scholar] [CrossRef] [PubMed]
- Vilas-boas, A.A.; Magalhães, D.; Campos, D.A.; Porretta, S.; Dellapina, G.; Poli, G.; Istanbullu, Y.; Demir, S.; Mart, Á.; Mart, S.; et al. Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods 2022, 11, 3859. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized Biscuits with Bioactive Ingredients Obtained by Citrus Lemon Pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef] [PubMed]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal Edible Coatings for Fresh Citrus Fruit: A Review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef] [Green Version]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C.; Bifani, V. Natural Additives in Bioactive Edible Films and Coatings: Functionality and Applications in Foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Monteiro, M.J.; Gomes, A.; Pintado, M. Impact of Whey Protein Coating Incorporated with Bifidobacterium and Lactobacillus on Sliced Ham Properties. Meat Sci. 2018, 139, 125–133. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart Packaging Systems for Food Applications: A Review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Delŕio, M.A.; Pérez-Gago, M.B. Antimicrobial Edible Films and Coatings for Fresh and Minimally Processed Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef]
- Raghav, P.K.; Agarwal, N.; Saini, M.; Vidhyapeeth, J.; Vidhyapeeth, J. Edible Coating of Fruits and Vegetables. Int. J. Sci. Mod. Educ. 2016, 1, 1. [Google Scholar]
- Dhanapal, A.; Rajamani, L.; Banu, M. Edible Films from Polysaccharides. Food Sci. Qual. 2012, 3, 9–18. [Google Scholar]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel Edible Coating Containing Essential Oil Nanoemulsions to Prolong the Shelf Life of Vegetable Products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Vijay-Kumar, M. Beneficial and Detrimental Effects of Processed Dietary Fibers on Intestinal and Liver Health: Health Benefits of Refined Dietary Fibers Need to Be Redefined! Gastroenterol. Rep. 2020, 8, 85–89. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services ; U.S. Department of Agriculture. Dietary Guidelines for Americans. Health 2015, 47, 245–251. [Google Scholar]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Antioxidant Dietary Fibre Enriched Meat-Based Functional Foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A. Lemon Albedo as a New Source of Dietary Fiber: Application to Bologna Sausages. Meat Sci. 2004, 67, 7–13. [Google Scholar] [CrossRef]
