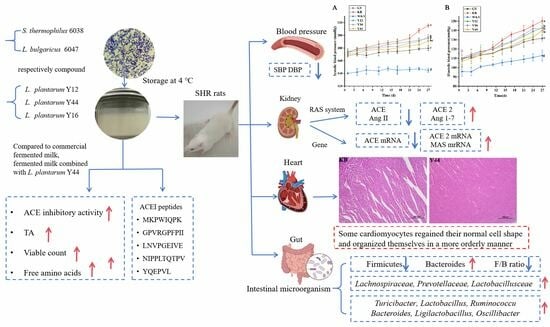
Oral Administration of Fermented Milk from Co-Starter Containing Lactobacillus plantarum Y44 Shows an Ameliorating Effect on Hypertension in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Combined Starter Culture
2.3. Fermentation of the Mixture of Soybean Milk and Skim Milk
2.4. Determination of Fermented Mixed Milk Characteristics
2.4.1. Titratable Acidity
2.4.2. Viable Counts
2.4.3. Free Amino Acids
2.4.4. Sensory Evaluation
2.4.5. Determination of Angiotensin-Converting-Enzyme Inhibitory (ACE I) Activity
2.4.6. Peptide Sequence Analysis
2.5. Assay to Determine the Effects of the Fermented Milk Oral Administration on the Spontaneously Hypertensive Rats (SHRs)
2.5.1. Histological Analyses and Organ Index
2.5.2. Determination of Biochemical Indexes in Rat Serum
2.5.3. RT-PCR Analysis
2.5.4. Fecal SCFA Analysis
2.5.5. Rat Fecal Microbiota
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Properties of the Fermented Mixed Milk from Combined Starter Cultures
3.2. Analysis of Differential Peptides of the Fermented Mixed Milk from Combined Starter Cultures
3.3. Effects of Fermented Milk Oral Administration on Blood Pressures of SHRs and WKY Rats
3.4. Effect of Fermented Milk Oral Administration on Renal RAS in SHRs and WKY Rats
3.5. Effects of Fermented Milk Oral Administration on Blood Lipid Indexes and Oxidation Indexes in the Sera of SHRs and WKY Rats
3.6. Ameliorating Effects of Fermented Milk Oral Administration on the Liver, Heart, and Kidney Injury of SHRs and WKY Rats
3.7. Effect of Fermented Milk Oral Administration on SCFA Concentration in Rats Feces
3.8. Effect of Fermented Milk Oral Administration on the Gut Microbiota of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Evaluation Index | Evaluation Index | Score |
|---|---|---|
| Sour–sweet ratio (15) | Moderate sweet and sour | 11–15 |
| Slightly sour or slightly sweet | 6–10 | |
| Bitter, too sour, or too sweet | 0–5 | |
| Viscosity (15) | Medium viscosity, thick | 11–15 |
| Viscosity is viscous or thin | 6–10 | |
| Viscosity is too sticky or too thin | 0–5 | |
| Consistency (15) | Good viscosity, fine and silky | 11–15 |
| Better viscosity and fineness | 6–10 | |
| Poor viscosity and no wire drawing | 0–5 | |
| Taste (20) | No sense of graininess, smoothness and astringency | 16–20 |
| The taste of the granules is slightly soft or hard and slightly powdery | 11–15 | |
| The texture of the granules is soft or hard and slightly powdery | 6–10 | |
| The taste of the granules is too soft or too hard, and the powder is very astringent | 0–5 | |
| Flavor (20) | Fermented milk has an obvious flavor, natural fermentation flavor and smell, and no peculiar smell | 16–20 |
| The flavor of the fermented milk is not enough, and the flavor of natural fermentation is low | 11–15 | |
| The flavor of the fermented milk is poor, and the flavor of natural fermentation is poor | 6–10 | |
| Fermented milk has no characteristic flavor or produces an unpleasant flavor | 0–5 | |
| Texture (15) | Uniform color, milky white, good organization, no bubbles, no whey precipitation | 11–15 |
| Light gray or grayish white color, good structure, slight whey precipitation | 6–10 | |
| Abnormal color, rough tissue and severe whey precipitation | 0–5 |
References
