Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population, Inclusion and Exclusion Criteria
2.2. Brain MRI Acquisition and Processing
2.3. Calculation of Accumulated PM2.5 Exposure
2.4. Neurocognitive Performance
2.5. Covariates
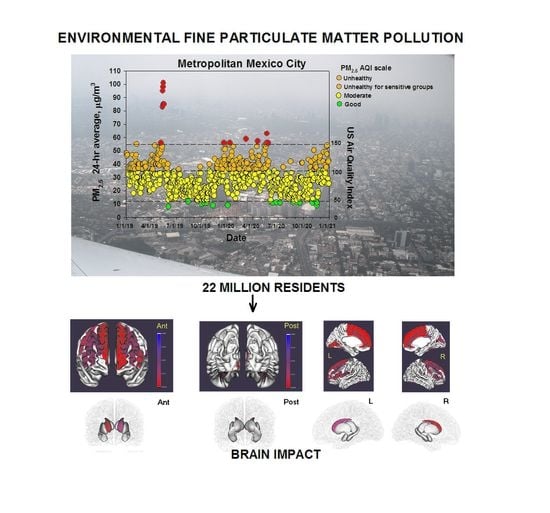
2.6. Study City and Air Quality
2.7. Statistical Analysis
3. Results
3.1. Air Pollution
3.2. Study Population and Demographics
3.3. Total Gray and White Matter Volumes and CSF, Cortical Thickness, Cortical Surface Area, and Intracranial Volume ICV
3.4. Subcortical Volume
3.5. MoCA Results
3.6. MoCA Total Score, Index Scores, Cortical Thickness and Subcortical Volumes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calderón-Garcidueñas, L.; Pérez-Calatayud, A.A.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Ramos-Morales, A.; Torres-Jardón, R.; Soberanes-Cerino, C.D.J.; Carrillo-Esper, R.; Briones-Garduño, J.C.; et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines 2022, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. Environ Res. 2018, 164, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles (and engineered Ti-rich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ. Res. 2020, 191, 110139. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimer’s Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Russ, T.C.; Cherrie, M.P.; Dibben, C.; Tomlinson, S.; Reis, S.; Dragosits, U.; Vieno, M.; Beck, R.; Carnell, E.; Shortt, N.K.; et al. Life Course Air Pollution Exposure and Cognitive Decline: Modelled Historical Air Pollution Data and the Lothian Birth Cohort 1936. J. Alzheimer’s Dis. 2021, 79, 1063–1074. [Google Scholar] [CrossRef]
- Mortamais, M.; Gutierrez, L.-A.; de Hoogh, K.; Chen, J.; Vienneau, D.; Carrière, I.; Letellier, N.; Helmer, C.; Gabelle, A.; Mura, T.; et al. Long-term exposure to ambient air pollution and risk of dementia: Results of the prospective Three-City Study. Environ. Int. 2021, 148, 106376. [Google Scholar] [CrossRef]
- Grande, G.; Ljungman, P.L.S.; Eneroth, K.; Bellander, T.; Rizzuto, D. Association Between Cardiovascular Disease and Long-term Exposure to Air Pollution with the Risk of Dementia. JAMA Neurol. 2020, 77, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Tham, R.; Schikowski, T. The Role of Traffic-Related Air Pollution on Neurodegenerative Diseases in Older People: An Epidemiological Perspective. J. Alzheimer’s Dis. 2021, 79, 949–959. [Google Scholar] [CrossRef]
- Porta, D.; Narduzzi, S.; Badaloni, C.; Bucci, S.; Cesaroni, G.; Colelli, V.; Davoli, M.; Sunyer, J.; Zirro, E.; Schwartz, J.; et al. Air pollution and cognitive development at age seven in a prospective Italian birth cohort. Epidemiology 2016, 27, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kicinski, M.; Vermeir, G.; Van Larebeke, N.; Hond, E.D.; Schoeters, G.; Bruckers, L.; Sioen, I.; Bijnens, E.; Roels, H.A.; Baeyens, W.; et al. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ. Int. 2015, 75, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.; Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008, 68, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Engle, R.; Mora-Tiscareño, A.; Styner, M.; Gómez-Garza, G.; Zhu, H.; Jewells, V.; Torres-Jardón, R.; Romero, L.; Monroy-Acosta, M.E.; et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 2011, 77, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Torres-Jardón, R.; Kulesza, R.J.; Mansour, Y.; González-González, L.O.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Mukherjee, P.S. Alzheimer disease starts in childhood in polluted Metropolitan Mexico City. A major health crisis in progress. Environ. Res. 2020, 183, 109137. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Hernández-Luna, J.; Ávila-Cervantes, R.; Macías-Escobedo, E.; González-González, O.; González-Maciel, A.; García-Hernández, K.; et al. Mild Cognitive Impairment and Dementia In-volving Multiple Cognitive Domains in Mexican Urbanites. J. Alzheimer’s Dis. 2019, 68, 1113–1123. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Macías-Escobedo, E.; Hernández-Castillo, A.; Carlos-Hernández, E.; Franco-Ortíz, A.; Castro-Romero, S.P.; Cortés-Flores, M.; Crespo-Cortés, C.N.; et al. Metals, Nanoparticles, Particulate Matter, and Cognitive Decline. Front. Neurol. 2022, 12, 794071. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Hagler, D.J., Jr.; Hatton, S.; Cornejo, M.D.; Makowski, C.; Fair, D.A.; Dick, A.S.; Sutherland, M.T.; Casey, B.; Barch, D.M.; Harms, M.P.; et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 2019, 202, 116091. [Google Scholar] [CrossRef]
- Secretaría del Medio Ambiente de la Ciudad de México. 2021. Available online: http://www.aire.cdmx.gob.mx/default.php (accessed on 10 September 2021).
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Julayanont, P.; Brousseau, M.; Chertkow, H.; Phillips, N.; Nasreddine, Z.S. Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 2014, 62, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Pugh, E.A.; Kemp, E.C.; van Dyck, C.H.; Mecca, A.P.; Sharp, E.S. Effects of Normative Adjustments to the Montreal Cognitive Assessment. Am. J. Geriatr. Psychiatry 2018, 26, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.; Garcia, C.; Adana, L.; Yacelga, T.; Rodriguez-Lorenzana, A.; Maruta, C. Use of the Montreal Cognitive Assessment (MoCA) in Latin America: A systematic review. Rev. De Neurol. 2018, 66, 397–408. [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013.