- Soncu, E.D.; Kolsar, N.; Çiçek, N.; Öztürk, G.S.; Akog ˘lu, T.; Ka¸sko, Y.; Ar, K. The Comparative Effect of Carrot and Lemon Fiber as a Fat Replacer on Physico-Chemical, Textural, and Organoleptic Quality of Low-Fat Beef Hamburger. Korean J. Food Sci. Anim. Resour. 2015, 35, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan-Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Dias, M.V.; de Medeiros, H.S.; de Fátima Ferreira Soares, N.; de Melo, N.R.; Borges, S.V.; de Deus Souza Carneiro, J.; de Assis Kluge Pereira, J.M.T. Development of Low-Density Polyethylene Films with Lemon Aroma. LWT-Food Sci. Technol. 2013, 50, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Li, Y. Combined Effects of Two Kinds of Essential Oils on Physical, Mechanical and Structural Properties of Chitosan Films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B.; Imeneo, V.; Piscopo, A.; Martín-Belloso, O.; Soliva-Fortuny, R. Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots. Foods 2022, 11, 1314. [Google Scholar] [CrossRef]
- Song, J.; Pan, T.; Wu, J.; Ren, F. The Improvement Effect and Mechanism of Citrus Fiber on the Water-Binding Ability of Low-Fat Frankfurters. J. Food Sci. Technol. 2016, 53, 4197–4204. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, D.; Scurria, A.; Picone, P.; Guiducci, A.; Pagliaro, M.; Pantaleo, G.; Albanese, L.; Meneguzzo, F.; Ciriminna, R. A Gluten-Free Biscuit Fortified with Lemon IntegroPectin. ChemistrySelect 2022, 7, e202104247. [Google Scholar] [CrossRef]
- Fu, J.T.; Chang, Y.H.; Shiau, S.Y. Rheological, Antioxidative and Sensory Properties of Dough and Mantou (Steamed Bread) Enriched with Lemon Fiber. LWT-Food Sci. Technol. 2015, 61, 56–62. [Google Scholar] [CrossRef]




| By-Product | Cultivars/Variety | Content of Terpenoids: Identification and Quantification | References |
|---|---|---|---|
| Lemon Peel | C. limon, Variety: Fino and Verna (Spain) | Limonene (59.1%), γ-terpinene (9.7%), β-pinene (5.2%), β-bisabolene (3.6%). | [16] |
| Lemon: Flavedo, Albedo, Juice vesicles and Segment wall | C. limon, Eureka variety (China) | Flavedo: Limonene (14.0 mg/g), citral (9.3 mg/g), γ-terpinene (4.1 mg/g), β-pinene (3.3 mg/g). | [24] |
| Albedo: Limonene (2.7 mg/g), citral (0.9 mg/g), γ-terpinene (0.4 m/g), β-pinene (0.2 mg/g). | |||
| Juice vesicles: Limonene (1.9 mg/g), citral (0.7 mg/g), γ-terpinene (0.4 m/g). | |||
| Segment Wall: Limonene (1.1 mg/g), citral (0.4 mg/g), γ-terpinene (0.3 m/g), β-pinene (0.1 mg/g). | |||
| Lemon Peel | C. limon, Four different cultivars: Ovale di Sorrento Sfusato Amalfitano Femminello Cerza Femminello Adamo at different ripening stages (Italy) | Limonene (63.7–76.9%), β-pinene (7.7–14.7%), γ-terpinene (5.5–10.4%), p-cymene (0.7–1.9%). | [19] |
| Lemon Peel | C. limon (Italy) | Limonene (57.7%), γ-terpinene (10.5%), β-pinene (9.3%), citronellol (8.2%). | [27] |
| Lemon Peel | C. limon, Five Varieties: Verna, Betera, Eureka, Fino 49, and Fino 95 (Spain) | Limonene (19,760–22,716 mg/kg), β-pinene (3757–5011 mg/kg), γ-terpinene (3226–3849 mg/kg), α-pinene (648.5–797.3 mg/kg), sabinene (611.8–853.0 mg/kg). | [28] |
| Lemon Peel | C. limon, Nanjing Wensenbauer International Trade Co., Ltd. (China) | Limonene (47.3%), β-pinene (13.7%), γ-pinene (10.6%), trans-citral (4.5%). | [29] |