- Hall, J.E.; Granger, J.P.; do Carmo, J.M.; da Silva, A.A.; Dubinion, J.; George, E.; Hamza, S.; Speed, J.; Hall, M.E. Hypertension: Physiology and pathophysiology. Compr. Physiol. 2012, 2, 2393–2442. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D.; Jiang, B.; Milani, F. Three key proteases—angiotensin-I-converting enzyme (ACE), ACE2 and renin—within and beyond the renin-angiotensin system. Arch. Cardiovasc. Dis. 2012, 105, 373–385. [Google Scholar] [CrossRef]
- Shen, X.; Xie, A.; Li, Z.; Jiang, C.; Wu, J.; Li, M.; Yue, X. Research Progress for Probiotics Regulating Intestinal Flora to Improve Functional Dyspepsia: A Review. Foods 2024, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Companys, J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Pedret, A.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 834–863. [Google Scholar] [CrossRef]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124784. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2011, 34, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kwok, L.Y.; Mi, Z.; Bala, J.; Xue, J.; Yang, J.; Ma, Y.; Zhang, H.; Chen, Y. Characterization of the angiotensin-converting enzyme inhibitory activity of fermented milks produced with Lactobacillus casei. J. Dairy Sci. 2017, 100, 9495–9507. [Google Scholar] [CrossRef]
- Song, T.; Lv, M.; Sun, B.; Zheng, L.; Zhao, M. Tripeptides Val-Pro-Pro (VPP) and Ile-Pro-Pro (IPP) Regulate the Proliferation and Migration of Vascular Smooth Muscle Cells by Interfering Ang II-Induced Human Umbilical Vein Endothelial Cells Derived EVs Delivering RNAs to VSMCs in the Co-Culture Model. J. Agric. Food Chem. 2020, 68, 6628–6637. [Google Scholar] [CrossRef]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Li, H.; Prairie, N.; Udenigwe, C.C.; Adebiyi, A.P.; Tappia, P.S.; Aukema, H.M.; Jones, P.J.; Aluko, R.E. Blood pressure lowering effect of a pea protein hydrolysate in hypertensive rats and humans. J. Agric. Food Chem. 2011, 59, 9854–9860. [Google Scholar] [CrossRef]
- Liu, C.F.; Hu, C.L.; Chiang, S.S.; Tseng, K.C.; Yu, R.C.; Pan, T.M. Beneficial preventive effects of gastric mucosal lesion for soy-skim milk fermented by lactic acid bacteria. J. Agric. Food Chem. 2009, 57, 4433–4438. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Song, Y.; Gao, Y.; Zhao, F.; Luo, Y.; Qian, F.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020, 11, 5205–5222. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Tang, N.; Huang, Y.; Chen, X.; Dong, M.; Rui, X.; Zhang, Q.; Li, W. Fermentative and physicochemical properties of fermented milk supplemented with sea buckthorn (Hippophae eleagnaceae L.). LWT 2022, 153, 112484. [Google Scholar] [CrossRef]
- Yu, J.; Guo, H.; Sun, M.; Jiang, C.; Jiang, S.; Mu, G.; Tuo, Y.; Gao, P. Milk fermented by combined starter cultures comprising three Lactobacillus strains exerts an alleviating effect on loperamide-induced constipation in BALB/c mice. Food Funct. 2023, 14, 5264–5276. [Google Scholar] [CrossRef]
- GB 5009.239-2016; National Food Safety Standard Determination of Acidity in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, É.; Dufossé, L.; Guérard, F. Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- GB 19302-2010; National Food Safety Standard Fermented Milk. National Standards of the People’s Republic of China: Beijing, China, 2010.
