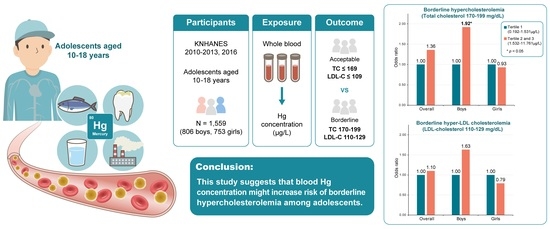
Association between Blood Mercury Concentration and Prevalence of Borderline Hypercholesterolemia among Adolescents: The Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2013 and 2016
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Laboratory Analysis
2.3. Ascertainment of Cases
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Distribution of Blood Hg Concentration and Differences in Baseline Characteristics
3.3. Baseline Characteristics According to Sex
3.4. Associations between Blood Hg Concentration and Prevalence of Borderline and Overt Hypercholesterolemia
3.5. Associations between Blood Hg Concentration and Prevalence of Borderline and Overt Hyper-LDL Cholesterolemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes Azevedo, B.; Barros Furieri, L.; Peçanha, F.M.; Wiggers, G.A.; Frizera Vassallo, P.; Ronacher Simões, M.; Fiorim, J.; Rossi de Batista, P.; Fioresi, M.; Rossoni, L.; et al. Toxic Effects of Mercury on the Cardiovascular and Central Nervous Systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Rissanen, T.H.; Voutilainen, S.; Tuomainen, T.-P. Mercury as a risk factor for cardiovascular diseases. J. Nutr. Biochem. 2007, 18, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.; Nieuwenhuijsen, M.J.; Elliott, P.; Jarup, L. Kidney Disease Mortality and Environmental Exposure to Mercury. Am. J. Epidemiol. 2007, 165, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, C.; Tremblay, P.-Y.; Ayotte, P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ. Res. 2017, 156, 747–760. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Voutilainen, S.; Rissanen, T.H.; Mursu, J.; Tuomainen, T.-P.; Korhonen, M.J.; Valkonen, V.-P.; Seppänen, K.; Laukkanen, J.A.; Salonen, J.T. Mercury, Fish Oils, and Risk of Acute Coronary Events and Cardiovascular Disease, Coronary Heart Disease, and All-Cause Mortality in Men in Eastern Finland. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs); WHO: Geneva, Switzerland, 2017. [Google Scholar]
- NCD Risk Factor Collaboration. National trends in total cholesterol obscure heterogeneous changes in HDL and non-HDL cholesterol and total-to-HDL cholesterol ratio: A pooled analysis of 458 population-based studies in Asian and Western countries. Int. J. Epidemiol. 2020, 49, 173–192. [Google Scholar] [CrossRef]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2005: Korea National Health and Nutrition Examination Survey (KNHANES III); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2012.
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2019: Korea National Health and Nutrition Examination Survey (KNHANES VIII-1); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2020.
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C.; McMahan, C.A.; Zieske, A.W.; Sloop, G.D.; Walcott, J.V.; Troxclair, D.A.; Malcom, G.T.; Tracy, R.E.; Oalmann, M.C.; Strong, J.P.; et al. Associations of Coronary Heart Disease Risk Factors With the Intermediate Lesion of Atherosclerosis in Youth. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1998–2004. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Zhang, C.; Bu, J. Relationship between Selected Serum Metallic Elements and Obesity in Children and Adolescent in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Fu, Z.; Shu, Y.; Zhang, J.; Lu, C.; Mo, X. Associations between total mercury and methyl mercury exposure and cardiovascular risk factors in US adolescents. Environ. Sci. Pollut. Res. 2018, 25, 6265–6272. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, X.; Liu, C.; Zhu, D.; Hua, J.; Hu, Y.; Peng, J.; Xu, D. Prehypertension and the Risk of Coronary Heart Disease in Asian and Western Populations: A Meta-analysis. J. Am. Heart Assoc. 2015, 4, e001519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Expert Panel on Integrated Guideline for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl 5), S213–S256. [Google Scholar] [CrossRef] [Green Version]
- Kweon, S.; Kim, Y.; Jang, M.-j.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-S.; Lee, B.-K. National estimates of blood lead, cadmium, and mercury levels in the Korean general adult population. Int. Arch. Occup. Environ. Health 2011, 84, 53–63. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, S.D.; Heumann, K.J.; Der Ananian, C.A.; Ainsworth, B.E. Validity and Reliability of the Global Physical Activity Questionnaire (GPAQ). Meas. Phys. Educ. Exerc. Sci. 2013, 17, 221–235. [Google Scholar] [CrossRef]
- Ministry of Education; Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention. The 16th Korea Youth Risk Behavior Web-Based Survey; Korea Centers for Disease Control and Prevention: Osong, Korea, 2020.