| Lemon Flesh | C. limon, Juice company (Spain) | Limonene (52.7%), p-cymene (13.7%), γ-terpinene (7.4%), α-terpinolene (5.1%), α-terpineol (4.7%). | [30] |
| Lemon Peel | C. limon, Five Varieties: Lime, Eureka, Volkamer, Limonia, and Red Limonia (China) | Total volatile concentration (12.0–31.6 mg.g−1), limonene (5.44–20.5 mg/g; 59.3%–73.3%). | [31] |
| Lemon Peel | C. limonum (Algeria) | Limonene (51.4%), β-pinene (17.0%), γ-terpinene (13.5%). | [32] |
| Lemon: (Peel and Pulp) | C. limon, Osbeck cv. Femminello Santa Teresa (Italy) | Limonene (60.6%), γ-terpinene (13.3%), myrcene (1.9%), β-pinene (14.8%), sabinene (3.3%), α-pinene (3.1%). | [33] |
| Lemon Waste | Lemon (C. limon) non-conforming fruits (inadequate appearance or size) (Spain) | Limonene (65.1%), γ-terpinene (9.7%), β-myrcene (1.4%), β-pinene (14.5%), sabinene (2.4%), α-pinene (1.9%). | [34] |
| Lemon Peel | C. limon, Eighteen cultivars: Sfusato Amalfitano, Ovale di Sorrento, Femminello Fior d’Arancio m 79, Femminello Siracusano m 296, Femminello Dosaco, Femminello S, Femminello Siracusano 2KR, Femminello Scandurra, Iniasel 95, Femminello Continella m 84, CNR L58, Femminello Adamo, Femminello Cerza, Akragas, Selinunte, Segesta, Erice, Kamarina (Italy) | Limonene (72.5–76.4%), β-pinene (11.6–18.7%), terpinene (2.9–8.3%), α-pinene (1.4–1.5%) and myrcene (0.95–1.1%). | [35] |
| By-Product | Lemon Fractions | Extraction Technologies | Extraction Conditions | Antioxidant Properties | Antimicrobial Properties | References |
|---|---|---|---|---|---|---|
| Lemon Flavedo | Essential oils | Microwave-assisted hydrodistillation (MAHD) | 525–1800 W, 5–10 min | Growth inhibition at a concentration of 50 ppm for S. aureus; Bacteriostatic effect was reached by using 150 ppm (S. aureus) and 500 ppm (Escherichia coli). | [34] | |
| Lemon Peels | Essential oils | Solvent extraction (Ethanol) | 180 min, 4 °C | ABTS assay showed antioxidant effectiveness of peel ethanolic extract of 18 lemon cultivars. | [35] | |
| Lemon: Peels (HD and MAD); Whole fruit (CP) | Essential oils | Hydro distillation (HD); Cold pressing (CP); Microwave accelerated distillation (MAD) | HD: 180 min | The antimicrobial activity for the six microorganisms tested by HD, CP or MAD were similar to MIC. | [40] | |
| CP: 60 min | ||||||
| MAD: 1000 W, 30 min, 100 °C | ||||||
| Lemon Peels | Essential oils | Steam distillation (SD) | 180 min | EO showed 55.1% inhibition of DPPH free radical. | The MIC and MBC value of EO against S. aureus was 1.3% and 5%, respectively. | [41] |
| Lemon Peels | Essential oils | HD; MAHD; Solvent-free microwave extraction (SFME) | HD: 335 W, 120 min | DPPH: IC50 values of the EOs extracted by HD, MAHD, and SFME were 44.1, 42.0, and 97.2 mg/mL. | [43] | |
| MAHD: 1200 W, 15 min | ||||||
| SFME: 1200 W, 15 min | ||||||