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Torres-Jardón, R. Politicas públicas y su efecto en la calidad del aire de la Zona metropolitana de la Ciudad de Mexico. In Transversalidad de la Politica del Aire en Mexico; Núñez, G.S., Ed.; Instituto de Investigaciones Dr. José Maria Luis Mora: Mexico City, Mexico, 2018; pp. 43–74. [Google Scholar]
- Velasco, E.; Retama, A. Ozone’s threat hits back Mexico City. Sustain. Cities Soc. 2017, 31, 260–263. [Google Scholar] [CrossRef]
- Zavala, M.; Brune, W.H.; Velasco, E.; Retama, A.; Cruz-Alvarez, L.A.; Molina, L.T. Changes in ozone production and VOC reac-tivity in the atmosphere of the Mexico City Metropolitan Area. Atmos. Environ. 2020, 238, 117747. [Google Scholar] [CrossRef]
- Molina, L.T.; Velasco, E.; Retama, A.; Zavala, M. Experience from Integrated Air Quality Management in the Mexico City Metropolitan Area and Singapore. Atmosphere 2019, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Dzepina, K.; Arey, J.; Marr, L.C.; Worsnop, D.R.; Salcedo, D.; Zhang, Q.; Onasch, T.B.; Molina, L.T.; Molina, M.J.; Jimenez, J.L. Detection of particle-phase polycyclic aromatic hydrocarbons in Mexico City using an aerosol mass spectrometer. Int. J. Mass Spectrom. 2007, 263, 152–170. [Google Scholar] [CrossRef]
- Mugica, V.; Mugica, F.; Torres, M.; Figueroa, J. PM2.5Emission Elemental Composition from Diverse Combustion Sources in the Metropolitan Area of Mexico City. Sci. World J. 2008, 8, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Zavala, M.; Molina, L.T.; Yacovitch, T.I.; Fortner, E.C.; Roscioli, J.R.; Floerchinger, C.; Herndon, S.C.; Kolb, C.E.; Knighton, W.B.; Paramo, V.H.; et al. Emission factors of black carbon and co-pollutants from diesel vehicles in Mexico City Atmos. Chem. Phys. 2017, 17, 15293–15305. [Google Scholar]
- Ladino, L.A.; Raga, G.B.; Baumgardner, D. On particle-bound polycyclic aromatic hydrocarbons (PPAH) and links to gaseous emissions in Mexico city. Atmos. Environ. 2018, 194, 31–40. [Google Scholar] [CrossRef]
- Amador-Muñoz, O.; Martínez-Domínguez, Y.; Gómez-Arroyo, S.; Peralta, O. Current situation of polycyclic aromatic hydrocarbons (PAH) in PM2.5 in a receptor site in Mexico City and estimation of carcinogenic PAH by combining non-real-time and real-time measurement techniques. Sci. Total Environ. 2019, 703, 134526. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Buseck, P.R. Hosted and Free-Floating Metal-Bearing Atmospheric Nanoparticles in Mexico City. Environ. Sci. Technol. 2010, 44, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Caudillo, L.; Salcedo, D.; Peralta, O.; Castro, T.; Alvarez-Ospina, H. Nanoparticle size distributions in Mexico city. Atmospheric Pollut. Res. 2019, 11, 78–84. [Google Scholar] [CrossRef]
- Velasco, E.; Retama, A.; Segovia, E.; Ramos, R. Particle exposure and inhaled dose while commuting by public transport in Mexico City. Atmos. Environ. 2019, 219, 117044. [Google Scholar] [CrossRef]
- Múgica-Alvarez, V.; Figueroa-Lara, J.; Romero-Romo, M.; Sepulveda-Sanchez, J.; Lopez-Moreno, T. Concentrations and pro-perties of airborne particles in the Mexico City subway system. Atmos. Environ. 2012, 49, 284–293. [Google Scholar] [CrossRef]
- CCA. Contaminación Ambiental en Hermosillo II: Expediente de Hechos Relativo a la Petición SEM-05-003; Comisión para la Cooperación Ambiental: Montreal, QC, Canada, 2014; 116p. [Google Scholar]
- Del Río-Salas, R.; Ruiz, J.; De la O-Villanueva, M.; Valencia-Moreno, M.; Moreno-Rodríguez, V.; Gómez-Alvarez, A.; Grijalva, T.; Mendivil, H.; Paz-Moreno, F.; Meza-Figueroa, D. Tracing geogenic and anthropogenic sources in urban dusts: Insights from lead isotopes. Atmos. Environ. 2012, 60, 202–210. [Google Scholar] [CrossRef]
- Cruz-Campas, M.E.; Gomez-Alvarez, A.; Quintero-Nuñez, M.; Varela-Salazar, J. One year air quality evaluation regarding total suspended particles (TSP) and heavy metals (Pb, Cd, Ni, Cu, Cr) in Hermosillo, Sonora, Mexico. Rev. Int. Contam. Ambient. 2013, 29, 269–283. [Google Scholar]
- Meza-Figueroa, D.; González-Grijalva, B.; Romero, F.; Ruiz, J.; Pedroza-Montero, M.; Rivero, C.I.-D.; Acosta-Elías, M.; Ochoa-Landin, L.; Navarro-Espinoza, S. Source apportionment and environmental fate of lead chromates in atmospheric dust in arid environments. Sci. Total Environ. 2018, 630, 1596–1607. [Google Scholar] [CrossRef]
- Rangel-López, C.J. Diagnostic of the Origin and State of the Air Pollution in Hermosillo, Sonora. Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional: Mexico City, Mexico, 2015; unpublished undergraduate thesis. (In Spanish) [Google Scholar]
- Iannopollo, E.; Garcia, K.; Alzheimer’s Disease Neuroimaging Initiative. Enhanced detection of cortical atrophy in Alzheimer’s disease using structural MRI with anatomically constrained longitudinal registration. Hum. Brain Mapp. 2021, 42, 3576–3592. [Google Scholar] [CrossRef]