- Wu, N.; Xu, W.; Liu, K.; Xia, Y.; Shuang, Q. Angiotensin-converting enzyme inhibitory peptides from Lactobacillus delbrueckii QS306 fermented milk. J. Dairy Sci. 2019, 102, 5913–5921. [Google Scholar] [CrossRef]
- Mao, J.; Zhou, Z.; Yang, H. Microbial succession and its effect on the formation of umami peptides during sufu fermentation. Front. Microbiol. 2023, 14, 1181588. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, P.; Ma, F.; Li, X.; Mu, G.; Tuo, Y. Mixed Lactiplantibacillus plantarum strains alleviated DSS-induced intestinal inflammation of Balb/c mice via the 5-HT/5-HT7R/NF-κB signaling pathway. J. Funct. Foods 2023, 102, 105435. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Qi, H.; Wang, Y.; Cui, L.; Wen, Y.; Li, H.; Yin, C. The ACE2-angiotensin-(1-7)-Mas axis protects against pancreatic cell damage in cell culture. Pancreas 2015, 44, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, M.; Xu, C.; Wang, S.; Li, L.; Zou, S.; Yu, J.; Wei, Y. Positive Effect of a Pea-Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats. Nutrients 2022, 14, 4069. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; McBreairty, L.E.; Chizen, D.R.; Pierson, R.A.; Chilibeck, P.D.; Zello, G.A. A Comparison of a Pulse-Based Diet and the Therapeutic Lifestyle Changes Diet in Combination with Exercise and Health Counselling on the Cardio-Metabolic Risk Profile in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Nutrients 2018, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Kelleni, M.T.; Ibrahim, S.A.; Abdelrahman, A.M. Effect of captopril and telmisartan on methotrexate-induced hepatotoxicity in rats: Impact of oxidative stress, inflammation and apoptosis. Toxicol. Mech. Methods 2016, 26, 371–377. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Gao, Y.; An, N.; Li, X.; Pan, X.; Yang, X.; Tian, L.; Sun, J.; Xiong, X.; et al. Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomed. Pharmacother. 2020, 130, 110503. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary Fibers from Fruits and Vegetables and Their Health Benefits via Modulation of Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.P.; Hsieh, Y.M.; Kuo, W.W.; Lin, C.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Tsai, C.C. Inhibition of cardiac hypertrophy by probiotic-fermented purple sweet potato yogurt in spontaneously hypertensive rat hearts. Int. J. Mol. Med. 2012, 30, 1365–1375. [Google Scholar] [CrossRef]
- Ahrén, I.L.; Xu, J.; Önning, G.; Olsson, C.; Ahrné, S.; Molin, G. Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin. Nutr. 2015, 34, 719–726. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Li, Y.; Chen, M.; Xue, L.; Wang, J.; Ding, Y.; Zhang, J.; Wu, S.; Ye, Q.; Zhang, S.; et al. Antihypertensive Activity of Milk Fermented by Lactiplantibacillus plantarum SR37-3 and SR61-2 in L-NAME-Induced Hypertensive Rats. Foods 2022, 11, 2332. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Paparella, A.; Martuscelli, M.; Corsetti, A.; Tofalo, R.; Suzzi, G. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiol. 2014, 42, 117–121. [Google Scholar] [CrossRef]