| Lemon ‘dried zest’ | Essential oils | Aqueous distillation | 300 min | EO showed 86.1% inhbition of DPPH free radical. | Antimicrobial activity against saprophytic (Bacillus subtilis, Penicillium chrysogenum, Fusarium moniliforme, Aspergillus niger, Aspergillus flavus, Saccharomyces cerevisiae) and pathogenic microorganisms (E.coli, Salmonella abony, S. aureus, P.aeruginosa, Candida albicans). | [45] |
| Lemon Leaves | Essential oils | HD | - | Inhibition against S.aureus (32 mm) and P.aeruginosa (49 mm). | [42] | |
| Lemon Peels | Essential oils | SD | 360 min | DPPH: IC50 of 28.91–37.69 mg/mL. | Antimicrobial activity against all tested Gram-positive bacteria and yeasts, and for one Gram-negative (Pseudomonas fluorescens). | [46] |
| Lemon Peels | Essential oils | CP | - | DPPH (0.7 mg/mL); CUPRAC (0.6 mg/mL); Iron chelation (0.7 mg/mL). | [47] |
| By-Product | Cultivars/Variety | Content of Phenolic Compounds: Identification and Quantification | References |
|---|---|---|---|
| Lemon: Peel, Pulp, Juice, Seeds, and Whole fruit | Five lemon (C. limon) cultivars, (China) | Peel: Total PCs: 3.17–4.71 µg/g FW. Phenolic acids: Caffeic: 293.7–741.6 µg/g FW; Chlorogenic: 138.7–527.5 µg/g FW; Gallic: 1.63–90.69 µg/g FW. Flavanones: Eriocitrin: 7.73–49.61 µg/g FW; Hesperidin: 1465–3315 µg/g FW; Hesperetin: 5.79–88.12 µg/g FW. | [9] |
| Pulp: Total PCs: 2.43–3.46 µg/g FW. Phenolic acids: Caffeic: 44.67–233.5 µg/g FW; Chlorogenic: 9.28–83.92 µg/g FW; Gallic: 0.95–28.42 µg/g FW. Flavanones: Eriocitrin: ND; Hesperidin: 525.3–1419 µg/g FW; Hesperetin: ND. | |||
| Juice: Total PCs: 0.29–0.52 µg/g FW. Phenolic acids: Caffeic: 17.83–128.4 µg/g FW; Chlorogenic: 2.70–22.1 µg/g FW; Gallic: 0.38–7.62 µg/g FW; Flavanones: Eriocitrin: ND; Hesperidin: 105.5–210.3 µg/g FW; Hesperetin: 0.83–4.7 µg/g FW. | |||
| Seeds: Total PCs: 2.1–3.4 µg/g FW. Phenolic acids: Caffeic: 9.31–116.8 µg/g FW; Chlorogenic: 10.11–124.4 µg/g FW; Gallic: 3.50–11.95 µg/g FW. Flavanones: Eriocitrin: 6.7–150.9 µg/g FW; Hesperidin: 10.3–49.9 µg/g FW; Hesperetin: ND. | |||
| Whole fruit: Total PCs: 1.63–3.04 µg/g FW. Phenolic acids: Caffeic: 127.3–389.2 µg/g FW; Chlorogenic: 1.6–3.0 µg/g FW; Gallic: 1.6–9.0 µg/g FW. Flavanones: Eriocitrin: 0.6–19.6 µg/g FW; Hesperidin: 889.8–9260 µg/g FW; Hesperetin: 1.5–24.5 µg/g FW. | |||
| Lemon Peel and Pulp | C. limon (Osbeck cv. Femminello Santa Teresa) (Italy) | Peel: Flavonoids: Vicenin-2: 1.38 mg/100g FW, Diosmetin 8-C-glucoside or 6-C-glucoside: 2.08–2.17 mg/100g FW, Eriocitrin/Neoeriocitrin: 35.4 mg/100g FW, Narirutin/Naringin: 0.73 mg/100g FW, Hesperidin/Neohesperidin: 24.9 mg/100g FW, Total Flavonoids: 66.7 mg/100g FW. | [33] |
| Pulp: Flavonoids: Vicenin-2: 3.68 mg/100g FW, Lucenin-2 4′-methyl ether: 4.15 mg/100g FW, Diosmetin 8-C-glucoside or 6-C-glucoside: 2.6–4.9 mg/100g FW, Eriocitrin/Neoeriocitrin: 53.5 mg/100g FW, Narirutin/Naringin: 7.8 mg/100g FW, Hesperidin/Neohesperidin 91.1 mg/100g FW, Total Flavonoids: 168 mg/100g FW. | |||