- Park, S. Associations of physical activity with sleep satisfaction, perceived stress, and problematic Internet use in Korean adolescents. BMC Public Health 2014, 14, 1143. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.N.K.; Scott, A.J. On Chi-Squared Tests for Multiway Contingency Tables with Cell Proportions Estimated from Survey Data. Ann. Stat. 1984, 12, 46–60. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 14.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Song, K.; Jeon, S.; Lee, H.S.; Choi, H.S.; Suh, J.; Kwon, A.; Kim, H.-S.; Chae, H.W. Trends of Dyslipidemia in Korean Youth According to Sex and Body Mass Index: Based on the Korea National Health and Nutrition Examination Survey (2007–2018). J. Pediatrics 2021, 237, 71–78.e5. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Cheng, H.; Yan, Y.; Zhao, X.; Chen, F.; Huang, G.; Hou, D.; Mi, J. 10-Year Trends in Serum Lipid Levels and Dyslipidemia Among Children and Adolescents From Several Schools in Beijing, China. J. Epidemiol. 2016, 26, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouda, K.; Iki, M.; Fujita, Y.; Nakamura, H.; Ohara, K.; Tachiki, T.; Nishiyama, T. Trends in Serum Lipid Levels of a 10- and 13-Year-Old Population in Fukuroi City, Japan (2007–2017). J. Epidemiol. 2020, 30, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.W.; Kim, S.-H.; Park, M.J. An association of blood mercury levels and hypercholesterolemia among Korean adolescents. Sci. Total Environ. 2020, 709, 135965. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Mackness, M.; Mackness, B. Paraoxonase 1 and atherosclerosis: Is the gene or the protein more important? Free Radic. Biol. Med. 2004, 37, 1317–1323. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of Paraoxonase 1 (PON1) Gene Polymorphisms and Functional Activity With Systemic Oxidative Stress and Cardiovascular Risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef] [Green Version]
- Mackness, M.I.; Mackness, B.; Durrington, P.N.; Fogelman, A.M.; Berliner, J.; Lusis, A.J.; Navab, M.; Shih, D.; Fonarow, G.C. Paraoxonase and coronary heart disease. Curr. Opin. Lipidol. 1998, 9, 319–324. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Popova, E.V.; Sinitskii, A.I.; Nemereshina, O.N.; Gatiatulina, E.R.; Nikonorov, A.A.; Skalny, A.V. Mercury and metabolic syndrome: A review of experimental and clinical observations. Biometals 2015, 28, 231–254. [Google Scholar] [CrossRef]
- Ayotte, P.; Carrier, A.; Ouellet, N.; Boiteau, V.; Abdous, B.; Sidi Elhadji Anassour, L.; Château-Degat, M.-L.; Dewailly, É. Relation between Methylmercury Exposure and Plasma Paraoxonase Activity in Inuit Adults from Nunavik. Environ. Health Perspect. 2011, 119, 1077–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic. Res. 2000, 33, S85–S97. [Google Scholar] [PubMed]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Bonomo, L.; Silva, M.; de Paula Oliveira, R.; Silva, M.E.; Pedrosa, M.L. Iron overload potentiates diet-induced hypercholesterolemia and reduces liver ppar-α expression in hamsters. J. Biochem. Mol. Toxicol. 2012, 26, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Hanao, N.; Nishiyama, K.; Kadota, Y.; Inoue, M.; Sato, M.; Suzuki, S. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 2012, 258, 32–42. [Google Scholar] [CrossRef]