- Pereira, J.B.; Svenningsson, P.; Weintraub, D.; Brønnick, K.; Lebedev, A.; Westman, E.; Aarsland, D. Initial cognitive decline is as-sociated with cortical thinning in early Parkinson disease. Neurology 2014, 82, 2017–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korthauer, L.E.; Blujus, J.K.; Awe, E.; Frahmand, M.; Prost, R.; Driscoll, I. Brain-behavior investigation of potential cognitive markers of Alzheimer’s disease in middle age: A multi-modal imaging study. Brain Imaging Behav. 2021. [Google Scholar] [CrossRef] [PubMed]
- Contador, J.; Pérez-Millan, A.; Guillen, N.; Tort-Merino, A.; Balasa, M.; Falgàs, N.; Olives, J.; Castellví, M.; Borrego-Écija, S.; Bosch, B.; et al. Baseline MRI atrophy predicts 2-year cognitive outcomes in early-onset Alzheimer’s disease. J. Neurol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Koenig, L.N.; LaMontagne, P.; Glasser, M.F.; Bateman, R.; Holtzman, D.; Yakushev, I.; Chhatwal, J.; Day, G.S.; Jack, C.; Mummery, C.; et al. Regional age-related atrophy after screening for preclinical Alzheimer disease. Neurobiol. Aging 2021, 109, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.; Becerra, S.; Duncan, J.; Spivak, N.; Dang, B.H.; Habelhah, B.; Mahdavi, K.D.; Mamoun, M.; Whitney, M.; Pereles, F.S.; et al. Translating state-of-the-art brain magnetic resonance imaging (MRI) techniques into clinical practice: Multimodal MRI differentiates dementia subtypes in a traditional clinical setting. Quant. Imaging Med. Surg. 2021, 11, 4056–4073. [Google Scholar] [CrossRef]
- Contador, J.; Pérez-Millán, A.; Tort-Merino, A.; Balasa, M.; Falgàs, N.; Olives, J.; Castellví, M.; Borrego-Écija, S.; Bosch, B.; Fernández-Villullas, G.; et al. Longitudinal brain atrophy and CSF biomarkers in early-onset Alzheimer’s disease. NeuroImage Clin. 2021, 32, 102804. [Google Scholar] [CrossRef]
- Platero, C.; Tobar, M.C.; Alzheimer’s Disease Neuroimaging Initiative. Predicting Alzheimer’s conversion in mild cognitive impairment patients using longitudinal neuroimaging and clinical markers. Brain Imaging Behav. 2020, 15, 1728–1738. [Google Scholar] [CrossRef]
- E Williams, M.; A Elman, J.; McEvoy, L.K.; A Andreassen, O.; Dale, A.M.; Eglit, G.M.L.; Eyler, L.T.; Fennema-Notestine, C.; E Franz, C.; A Gillespie, N.; et al. 12-year prediction of mild cognitive impairment aided by Alzheimer’s brain signatures at mean age 56. Brain Commun. 2021, 3, fcab167. [Google Scholar] [CrossRef]
- Amini, M.; Pedram, M.M.; Moradi, A.; Jamshidi, M.; Ouchani, M. Single and Combined Neuroimaging Techniques for Alzheimer’s Disease Detection. Comput. Intell. Neurosci. 2021, 2021, 9523039. [Google Scholar] [CrossRef]
- Falcón, C.; Gascon, M.; Molinuevo, J.L.; Operto, G.; Cirach, M.; Gotsens, X.; Fauria, K.; Arenaza-Urquijo, E.M.; Pujol, J.; Sunyer, J.; et al. Brain correlates of urban environmental exposures in cognitively unimpaired individuals at increased risk for Alzheimer’s disease: A study on Barcelona’s population. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2021, 13, e12205. [Google Scholar] [CrossRef]
- Ingala, S.; De Boer, C.; A Masselink, L.; Vergari, I.; Lorenzini, L.; Blennow, K.; Chételat, G.; Di Perri, C.; Ewers, M.; van der Flier, W.M.; et al. Application of the ATN classification scheme in a population without dementia: Findings from the EPAD cohort. Alzheimer’s Dement. 2021, 17, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.E.; Péron, J. The basal ganglia and the cerebellum in human emotion. Soc. Cogn. Affect. Neurosci. 2020, 15, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Holtbernd, F.; Romanzetti, S.; Oertel, W.H.; Knake, S.; Sittig, E.; Heidbreder, A.; Maier, A.; Krahe, J.; Wojtala, J.; Dogan, I.; et al. Convergent patterns of structural brain changes in rapid eye movement sleep behavior disorder and Parkinson’s disease on behalf of the German rapid eye movement sleep behavior disorder study group. Sleep 2021, 44, zsaa199. [Google Scholar] [CrossRef] [PubMed]
- Devignes, Q.; Viard, R.; Betrouni, N.; Carey, G.; Kuchcinski, G.; Defebvre, L.; Leentjens, A.F.G.; Lopes, R.; Dujardin, K. Posterior Cortical Cognitive Deficits Are Associated with Structural Brain Alterations in Mild Cognitive Impairment in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 668559. [Google Scholar] [CrossRef]
- Crowley, S.J.; Banan, G.; Amin, M.; Tanner, J.J.; Hizel, L.; Nguyen, P.; Brumback, B.; Rodriguez, K.; McFarland, N.; Bowers, D.; et al. Statistically Defined Parkinson’s Disease Executive and Memory Cognitive Phenotypes: Demographic, Behavioral, and Structural Neuroimaging Comparisons. J. Park. Dis. 2021, 11, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Shahmaei, V.; Faeghi, F.; Mohammadbeigi, A.; Hashemi, H.; Ashrafi, F. Evaluation of iron deposition in brain basal ganglia of patients with Parkinson’s disease using quantitative susceptibility mapping. Eur. J. Radiol. Open 2019, 6, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Camarda, C.; Torelli, P.; Pipia, C.; Battaglini, I.; Azzarello, D.; Rosano, R.; Ventimiglia, C.C.; Sottile, G.; Cilluffo, G.; Camarda, R. Mild Parkinsonian Signs in a Hospital-based Cohort of Mild Cognitive Impairment Types: A Cross-sectional Study. Curr. Alzheimer Res. 2019, 16, 633–649. [Google Scholar] [CrossRef]