| Lemon Peel | C. limon, juice production company (Tunisia) | Phenolic acids: Hydroxybenzoic acid hexose: 987.9 μg/g DM, Dimer of caffeic acid-O-hexoside: 263 μg/g DM, Sinapic acid hexoside I: 208.6 μg/g DM. Flavonols: Limocitrol-O-glucoside-HMG: 18.3 μg/g DM, Rutin: 14.4 μg/g DM, Quercetin-rutinoside: 12.3 μg/g DM. Flavones: Diosmin or neodiosmin: 102.6–296.4 μg/g DM, Apigenin-7-O-(malonyl-apyosil)-hexoside: 82.5 μg/g DM. Flavanones: Hesperidin: 1234.8 μg/g DM, Neohesperidin: 950.8 μg/g DM, Eriocitrin: 955.4 μg/g DM, Diosmetin 6,8-di-C-β-glucoside: 51.7 μg/g DM. | [50] |
| Lemon: Peel, Pulp, and Whole fruit | C. limon, Interdonato cultivar (Turkey) | Peel: Total PCs: 251.1 mg/100g. Phenolic acids: Ferulic: 32.6 mg/100g; Caffeic: 23.0 mg/100g; Chlorogenic: 20.9 mg/100g. | [51] |
| Pulp: Total PCs: 78.6 mg/100g. Phenolic acids: Ferulic: 19.6 mg/100g; Caffeic: 9.9 mg/100g; Chlorogenic: 8.1 mg/100g. | |||
| Whole fruit: Total PCs: 174.4 mg/100g. Phenolic acids: Ferulic: 28.1 mg/100g; Caffeic: 16.8 mg/100g; Chlorogenic: 15.5 mg/100g. | |||
| Lemon Peel | C. limon, local store (Brasil) | Phenolic acids: p-coumaric acid, Dihydroferulic acid. Flavanones: Diosmetin-6.8-di-C-glucosid, Apigenin 6,8-di-C-glucoside, Eriocitrin, Chrysoeriol 6,8-di-C-glucoside, Vitexin 2′′-xyloside, Neodiosmin, Rhoifolin 4-glucoside, Neoeriocitrin, Quercetin-3-O-neohesperidoside, Luteolin-neohesperidosidose, Diosmetin-7-O-rutinoside diosmin, Kaempferol-3-O-Rutinose, Diosmetin 8-C-glucoside, Rhoifolin, Isorhamnetin-3-O-neohesperidoside, Limocitrin-neohesperidoside, Hesperidin. | [52] |
| Lemon Peel | C. limon, local suppliers (China) | Flavanones: Eriocitrin: 27.7 mg/g DM; Hesperidin: 24.5 mg/g DM; Polymethoxyflavones: Sinensetin: 0.7 mg/g DM; Nobiletin: 0.3 mg/g DM. | [53] |
| Lemon Peel | Edible lemons (C. limon), local market (Spain) | Flavanones: Eriocitrin, Eriodictyol, Neoeriocitrin, Naringin, Hesperidin, Hesperetin, Neohesperidin; Flavones: Apigenin, Luteolin, Homoorientin, Orientin, Vitexin, Diosmetin, Rhoifolin, Diosmin, Neodismin; Flavanols: Quercetin, Rutin, Limocitrin, Spinacetin. | [54] |
| Lemon Peel | C. limon, local market (Egypt) | Benzoic acid: 758.7 μg/g DM, p-Hydroxybenzoic acid: 281.4 μg/g DM, Chlorogenic acid: 127.3 μg/g DM, Myricetin: 153.4 μg/g DM, Quercetin: 364.2 μg/g DM, Rutin: 181.1 μg/g DM, Naringin: 269.9 μg/g DM, Total: 2534.1 μg/g DM. | [55] |
| By-Product | Lemon Fractions | Extraction Technologies | Extraction Conditions | Antioxidant Properties | Antimicrobial Properties | References |
|---|---|---|---|---|---|---|
| Lemon: Peel, Pulp, Juice, Seed, and Whole fruit | Phenolic compounds | Methanolic extraction | 720 min | Peel: DPPH: 1.08–8.20%. ABTS: 8.65–14.40 mM. FRAP: 1.62–6.60 mM. | [9] | |
| Pulp: DPPH: 4.00–7.29%. ABTS: 0.94–3.85 mM. FRAP: 0.37–1.85 mM. | ||||||