- Olivieri, G.; Novakovic, M.; Savaskan, E.; Meier, F.; Baysang, G.; Brockhaus, M.; Müller-Spahn, F. The effects of β-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and β-amyloid secretion. Neuroscience 2002, 113, 849–855. [Google Scholar] [CrossRef]
- Benz, V.; Kintscher, U.; Foryst-Ludwig, A. Sex-Specific Differences in Type 2 Diabetes Mellitus and Dyslipidemia Therapy: PPAR Agonists. In Sex and Gender Differences in Pharmacology; Regitz-Zagrosek, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 387–410. [Google Scholar]
- Jalouli, M.; Carlsson, L.; Améen, C.; Lindén, D.; Ljungberg, A.; Michalik, L.; Edén, S.; Wahli, W.; Oscarsson, J. Sex Difference in Hepatic Peroxisome Proliferator-Activated Receptor α Expression: Influence of Pituitary and Gonadal Hormones. Endocrinology 2003, 144, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Yoon, M. Inhibition of the Actions of Peroxisome Proliferator-activated Receptor α on Obesity by Estrogen. Obesity 2007, 15, 1430–1440. [Google Scholar] [CrossRef]
- Siblerud, R.L. The relationship between mercury from dental amalgam and the cardiovascular system. Sci. Total Environ. 1990, 99, 23–35. [Google Scholar] [CrossRef]
- Björkman, L.; Lundekvam, B.F.; Lægreid, T.; Bertelsen, B.I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahter, M. Mercury in human brain, blood, muscle and toenails in relation to exposure: An autopsy study. Environ. Health 2007, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2012.
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2011: Korea National Health and Nutrition Examination Survey (KNHANES V-2); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2012.
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2012: Korea National Health and Nutrition Examination Survey (KNHANES V-3); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2013.
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANES VI-1); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2014.
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2016: Korea National Health and Nutrition Examination Survey (KNHANES VII-1); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2017.
- Sanders, A.P.; Mazzella, M.J.; Malin, A.J.; Hair, G.M.; Busgang, S.A.; Saland, J.M.; Curtin, P. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ. Int. 2019, 131, 104993. [Google Scholar] [CrossRef]
- Almerud, P.; Zamaratskaia, G.; Lindroos, A.K.; Bjermo, H.; Andersson, E.M.; Lundh, T.; Ankarberg, E.H.; Lignell, S. Cadmium, total mercury, and lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ. Res. 2021, 197, 110991. [Google Scholar] [CrossRef]
- Apel, P.; Angerer, J.; Wilhelm, M.; Kolossa-Gehring, M. New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2017, 220, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.-Y.; Li, M.-M.; Wang, J.; Yan, J.; Zhou, C.-C.; Yan, C.-H. Blood mercury concentration, fish consumption and anthropometry in Chinese children: A national study. Environ. Int. 2018, 110, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ilmiawati, C.; Yoshida, T.; Itoh, T.; Nakagi, Y.; Saijo, Y.; Sugioka, Y.; Sakamoto, M.; Ikegami, A.; Ogawa, M.; Kayama, F. Biomonitoring of mercury, cadmium, and lead exposure in Japanese children: A cross-sectional study. Environ. Health Prev. Med. 2015, 20, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy Scott, M.; Stone Neil, J.; Bailey Alison, L.; Beam, C.; Birtcher Kim, K.; Blumenthal Roger, S.; Braun Lynne, T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman Daniel, E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 1559) | TC | LDL-Cholesterol | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Acceptable (n = 1034) | Borderline High (n = 395) | High (n = 130) | pa | Acceptable (n = 1242) | Borderline High (n = 224) | High (n = 93) | pa | ||