- Owens-Walton, C.; Jakabek, D.; Li, X.; Wilkes, F.A.; Walterfang, M.; Velakoulis, D.; van Westen, D.; Looi, J.; Hansson, O. Striatal changes in Parkinson disease: An investigation of morphology, functional connectivity and their relationship to clinical symptoms. Psychiatry Res. Neuroimaging 2018, 275, 5–13. [Google Scholar] [CrossRef]
- Levy, R.; Czernecki, V. Apathy and the basal ganglia. J. Neurol. 2006, 253, vii54–vii61. [Google Scholar] [CrossRef]
- Li, R.; Zou, T.; Wang, X.; Wang, H.; Hu, X.; Xie, F.; Meng, L.; Chen, H. Basal ganglia atrophy–associated causal structural network degeneration in Parkinson’s disease. Hum. Brain Mapp. 2021, 43, 1145–1156. [Google Scholar] [CrossRef]
- Van den Berg, K.R.E.; Helmich, R.C. The Role of the Cerebellum in Tremor—Evidence from Neuroimaging. Tremor Other Hyperkinetic Mov. 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Chipika, R.H.; Christidi, F.; Hengeveld, J.C.; Karavasilis, E.; Argyropoulos, G.D.; Lope, J.; Shing, S.L.H.; Velonakis, G.; Dupuis, L.; et al. Genotype-associated cerebellar profiles in ALS: Focal cerebellar pathology and cerebro-cerebellar connectivity alterations. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Chipika, R.H.; Shing, S.L.H.; Christidi, F.; Lope, J.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; McLaughlin, R.L.; Hardiman, O.; et al. Infratentorial pathology in frontotemporal dementia: Cerebellar grey and white matter alterations in FTD phenotypes. J. Neurol. 2021, 268, 4687–4697. [Google Scholar] [CrossRef] [PubMed]
- Nestor, P.G.; Nakamura, M.; Niznikiewicz, M.; Thompson, E.; Levitt, J.J.; Choate, V.; Shenton, M.E.; McCarley, R. In search of the functional neuroanatomy of sociality: MRI subdivisions of orbital frontal cortex and social cognition. Soc. Cogn. Affect. Neurosci. 2013, 8, 460–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarbo, K.; Verstynen, T.D. Converging Structural and Functional Connectivity of Orbitofrontal, Dorsolateral Prefrontal, and Posterior Parietal Cortex in the Human Striatum. J. Neurosci. 2015, 35, 3865–3878. [Google Scholar] [CrossRef] [Green Version]
- Nestor, P.G.; Forte, M.; Ohtani, T.; Levitt, J.J.; Newell, D.T.; Shenton, M.E.; Niznikiewicz, M.; McCarley, R.W. Faulty Executive Attention and Memory Interactions in Schizophrenia: Prefrontal Gray Matter Volume and Neuropsychological Impairment. Clin. EEG Neurosci. 2020, 51, 267–274. [Google Scholar] [CrossRef]
- Levitt, J.J.; Zhang, F.; Vangel, M.; Nestor, P.G.; Rathi, Y.; Kubicki, M.; E Shenton, M.; O’Donnell, L.J. The Organization of Frontostriatal Brain Wiring in Healthy Subjects Using a Novel Diffusion Imaging Fiber Cluster Analysis. Cereb. Cortex 2021, 31, 5308–5318. [Google Scholar] [CrossRef]
- Fariña, A.; Rojek-Giffin, M.; Gross, J.; De Dreu, C.K.W. Social preferences correlate with cortical thickness of the orbito-frontal cortex. Soc. Cogn. Affect. Neurosci. 2021, 16, 1191–1203. [Google Scholar] [CrossRef]
- Burks, J.D.; Conner, A.K.; Bonney, P.A.; Glenn, C.A.; Baker, C.M.; Boettcher, L.B.; Briggs, R.G.; O’Donoghue, D.L.; Wu, D.H.; Sughrue, M.E. Anatomy and white matter connections of the orbitofrontal gyrus. J. Neurosurg. 2018, 128, 1865–1872. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Torres-Solorio, A.K.; Kulesza, R.J.; Torres-Jardón, R.; González-González, L.O.; García-Arreola, B.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Hernández-Castillo, A.; Carlos-Hernández, E.; et al. Gait and balance disturbances are common in young urbanites and associated with cognitive impairment. Air pollution and the historical development of Alzheimer’s disease in the young. Environ. Res. 2020, 191, 110087. [Google Scholar] [CrossRef]
- Llano, D.A.; Kwok, S.S.; Devanarayan, V.; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Reported Hearing Loss in Alzheimer’s Disease Is Associated with Loss of Brainstem and Cerebellar Volume. Front. Hum. Neurosci. 2021, 15, 739754. [Google Scholar] [CrossRef] [PubMed]
- Droby, A.; El Mendili, M.M.; Giladi, N.; Hausdorff, J.M.; Maidan, I.; Mirelman, A. Gait and cognitive abnormalities are associated with regional cerebellar atrophy in elderly fallers—A pilot study. Gait Posture 2021, 90, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Booth, S.; Ko, J.H. Hypermetabolic Cerebellar Connectome in Alzheimer’s Disease. Brain Connect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Scamarcia, P.G.; Agosta, F.; Caso, F.; Filippi, M. Update on neuroimaging in non-Alzheimer’s disease dementia: A focus on the Lewy body disease spectrum. Curr. Opin. Neurol. 2021, 34, 532–538. [Google Scholar] [CrossRef]
- Sarasso, E.; Agosta, F.; Piramide, N.; Filippi, M. Progression of grey and white matter brain damage in Parkinson’s disease: A critical review of structural MRI literature. J. Neurol. 2021, 268, 3144–3179. [Google Scholar] [CrossRef]