- Rubattu, S.; Pagliaro, B.; Pierelli, G.; Santolamazza, C.; Di Castro, S.; Mennuni, S.; Volpe, M. Pathogenesis of target organ damage in hypertension: Role of mitochondrial oxidative stress. Int. J. Mol. Sci. 2014, 16, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Lob, H.E.; Marvar, P.J.; Guzik, T.J.; Sharma, S.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 2010, 55, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Díaz, A.G.; Pazarín-Villaseñor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, H.; Cai, Y.; Halabi, C.M.; Becker, L.K.; Santos, R.A.; Speth, R.C.; Sigmund, C.D.; Lazartigues, E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ. Res. 2010, 106, 373–382. [Google Scholar] [CrossRef]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. American journal of physiology. Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef]
- Goswami, C.; Iwasaki, Y.; Yada, T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 2018, 57, 130–135. [Google Scholar] [CrossRef]
- Chappell, M.C. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: More than regulation of blood pressure. Hypertension 2007, 50, 596–599. [Google Scholar] [CrossRef]
- Duprez, D.A. Role of the renin–angiotensin–aldosterone system in vascular remodeling and inflammation: A clinical review. J. Hypertens. 2006, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Sriramula, S.; Lazartigues, E. ACE2/ANG-(1–7)/Mas pathway in the brain: The axis of good. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R804–R817. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Q.; Liu, Y.; Wang, L.; Ge, Z.; Li, Z.; Feng, S.; Wu, C. Gut microbiota and hypertension: Association, mechanisms and treatment. Clin. Exp. Hypertens. 2023, 45, 2195135. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.Y.; Li, Z.M.; Mao, Y.Q.; Chen, H.L.; Hu, W.; Han, B.; Wang, L.S. Probiotic yogurt blunts the increase of blood pressure in spontaneously hypertensive rats via remodeling of the gut microbiota. Food Funct. 2021, 12, 9773–9783. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflug. Arch. Eur. J. Physiol. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]












| Animals | Groups | Feeding |
|---|---|---|
| SHR | Positive control group (GY) | Captopril |
| SHR | Negative control group (KB) | Pasteurized milk |
| WKY | Blank group (WKY) | Pasteurized milk |
| SHR | Y12 group (Y12) | 6038 + 6047 + Y12 fermented milk |
| SHR | Y44 group (Y44) | 6038 + 6047 + Y44 fermented milk |
| SHR | Y16 group (Y16) | 6038 + 6047 + Y16 fermented milk |
| The gavage dose was consistent among the groups, with a dosage of 10 mg/kg/d. | ||
| Type of fermented milk | 6038 + 6047 | 6038 + 6047 + Y12 | 6038 + 6047 + Y44 | 6038 + 6047 + Y16 | |
| Chemical characteristics | |||||
| TA (°T) | 90.09 ± 3.09 b | 98.96 ± 2.27 a | 97.75 ± 5.91 a | 95.80 ± 1.77 a | |
| Viable count (CFU mL−1) | 3.88 ± 0.52 × 109 b | 4.35 ±0.47 × 109 a | 4.59 ± 0.54 × 109 a | 4.54 ± 0.36 × 109 a | |
| Free amino acids (mg/mL) | 0.303 ± 0.097 c | 0.390 ± 1.06 b | 0.417 ± 0.93 b | 0.493 ± 0.94 a | |
| Sensory score (points) | 85.25 ± 3.39 c | 91.25 ± 4.11 a | 88.67 ± 3.17 b | 92.65 ± 2.47 a | |
| ACE I activity (%) | 25.98 ± 1.19 c | 46.80 ± 2.10 ab | 53.56 ± 0.69 a | 42.98 ± 1.19 b | |
| Comparisons | All | Up | Down |
|---|---|---|---|
| Y vs. Y12 | 750 | 331 | 419 |
| Y vs. Y44 | 982 | 374 | 608 |
| Y vs. Y16 | 96 | 37 | 59 |
| Y12 vs. Y44 | 887 | 511 | 376 |
| Y12 vs. Y16 | 673 | 346 | 337 |
| Y44 vs. Y16 | 886 | 371 | 443 |
| Polypeptide Sequence | Mass | Length | m/z | Relative Content | |||
|---|---|---|---|---|---|---|---|
| Y | Y12 | Y44 | Y16 | ||||
| DAYPSGAW | 1141.5079 | 8 | 571.7617 | 0.0429 | 0 | 0 | 0 |
| EMPFPK | 1007.4786 | 6 | 504.745 | 0.2865 | 0.1977 | 0 | 0 |