| Juice: DPPH: 0.22–0.59%. ABTS: 0.42–0.71 mM. FRAP: 0.07–0.71 mM. | ||||||
| Seed: DPPH: 0.50–4.01%. ABTS: 7.74–11.97 mM. FRAP: 2.30–3.40 mM. | ||||||
| Whole fruit: DPPH: 3.10–7.96%. ABTS: 8.79 -13.09 mM. FRAP: 1.15–3.65 mM. | ||||||
| Lemon Peel | Phenolic compounds | UAE | 325 W, 17 min | DPPH: 43.16 mg TE/g DM; ABTS: 18.95 mg TE/g DM; FRAP: 74.95 mg TE/g DM; CUPRAC: 29.35 mg TE/g DM. | [53] | |
| Lemon Peel | Phenolic compounds | Maceration (M) and ultrasound-assisted extraction (UAE) | M: 30 min, 37 °C | DPPH activity using maceration was 22.46% and 39.73% with UAE. | [58] | |
| UAE: 30 min, 37 °C | ||||||
| Lemon Albedo and Flavedo | Phenolic compounds | Pulsed Electric Fields (PEF) | 0–300 μs, 3–9 kV/cm | Samples with higher phenol content rate showed higher antioxidant activity. | [59] | |
| Lemon Peel | Phenolic compounds | Hydro-ethanolic extraction | 120 min, 60 °C | Inhibition against Aspergillus flavus of 13.51% after 7 days of incubation. | [63] | |
| Lemon Peel | Phenolic compounds | UAE, Microwave-assisted extraction (MAE) and conventional solvent extraction (CSE) | UAE: 5–20 min, room temperature | DPPH: IC50: UAE (268.24 µg GAE/mL); MAE (203.59 µg GAE/mL); CSE (298.82 mg GAE/mL).Higher antioxidant activity for the MAE extracts. | [60] | |
| MAE: 300–600 W, 1.5–4 min | ||||||
| CSE: 120 min, 60 °C | ||||||
| Lemon Peel | Phenolic compounds | Soxhlet and UAE | Soxhlet: 960 min, 65 °C | The antioxidant activity of samples extracted by UAE was 1.5 to 2.0 times more in comparison to Soxhlet extraction. | [64] | |
| UAE: 200W, 30–50 min, 40–60 °C | ||||||
| Lemon Peel | Phenolic compounds | High-pressure extraction | 300–500 MPa, 3–10 min | DPPH: 80.93 mg TE/100 g. | LP extract at 0.6 and 0.3 mg/mL concentrations demonstrated antibacterial activity against 16 microorganisms. | [65] |
| Lemon extract (peel, bagasse, and seed) | Phenolic compounds | Metanolic extraction | - | Antioxidant activity of 74%. | [66] |
| By-Product | Cultivars/Variety | Content of Dietary Fibre | References |
|---|---|---|---|
| Lemon Peel | C. limon from Pokka Sapporo Food and Beverage (Japan) | Total DF: 47.1%; Insoluble DF: 34.8%; Soluble DF: 12.3%. | [8] |
| Lemon Powder with (Pulp, seed, and peels) | C. limon, juice production company (Tunisia) | Total DF in final powder (pulp, seed an peels): 78.68 g/100 g DM. | [50] |
| Lemon: Peel, pulp, and whole fruit | C. limon, Interdonato cultivar (Turkey) | Peel: Total DF: 64.64 g/100g. | [51] |
| Pulp: Total DF: 28.29 g/100g. | |||
| Whole fruit: Total DF: 37.88 g/100g. | |||
| Lemon: Peels and Peeled | C. limon, local farmer (Spain) | Peels: Total DF: 2.49 g/100g of FW; Insoluble DF: 67%; Soluble 33%. | [68] |
| Peeled: Total DF: 1.31 g/100g of FW; Insoluble DF: 67%; Soluble: 33%. | |||
| Lemon: Peels and Pulp | C. limon, from commercial orchar (Spain) | Peels: Pectin: 13% of DW; Lignin: 7.56% of DW; Cellulose: 23.06% of DW; Hemicellulose: 8.09% of DW. | [73] |