- Stage, E.C.; Svaldi, D.; Phillips, M.; Canela, V.H.; Duran, T.; Goukasian, N.; Risacher, S.L.; Saykin, A.J.; Apostolova, L.G.; Alzheimer’s Disease Neuroimaging Initiative. Neurodegenerative changes in early- and late-onset cognitive impairment with and without brain amyloidosis. Alzheimer’s Res. Ther. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Perry, D.; A Brown, J.; Possin, K.L.; Datta, S.; Trujillo, A.; Radke, A.; Karydas, A.; Kornak, J.; Sias, A.C.; Rabinovici, G.D.; et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 2017, 140, 3329–3345. [Google Scholar] [CrossRef]
- Okada, T.; Tanaka, S.; Nakai, T.; Nishizawa, S.; Inui, T.; Sadato, N.; Yonekura, Y.; Konishi, J. Naming of animals and tools: A functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neurosci. Lett. 2000, 296, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.M.; Jiang, T.; Wang, W.M.; Li, T.D.; Liu, Y.; Lu, Y.C. Functional MRI mapping of category-specific sites associated with naming of famous faces, animals and man-made objects. Neurosci. Bull. 2011, 27, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Meyers, J.E.; Volkert, K.; Diep, A. Sentence Repetition Test: Updated Norms and Clinical Utility. Appl. Neuropsychol. 2000, 7, 154–159. [Google Scholar] [CrossRef]
- Small, J.A.; Kemper, S.; Lyons, K. Sentence repetition and processing resources in Alzheimer’s disease. Brain Lang. 2000, 75, 232–258. [Google Scholar] [CrossRef] [PubMed]
- Beales, A.; Whitworth, A.; Cartwright, J.; Panegyres, P.K.; Kane, R.T. Profiling sentence repetition deficits in primary progressive aphasia and Alzheimer’s disease: Error patterns and association with digit span. Brain Lang. 2019, 194, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Taler, V.; Phillips, N.A. Language performance in Alzheimer’s disease and mild cognitive impairment: A comparative review. J. Clin. Exp. Neuropsychol. 2008, 30, 501–556. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.D.; Hermann, B.; Mecollari, J.; Turkstra, L.S. Connected speech and language in mild cognitive impairment and Alzheimer’s disease: A review of picture description tasks. J. Clin. Exp. Neuropsychol. 2018, 40, 917–939. [Google Scholar] [CrossRef]
- Kim, H.; Yu, K.-H.; Lee, B.-C.; Kim, B.-C.; Kang, Y. Validity of the Montreal Cognitive Assessment (MoCA) Index Scores: A Comparison with the Cognitive Domain Scores of the Seoul Neuropsychological Screening Battery (SNSB). Dement. Neurocogn. Disord. 2021, 20, 28–37. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; D’Angiulli, A.; Kulesza, R.J.; Torres-Jardón, R.; Osnaya, N.; Romero, L.; Keefe, S.; Herritt, L.; Brooks, D.M.; Avila-Ramirez, J.; et al. Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int. J. Dev. Neurosci. 2011, 29, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; González-González, L.O.; Kulesza, R.J.; Fech, T.M.; Pérez-Guillé, G.; Luna, M.A.J.-B.; Soriano-Rosales, R.E.; Solorio, E.; Miramontes-Higuera, J.D.J.; Chew, A.G.-M.; et al. Exposures to fine particulate matter (PM2.5) and ozone above USA standards are associated with auditory brainstem dysmorphology and abnormal auditory brainstem evoked potentials in healthy young dogs. Environ. Res. 2017, 158, 324–332. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Kulesza, R.J.; Mansour, Y.; Aiello-Mora, M.; Mukherjee, P.S.; González-González, L.O. Increased Gain in the Auditory Pathway, Alzheimer’s Disease Continuum, and Air Pollution: Peripheral and Central Auditory System Dysfunction Evolves Across Pediatric and Adult Urbanites. J. Alzheimer’s Dis. 2019, 70, 1275–1286. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Rajkumar, R.; Stommel, E.; Kulesza, R.; Mansour, Y.; Rico-Villanueva, A.; Flores-Vázquez, J.; Brito-Aguilar, R.; Ramírez-Sánchez, S.; García-Alonso, G.; et al. Brainstem Quadruple Aberrant Hyperphosphorylated Tau, Beta-Amyloid, Alpha-Synuclein and TDP-43 Pathology, Stress and Sleep Behavior Disorders. Int. J. Environ. Res. Public Health 2021, 18, 6689. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Ávila-Ramírez, J.; Calderón-Garcidueñas, A.; González-Heredia, T.; Acuna-Ayala, H.; Chao, C.K.; Thompson, C.; Ruiz-Ramos, R.; Cortés-González, V.; Martinez-Martinez, L.; et al. Cerebrospinal Fluid Biomarkers in Highly Ex-posed PM 2∙5 Urbanites: The Risk of Alzheimer’s and Parkinson’s Diseases in Young Mexico City Residents. J. Alzheimer’s Dis. 2016, 54, 597–613. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Melo-Sánchez, G.; Rodriguez-Diaz, J.; Torres-Jardon, R.; Styner, M.; Mukherjee, P.S.; Lin, W.; Jewells, V. A critical Proton MR Spectroscopy marker of Alz-heimer’s disease early neurodegenerative change: Low hippocampal NAA/Cr ratio impacts APOE ɛ4 Mexico City children and their parents. J. Alzheimer’s Dis. 2015, 48, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Franco-Lira, M.; Zhu, H.; Lu, Z.; Solorio, E.; Torres-Jardón, R.; D’Angiulli, A. Decreases in short term memory, IQ and altered brain metabolic ratios in urban apolipoprotein ε4 children exposed to air pollution. APOE modulates children’s brain air pollution responses. J. Alzheimer’s Dis. 2015, 45, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Votinov, M.; Myznikov, A.; Zheltyakova, M.; Masharipov, R.; Korotkov, A.; Cherednichenko, D.; Habel, U.; Kireev, M. The Interaction between Caudate Nucleus and Regions Within the Theory of Mind Network as a Neural Basis for Social Intelligence. Front. Neural Circuits 2021, 15, 727960. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Guilford, J.P. Four Factor Tests of Social Intelligence (Behavioral Cognition): Manual of Instructions and Interpretations; Sheridan Psychological Services: Orange, CA, USA, 1976. [Google Scholar]