| FVAPFPEVFG | 1108.5593 | 10 | 555.2863 | 0.3365 | 0.4932 | 0.1881 | 0.6551 |
| HLPLP | 575.3431 | 5 | 288.6788 | 0.3818 | 0.2455 | 0 | 0 |
| AMKPWIQPK | 1097.6056 | 9 | 549.8098 | 9.1141 | 56.9335 | 57.9351 | 18.3578 |
| DKIHPF | 755.3966 | 6 | 378.7053 | 1 | 4.1244 | 2.9753 | 4.7795 |
| EIVPNSAEERLH | 1392.6997 | 12 | 465.2398 | 0 | 0 | 0.0714 | 0 |
| FALPQY | 737.3748 | 6 | 369.6942 | 2.4255 | 0 | 0 | 0.4849 |
| FALPQYLK | 978.5538 | 8 | 490.2833 | 0 | 0 | 0 | 0.1396 |
| FPEVFGK | 822.4276 | 7 | 412.2204 | 0.0138 | 0.0573 | 0 | 0 |
| FVAPFPEVFG | 1108.5593 | 10 | 555.2863 | 0.3365 | 0.3817 | 0.1881 | 0.6551 |
| GPVRGPFPII | 1051.6178 | 10 | 526.8159 | 1.9852 | 18.2349 | 68.8615 | 3.4358 |
| IPPLTQTPV | 964.5593 | 9 | 483.287 | 0 | 1.0812 | 6.5293 | 0 |
| KAAAAP | 726.4388 | 6 | 364.2252 | 0 | 0 | 0.0717 | 0 |
| KYIPIQ | 760.4483 | 6 | 381.2311 | 0 | 0.4559 | 0.8266 | 0 |
| LDAQSAPLR | 969.5243 | 9 | 485.77 | 0.7855 | 0 | 0 | 0.2509 |
| LGPVRGPFP | 938.5338 | 9 | 470.2735 | 0.4908 | 0.7913 | 0 | 0.2365 |
| LHLPLPL | 801.5112 | 7 | 401.7628 | 9.2085 | 1.8828 | 3.6409 | 1.8227 |
| LNVPGEIVE | 968.5178 | 9 | 485.2659 | 0.0144 | 0.6265 | 16.0615 | 0.8688 |
| LTQTPVVVPPF | 1196.6804 | 11 | 599.3472 | 6.9091 | 0 | 0 | 1.2390 |
| LVYPFPGPIH | 1138.6174 | 10 | 570.316 | 0 | 2.2781 | 0 | 0.1767 |
| LVYPFPGPIPNSLPQN | 1751.9246 | 16 | 876.968 | 0 | 0.0204 | 0.0696 | 0.1572 |
| LVYPFPGPIPNSLPQNIPP | 2059.1143 | 19 | 1030.5667 | 0.7600 | 0 | 0 | 0.6918 |
| MKPWIQPK | 1026.5685 | 8 | 514.2914 | 1.2883 | 8.5013 | 2.3931 | 1.8913 |
| NIPPLTQTPV | 1078.6023 | 10 | 540.308 | 5.0910 | 56.5832 | 76.8847 | 15.6471 |
| NLHLPLP | 802.4701 | 7 | 402.2415 | 0 | 4.5065 | 1.0877 | 0 |
| PFPEVFGK | 919.4803 | 8 | 460.7472 | 7.9391 | 6.6073 | 1.0813 | 13.6954 |
| QEPVLGPVRGPFP | 1391.7561 | 13 | 696.8857 | 37.6619 | 95.2655 | 1.9809 | 80.9390 |
| TTMPLW | 747.3625 | 6 | 374.6879 | 0 | 0.6130 | 0.1559 | 0 |
| VPSERYL | 862.4548 | 7 | 432.2333 | 0.0000 | 0.0983 | 0.5551 | 0.2403 |
| VRGPFP | 671.3755 | 6 | 336.6948 | 1.6800 | 0 | 0 | 0 |
| VRGPFPIIV | 996.612 | 9 | 499.313 | 1.7564 | 2.6911 | 0.6419 | 1.0535 |
| VVVPPF | 656.3897 | 6 | 329.2016 | 0.2691 | 0 | 0.2359 | 0 |
| YAKPA | 548.2958 | 5 | 275.1553 | 0 | 0 | 1 | 0 |
| YIPIQY | 795.4167 | 6 | 398.715 | 0 | 0.3886 | 0.5081 | 0 |
| YPQRDMPIQ | 1146.5492 | 9 | 574.2802 | 0 | 0 | 1 | 0 |
| YQEPVL | 747.3802 | 6 | 374.6969 | 0.2386 | 6.2676 | 5.2048 | 0.6514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Sun, M.; Jiang, S.; Jiang, C.; Mu, G.; Tuo, Y. Oral Administration of Fermented Milk from Co-Starter Containing Lactobacillus plantarum Y44 Shows an Ameliorating Effect on Hypertension in Spontaneously Hypertensive Rats. Foods 2024, 13, 641. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13050641
Yu J, Sun M, Jiang S, Jiang C, Mu G, Tuo Y. Oral Administration of Fermented Milk from Co-Starter Containing Lactobacillus plantarum Y44 Shows an Ameliorating Effect on Hypertension in Spontaneously Hypertensive Rats. Foods. 2024; 13(5):641. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13050641
Chicago/Turabian StyleYu, Jiang, Mengying Sun, Shilong Jiang, Chuqi Jiang, Guangqing Mu, and Yanfeng Tuo. 2024. "Oral Administration of Fermented Milk from Co-Starter Containing Lactobacillus plantarum Y44 Shows an Ameliorating Effect on Hypertension in Spontaneously Hypertensive Rats" Foods 13, no. 5: 641. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13050641