| Pulp: Pectin: 22.53% of DW; Lignin: 7.55% of DW; Cellulose: 36.22% of DW; Hemicellulose: 11.05% of DW. | |||
| Lemon Peels | C. limon (Eureka and Fino 49), juice extraction (Chile) | Eureka: Total DF: 60.1 g/100g DW; Insoluble DF: 50.9 g/100g DW; Soluble DF: 9.2 g/100g DW. Fino 49: Total DF: 68 g/100g DW; Insoluble DF: 62 g/100g DW; Soluble DF: 6.3 g/100g DW. | [74] |
| Lemon Pomace | C. limon, juice industry (Spain) | Total DF: 72.4 8 g/100g DW; Insoluble DF: 14.58 g/100g DW; Soluble DF: 57.8 g/100g DW. | [75] |
| Lemon: Defatted seed | C. limon (Kütdiken), food industry (Turkey) | Total DF: 86.1 g/100g DW; Insoluble DF: 79.6 g/100g DW; Soluble DF: 6.5 g/100g DW. | [76] |
| By-Product | Lemon Fractions | Extraction Technologies | Extraction Conditions | Yield (%) | Degree of Esterification (%) | Bioactive Properties | References |
|---|---|---|---|---|---|---|---|
| Lemon peel | Pectin | Microwave assisted extraction (MAE) | 300–700 W, 1–3 min | Citric acid: 5.80%–25.00% | 1.2–35.1% | [83] | |
| Lemon peel | Pectin | Acid hydrolisis | 180 min, 85 °C | Hydrochloric acid: 27%–39% | 87.19–92.6% | Antioxidant activity of 71.46 ± 1.39 mg TE/g sample in ABTS method. | [85] |
| Lemon peel | Pectin | Hydrodynamic cavitation | - | Antibacterial activity against S.aureus. | [86] | ||
| Lemon peel | Pectin | Hydrodynamic cavitation | - | Antioxidant activity: ORAC values are remarkably high for ‘’IntegroPectin’’ (122,200 μmol TE/100 g). | [87] | ||
| Lemon peel | Pectin | Acid hydrolisis | 30–60 min, 60–80 °C | Nitric acid: 17.4%–46.4% Citric acid: 21.4%–76.0% | 58.62% 38.46% | [88] | |
| Lemon peel | Pectin | Acid hydrolisis | 60 min, 40–90 °C | Citric acid: 36.71% | 1.50% | [89] | |
| Lemon peel | Pectin | Ultrasound assisted extraction (UAE) and (MAE) | UAE: 360–600 W, 1–3 min | UAE: Nitric acid: 7.19%–10.11% UAE: Hydrochloric acid: 5.97%–8.60% MAE: Nitric acid: 9.71% MAE: Hydrochloric acid: 7.31% | 50.51% 51.13% 50.65% 51.08% | [90] | |
| MAE: 15–45 min, 60–75°C | |||||||
| Lemon peel | Pectin | Acid hydrolisis | 240 min, 80 °C | Citric acid: 30.6% | 18.30–58.06% | [91] | |
| Lemon peel | Pectin | Acid hydrolisis | 90 min, 80–90 °C | Hydrochloric acid: 1.38%–2.22% | 88.6% | [92] | |
| Lemon waste | Pectin | MAE | 600 W, 60–80 min, 100 °C | 24–40% | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12051095
Magalhães D, Vilas-Boas AA, Teixeira P, Pintado M. Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review. Foods. 2023; 12(5):1095. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12051095
Chicago/Turabian StyleMagalhães, Daniela, Ana A. Vilas-Boas, Paula Teixeira, and Manuela Pintado. 2023. "Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review" Foods 12, no. 5: 1095. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12051095