- Myznikov, A.; Zheltyakova, M.; Korotkov, A.; Kireev, M.; Masharipov, R.; Jagmurov, O.D.; Habel, U.; Votinov, M. Neuroanatomical Correlates of Social Intelligence Measured by the Guilford Test. Brain Topogr. 2021, 34, 337–347. [Google Scholar] [CrossRef]
- Khan, A.R.; Hiebert, N.M.; Vo, A.; Wang, B.T.; Owen, A.M.; Seergobin, K.N.; MacDonald, P.A. Biomarkers of Parkinson’s disease: Striatal sub-regional structural morphometry and diffusion MRI. NeuroImage Clin. 2019, 21, 101597. [Google Scholar] [CrossRef]
- Lam, J.A.; Murray, E.R.; Yu, K.E.; Ramsey, M.; Nguyen, T.T.; Mishra, J.; Martis, B.; Thomas, M.L.; Lee, E.E. Neurobiology of loneliness: A systematic review. Neuropsychopharmacology 2021, 46, 1873–1887. [Google Scholar] [CrossRef]
- Boyes, A.; McLoughlin, L.T.; Anderson, H.; Schwenn, P.; Shan, Z.; Gatt, J.M.; Lagopoulos, J.; Hermens, D.F. Basal ganglia correlates of wellbeing in early adolescence. Brain Res. 2022, 1774, 147710. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban air pollution: Influences on olfactory function and pathology in exposed children and young adults. Exp. Toxicol. Pathol. 2010, 62, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; Pérez-Guillé, B.; Mukherjee, P.S.; Gónzalez-Maciel, A. Combustion-derived nanoparticles, the neuroenteric system, cervical vagus, hyperphosphorylated alpha synuclein and tau in young Mexico City residents. Environ. Res. 2017, 159, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Sieurin, J.; Wirdefeldt, K.; Pedersen, N.; Almqvist, C.; Larsson, H.; Valdimarsdóttir, U.A.; Fang, F. Association of Stress-Related Disorders with Subsequent Neurodegenerative Diseases. JAMA Neurol. 2020, 77, 700–709. [Google Scholar] [CrossRef]
- Desmarais, P.; Weidman, D.; Wassef, A.; Bruneau, M.-A.; Friedland, J.; Bajsarowicz, P.; Thibodeau, M.-P.; Herrmann, N.; Nguyen, Q.D. The Interplay between Post-traumatic Stress Disorder and Dementia: A Systematic Review. Am. J. Geriatr. Psychiatry 2020, 28, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Neylan, T.C. Post-traumatic Stress Disorder and Neurodegeneration. Am. J. Geriatr. Psychiatry 2020, 28, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Günak, M.M.; Billings, J.; Carratu, E.; Marchant, N.L.; Favarato, G.; Orgeta, V. Post-traumatic stress disorder as a risk factor for dementia: Systematic review and meta-analysis. Br. J. Psychiatry 2020, 217, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, Y.-J.; Park, K.W.; Hwang, Y.S.; Lee, S.H.; Kim, B.J.; Chung, S.J. Association of NO2 and Other Air Pollution Exposures With the Risk of Parkinson Disease. JAMA Neurol. 2021, 78, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Stommel, E.W.; Rajkumar, R.P.; Mukherjee, P.S.; Ayala, A. Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters. Int. J. Environ. Res. Public Health 2021, 18, 11568. [Google Scholar] [CrossRef]
- Fleury, V.; Himsl, R.; Joost, S.; Nicastro, N.; Bereau, M.; Guessous, I.; Burkhard, P.R. Geospatial analysis of individual-based Parkinson’s disease data supports a link with air pollution: A case-control study. Park. Relat. Disord. 2021, 83, 41–48. [Google Scholar] [CrossRef]
- Wiesman, A.I.; Mundorf, V.M.; Casagrande, C.C.; Wolfson, S.L.; Johnson, C.M.; May, P.E.; Murman, D.L.; Wilson, T.W. Somatosensory dysfunction is masked by variable cognitive deficits across patients on the Alzheimer’s disease spectrum. eBioMedicine 2021, 73, 103638. [Google Scholar] [CrossRef]
- Bessi, V.; Giacomucci, G. Hidden functional derangement of somatosensory cortices in Alzheimer’s Disease. eBioMedicine 2021, 74, 103708. [Google Scholar] [CrossRef]
- Zhang, Z.; Robinson, L.; Jia, T.; Quinlan, E.B.; Tay, N.; Chu, C.; Barker, E.D.; Banaschewski, T.; Barker, G.J.; Bokde, A.L.; et al. Development of Disordered Eating Behaviors and Comorbid Depressive Symptoms in Adolescence: Neural and Psychopathological Predictors. Biol. Psychiatry 2020, 90, 853–862. [Google Scholar] [CrossRef]
- Jueajinda, S.; Stiramon, O.; Ekpanyaskul, C. Social Intelligence Counseling Intervention to Reduce Bullying Behaviors Among Thai Lower Secondary School Students: A Mixed-method Study. J. Prev. Med. Public Health 2021, 54, 340–351. [Google Scholar] [CrossRef]
- Falgàs, N.; Illán-Gala, I.; Allen, I.E.; Mumford, P.; Essanaa, Y.M.; Le, M.M.; You, M.; Grinberg, L.T.; Rosen, H.J.; Neylan, T.C.; et al. Specific cortical and subcortical grey matter regions are associated with insomnia severity. PLoS ONE 2021, 16, e0252076. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, A.; Abdulkadir, A.; Jack, C.R., Jr.; Thompson, P.M.; Harvey, D.J.; Truelove-Hill, M.; Sreepada, L.P.; Davatzikos, C.; Lipton, R.B.; Alzheimer Disease neuroimaging Initiative. Predictive value of ATN biomarker profiles in estimating disease progression in Alzheimer’s disease dementia. Alzheimer’s Dement. 2021, 17, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Wiste, H.J.; Therneau, T.M.; Weigand, S.D.; Knopman, D.S.; Mielke, M.M.; Lowe, V.J.; Vemuri, P.; Machulda, M.M.; Schwarz, C.G.; et al. Associations of Amyloid, Tau, and Neurodegeneration Biomarker Profiles with Rates of Memory Decline Among Individuals Without Dementia. JAMA 2019, 321, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Tosun, D.; Demir, Z.; Veitch, D.P.; Weintraub, D.; Aisen, P.; Jack, C.R., Jr.; Jagust, W.J.; Petersen, R.C.; Saykin, A.J.; Shaw, L.M.; et al. Contribution of Alzheimer’s biomarkers and risk factors to cognitive impairment and decline across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2021. [Google Scholar] [CrossRef]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; DeCarli, C.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagust, W.; Landau, S.M.; et al. Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimer’s Dement. 2021. [Google Scholar] [CrossRef]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R., Jr.; Vergallo, A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]






| Residency | MoCA Scores | Average Age Years | BMI | Education Years | Memory |
|---|---|---|---|---|---|
| MMC ≥ 31 years old n: 83 | 20.4 ± 3.4 | 46.4 ± 11.8 | 27.8 ± 3.9 | 13.2 ± 3.3 | 1.4 ± 1.4 |
| MRI MMC ≥ 31 years old n: 14 | 23.3 ± 2.8 | 42.7 ± 9.3 | 28.1 ± 4.3 | 16.0 ± 2.1 | 2.0 ± 1.4 |
| CONTROL ≥ 31 years old n: 13 | 25.2 ± 2.3 | 44.0 ± 7.2 | 26.9 ± 4.3 | 15.2 ± 2.8 | 3.3 ± 1.7 |
| MMC ≤ 30 years old n: 150 | 24.2 ± 2.6 | 21.6 ± 3.5 | 24.2 ± 3.2 | 13.6 ± 1.7 | 2.7 ± 1.4 |
| MRI MMC ≤ 30 years old n: 20 | 24.5 ± 2.6 | 22.0 ± 3.3 | 23.8 ± 3.7 | 14.5 ± 1.6 | 2.5 ± 1.2 |
| CONTROL ≤ 30 years old n: 22 | 24.7 ± 2.1 | 19.3 ± 1.3 | 21.9 ± 2.7 | 13.7 ± 0.6 | 3.1 ± 1.3 |
| ALL MRI MMC n: 34 | 23.9 ± 2.7 | 32.4 ± 6.3 | 25.9 ± 4.0 | 15.2 ± 1.8 | 2.25 ± 1.3 |
| MMC n: 233 | 22.3 ± 3 | 33.95 ± 7.65 | 26 ± 3.55 | 13.4 ± 2.5 | 2.05 ± 1.4 |
| CONTROL City n: 35 | 24.95 ± 2.2 | 31.65 ± 4.25 | 24.4 ± 3.5 | 14.45 ± 1.7 | 3.2 ± 1.5 |
| Anatomical Region | ≤30 Years Mean SD | ≥31 Years Mean SD | p Value Corrected FDR |
|---|---|---|---|
| VOLUME DATA | |||
| WHITE MATTER | 455,195 ± 40,045 | 447,456 ± 38,578 | 0.9285 |
| GRAY MATTER | 754,913 ± 46,840 | 715,865 ± 59,604 | <0.0001 |
| CSF | 244,804 ± 26,320 | 270,899 ± 33,336 | 0.000851 |
| LEFT CEREBELLUM WM | 13,223 ± 1465 | 12,083 ± 1139 | 0.0715 |
| RIGHT CEREBELLUM WM | 13,101 ± 1408 | 11,762 ± 921 | 0.014468 |
| LEFT CEREBELLUM CORTEX | 48,482 ± 4679 | 42,265 ± 3688 | 0.000114 |
| RIGHT CEREBELLUM CORTEX | 49,084 ± 4538 | 42,353 ± 3409 | <0.0001 |
| LEFT CAUDATE | 3529 ± 378 | 3216 ± 319 | 0.0153 |
| RIGHT CAUDATE | 3682 ± 382 | 3320 ± 328 | 0.006498 |
| LEFT PUTAMEN | 5115 ± 639 | 4610 ± 656 | 0.0993 |
| RIGHT PUTAMEN | 5004 ± 731 | 4561 ± 682 | 0.1785 |
| LEFT PALLIDUM | 1829 ± 368 | 1716 ± 222 | 0.6175 |
| RIGHT PALLIDUM | 1753 ± 323 | 1654 ± 269 | 0.5656 |
| LEFT HIPPOCAMPUS | 3986 ± 321 | 4011 ± 346 | 0.5777 |
| RIGHT HIPPOCAMPUS | 4162 ± 434 | 4166 ± 388 | 0.4395 |
| LEFT AMYGDALA | 1503 ± 163 | 1472 ± 155 | 0.8599 |
| RIGHT AMYGDALA | 1600 ± 190 | 1559 ± 168 | 0.8045 |
| LEFT ACCUMBENS AREA | 634 ± 105 | 555 ± 115 | 0.0377 |
| RIGHT ACCUMBENS AREA | 583 ± 99 | 517 ± 83 | 0.0758 |
| OPTIC CHIASM | 231 ± 33 | 256 ± 24 | 0.007 |
| CORPUS CALLOSUM POST | 982 ± 176 | 939 ± 126 | 0.6781 |
| CORPUS CALLOSUM MIDPOSTERIOR | 534 ± 110 | 468 ± 76 | 0.1625 |
| CORPUS CALLOSUM CENTRAL | 511 ± 103 | 441 ± 67 | 0.0981 |
| SURFACE AREA DATA | |||
| LEFT GYRUS FRONTAL INF OPERCULAR | 3269 ± 491 | 2777 ± 426 | 0.013 |
| RIGHT GYRUS FRONTAL INF OPERCULAR | 3261 ± 475 | 2813 ± 490 | 0.0289 |
| LEFT GYRUS FRONTAL INF ORBITAL | 1193 ± 168 | 1057 ± 155 | 0.0385 |
| LEFT GYRUS FRONTAL MIDDLE | 9961 ± 931 | 8659 ± 1110 | 0.004605 |
| LEFT GYRUS OCC TEMP MEDIAL LINGUAL | 4653 ± 591 | 4095 ± 621 | 0.0397 |
| LEFT GYRUS ORBITAL | 5985 ± 702 | 5451 ± 787 | 0.0440 |
| LEFT PARIETAL INF ANGULAR | 5627 ± 970 | 4912 ± 871 | 0.0313 |
| LEFT GYRUS TEMPORAL INFERIOR | 7085 ± 1306 | 6307 ± 1085 | 0.0352 |
| LEFT GYRUS TEMPORAL MIDDLE | 7214 ± 757 | 6411 ± 627 | 0.000855 |
| LEFT LAT FIS ANTERIOR HORIZONTAL | 424 ± 86 | 323 ± 93 | 0.0082 |
| LEFT LAT FIS ANTERIOR VERTICAL | 480 ± 84 | 379 ± 85 | 0.002906 |
| LEFT SULCUS ORBITAL LATERAL | 513 ± 122 | 397 ± 104 | 0.005822 |
| LEFT SULCUS ORBITAL H SHAPED | 2453 ± 335 | 2149 ± 374 | 0.013217 |
| RIGHT GYRI & SULCUS OCCIPITAL INFERIOR | 2494 ± 364 | 2147 ± 500 | 0.0425 |
| RIGHT GYRI & SULCUS CINGULATE ANTERIOR | 5211 ± 456 | 4706 ± 749 | 0.015274 |
| RIGHT G CUNNEUS | 2984 ± 405 | 2692 ± 281 | 0.0675 |
| RIGHT G FRONTAL INF OPERCULAR | 3261 ± 475 | 2813 ± 490 | 0.0289 |
| RIGHT G OCCIPITAL TEMP LAT FUSIFORM | 4761 ± 592 | 4208 ± 661 | 0.014617 |
| RIGHT G PRECENTRAL | 6477 ± 794 | 5707 ± 995 | 0.0316 |
| RIGHT G TEMP SUP GT TRANSVERSE | 778 ± 115 | 677 ± 105 | 0.0349 |
| RIGHT G TEMP SUPERIOR LATERAL | 4754 ± 527 | 4329 ± 459 | 0.0320 |
| RIGHT G TEMPORAL INFERIOR | 6768 ± 1074 | 5917 ± 964 | 0.0255 |
| RIGHT G TEMPORAL MIDDLE | 8176 ± 892 | 7175 ± 864 | 0.00021 |
| RIGHT LAT ANT FIS HORIZONTAL | 531 ± 173 | 425 ± 114 | 0.0488 |
| RIGHT SULCUS OCCIP MIDDLE LUNATUS | 1191 ± 288 | 999 ± 175 | 0.0588 |
| RIGHT SULCUS orbital MED OLFACTORY | 972 ± 125 | 899 ± 111 | 0.0291 |
| RIGHT SULCUS H SHAPED | 2375 ± 339 | 2099 ± 249 | 0.013 |
| RIGHT SULCUS TEMPORAL SUPERIOR | 8880 ± 1142 | 8038 ± 898 | 0.0498 |
| LEFT G PARIETAL ING ANGULAR | 5628 ± 971 | 4913 ± 871 | 0.0313 |
| LEFT G TEMPORAL MIDDLE | 7215 ± 758 | 6412 ± 628 | 0.0009 |
| CORTICAL THICKNESS DATA | |||
| LEFT G&S SUBCENTRAL | 2.69 ± 0.17 | 2.55 ± 0.14 | 0.02358 |
| LEFT G&S TRANV FRONTAL POL | 2.71 ± 0.17 | 2.55 ± 0.13 | 0.00318 |
| LEFT G&S CINGULAR ANTERIOR | 2.66 ± 0.15 | 2.49 ± 0.15 | 0.004167 |
| LEFT G&S CINGULATE MID ANTERIOR | 2.68 ± 0.19 | 2.47 ± 0.19 | 0.008505 |
| LEFT G&S CINGULATE MID POSTERIOR | 2.57 ± 0.17 | 2.33 ± 0.33 | <0.0001 |
| LEFT FRONTAL INF OPERCULAR | 2.77 ± 0.15 | 2.61 ± 0.15 | 0.008622 |
| LEFT G FRONTAL INF TRIANGULAR | 2.69 ± 0.17 | 2.54 ± 0.14 | 0.00665 |
| LEFT FRONTAL MIDDLE | 2.64 ± 0.14 | 2.53 ± 0.09 | 0.01592 |
| LEFT G FRONTAL SUPERIOR | 2.98 ± 0.14 | 2.81 ± 0.12 | 0.00036 |
| LEFT G OCCIPITAL TEMP LAT FUSIFORM | 2.91 ± 0.15 | 2.81 ± 0.14 | 0.01941 |
| LEFT G OCC TEMP MEDIAL LINGUAL | 2.06 ± 0.07 | 1.95 ± 0.08 | 0.00075 |
| LEFT G PRECUNNEUS | 2.43 ± 0.12 | 2.32 ± 0.17 | 0.0292 |
| LEFT LAT FIS ANTERIOR HORIZONTAL | 2.34 ± 0.28 | 2.09 ± 0.22 | 0.0257 |
| LEFT LAT FIS ANTERIOR VERTICAL | 2.30 ± 0.34 | 2.02 ± 0.21 | 0.01803 |
| LEFT S CALCARINE | 1.89 ± 0.12 | 1.80 ± 0.095 | 0.0544 |
| LEFT S CINGULAR INSULA SUPERIOR | 2.57 ± 0.12 | 2.43 ± 0.14 | 0.00451 |
| LEFT S FRONTAL SUPERIOR | 2.44 ± 0.16 | 2.32 ± 0.13 | 0.0068 |
| LEFT S PRECENTRAL SUPERIOR PAR | 2.42 ± 0.11 | 2.28 ± 0.21 | 0.01671 |
| LEFT G&S CINGULATE ANTERIOR | 2.58 ± 0.17 | 2.43 ± 0.10 | 0.0097 |
| LEFT G&S CINGULATE MID ANTERIOR | 2.66 ± 0.14 | 2.50 ± 0.13 | 0.0053 |
| LEFT G&S CINGULATE MID POSTERIOR | 2.58 ± 0.12 | 2.43 ± 0.13 | 0.0020 |
| LEFT G CINGULATE POSTCENTRAL | 2.87 ± 0.24 | 2.68 ± 0.15 | 0.01919 |
| LEFT G CUNNEUS | 1.85 ± 0.11 | 1.75 ± 0.07 | 0.01230 |
| LEFT G FRONTAL INF OPERCULAR | 2.78 ± 0.18 | 2.59 ± 0.21 | 0.0245 |
| LEFT G FRONTAL INF TRIANGULAR | 2.76 ± 0.17 | 2.60 ± 0.18 | 0.0147 |
| LEFT G FRONTAL MIDDLE | 2.65 ± 0.11 | 2.55 ± 0.11 | 0.0172 |
| LEFT G FRONTAL SUPERIOR | 2.95 ± 0.14 | 2.79 ± 0.1 | 0.0016 |
| LEFT OCCIPITAL MIDDLE | 2.63 ± 0.12 | 2.55 ± 0.08 | 0.0319 |
| LEFT SUP TEMP LATERAL FUSIFORM | 2.99 ± 0.14 | 2.86 ± 0.16 | 0.0137 |
| LEFT G PARIETAL INF SUPRAMARGINAL | 2.73 ± 0.11 | 2.64 ± 0.13 | 0.0386 |
| LEFT G PRECENTRAL | 2.96 ± 0.14 | 2.78 ± 0.28 | 0.0103 |
| LEFT G CUNNEUS | 2.43 ± 0.13 | 2.34 ± 0.15 | 0.0676 |
| LEFT CIRCULAR INSULAR SUPERIOR | 2.61 ± 0.10 | 2.47 ± 0.16 | 0.011 |
| LEFT S FRONTAL SUPERIOR | 2.42 ± 0.16 | 2.28 ± 0.14 | 0.016 |
| LEFT S PRECENTRAL SUPERIOR | 2.44 ± 0.17 | 2.30 ± 0.22 | 0.026 |
| MoCA total Score | |
| Right Caudate | 0.0065 |
| Left Caudate | 0.009 |
| Left gyrus orbital | 0.0014 |
| Orientation | |
| Right Gyrus temporal sup transverse | 0.0045 |
| EIS | |
| Right cerebellar white matter | 0.029 |
| Left sulcus orbital lateral | 0.0019 |
| Left gyrus frontal inferior triangular | 0.023 |
| LIS | |
| Left gyrus orbital | 0.026 |
| Right gyrus temporal superior transverse | 0.0071 |
| Right gyrus temporal inferior | 0.026 |
| Left gyrus and sulcus cingulate middle posterior | 0.0084 |
| Left gyrus and sulcus cingulate middle anterior | 0.021 |
| VIS | |
| Left gyrus orbital | 0.0184 |
| Left sulcus orbital lateral | 0.0030 |
| AIS | |
| Right gyrus temporal superior transverse | 0.0063 |
| SUMMARY SCORE | |
| Left gyrus orbital | 0.0009 |
| Left sulcus orbital lateral | 0.021 |
| Left gyrus and sulcus subcentral | 0.023 |
| Right gyrus temporal superior lateral | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Garcidueñas, L.; Hernández-Luna, J.; Mukherjee, P.S.; Styner, M.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Crespo-Cortés, C.N.; Stommel, E.W.; Torres-Jardón, R. Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution. Toxics 2022, 10, 156. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040156
Calderón-Garcidueñas L, Hernández-Luna J, Mukherjee PS, Styner M, Chávez-Franco DA, Luévano-Castro SC, Crespo-Cortés CN, Stommel EW, Torres-Jardón R. Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution. Toxics. 2022; 10(4):156. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040156
Chicago/Turabian StyleCalderón-Garcidueñas, Lilian, Jacqueline Hernández-Luna, Partha S. Mukherjee, Martin Styner, Diana A. Chávez-Franco, Samuel C. Luévano-Castro, Celia Nohemí Crespo-Cortés, Elijah W. Stommel, and Ricardo Torres-Jardón. 2022. "Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution" Toxics 10, no. 4: 156. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040156