| Age, mean ± SE, years | 14.2 ± 0.1 | 14.3 ± 0.1 | 13.8 ± 0.1 | 13.6 ± 0.3 | <0.0001 | 14.2 ± 0.1 | 13.8 ± 0.2 | 14.1 ± 0.3 | 0.031 |
| 10–12 years, n (%) | 524 (33.6) | 316 (30.6) | 152 (38.5) | 56 (43.1) | 0.003 | 402 (32.4) | 87 (38.8) | 35 (37.6) | 0.208 |
| 13–15 years, n (%) | 568 (36.4) | 399 (38.6) | 134 (33.9) | 35 (26.9) | 467 (37.6) | 71 (31.7) | 30 (32.3) | ||
| 16–18 years, n (%) | 467 (30.0) | 319 (30.9) | 109 (27.6) | 39 (30.0) | 373 (30.0) | 66 (29.5) | 28 (30.1) | ||
| Sex | |||||||||
| Boys, n (%) | 806 (51.7) | 582 (56.3) | 171 (43.3) | 53 (40.8) | <0.0001 | 658 (53.0) | 109 (48.7) | 39 (41.9) | 0.006 |
| Girls, n (%) | 753 (48.3) | 452 (43.7) | 224 (56.7) | 77 (59.2) | 584 (47.0) | 115 (51.3) | 54 (58.1) | ||
| BMI, mean ± SE, kg/m2 | 20.9 ± 0.1 | 20.7 ± 0.1 | 21.0 ± 0.2 | 21.7 ± 0.5 | 0.005 | 20.6 ± 0.1 | 21.6 ± 0.3 | 22.2 ± 0.6 | <0.0001 |
| Normal weight, n (%) | 1229 (78.8) | 843 (81.5) | 297 (75.2) | 89 (68.5) | 0.005 | 1009 (81.2) | 161 (71.9) | 59 (63.4) | <0.0001 |
| Overweight and obesity, n (%) | 330 (21.2) | 191 (18.5) | 98 (24.8) | 41 (31.5) | 233 (18.8) | 63 (28.1) | 34 (36.6) | ||
| Smoking status | |||||||||
| Never smokers, n (%) | 1388 (89.0) | 910 (88.0) | 355 (89.9) | 123 (94.6) | 0.006 | 1098 (88.4) | 204 (91.1) | 86 (92.5) | 0.138 |
| Smokers, n (%) | 171 (11.0) | 124 (12.0) | 40 (10.1) | 7 (5.4) | 144 (11.6) | 20 (8.9) | 7 (7.5) | ||
| Household income level | |||||||||
| Quintile 1, n (%) | 216 (13.9) | 138 (13.3) | 62 (15.7) | 16 (12.3) | 0.362 | 169 (13.6) | 38 (17.0) | 9 (9.7) | 0.043 |
| Quintile 2, n (%) | 322 (20.7) | 212 (20.5) | 83 (21.0) | 27 (20.8) | 259 (20.9) | 46 (20.5) | 17 (18.3) | ||
| Quintile 3, n (%) | 343 (22.0) | 223 (21.6) | 94 (23.8) | 26 (20.0) | 267 (21.5) | 58 (25.9) | 18 (19.4) | ||
| Quintile 4, n (%) | 322 (20.7) | 223 (21.6) | 75 (19.0) | 24 (18.5) | 265 (21.3) | 39 (17.4) | 18 (19.4) | ||
| Quintile 5, n (%) | 356 (22.8) | 238 (23.0) | 81 (20.5) | 37 (28.5) | 282 (22.7) | 43 (19.2) | 31 (33.3) | ||
| Physical activity | |||||||||
| Inactive, n (%) | 703 (45.1) | 421 (40.7) | 219 (55.4) | 63 (48.5) | 0.001 | 547 (44.0) | 113 (50.4) | 43 (46.2) | 0.180 |
| Minimally active, n (%) | 484 (31.0) | 353 (34.1) | 97 (24.6) | 34 (26.2) | 405 (32.6) | 53 (23.7) | 26 (28.0) | ||
| Active, n (%) | 372 (23.9) | 260 (25.1) | 79 (20.0) | 33 (25.4) | 290 (23.3) | 58 (25.9) | 24 (25.8) | ||
| Menstruation (for girls) | |||||||||
| Premenarcheal, n (%) | 169 (22.4) | 90 (19.9) | 62 (27.7) | 17 (22.1) | 0.012 | 129 (22.1) | 27 (23.5) | 13 (24.1) | 0.540 |
| Postmenarcheal, n (%) | 584 (77.6) | 362 (80.1) | 162 (72.3) | 60 (77.9) | 455 (77.9) | 88 (76.5) | 41 (75.9) | ||
| Total energy intake, mean ± SE, kcal/day | 2188.9 ± 26.4 | 2267.5 ± 31.8 | 2036.3 ± 47.7 | 2016.1 ± 95.5 | <0.0001 | 2237.1 ± 29.2 | 2006.6 ± 60.7 | 2032.2 ± 118.9 | <0.0001 |
| Survey year | |||||||||
| 2010, n (%) | 323 (20.7) | 208 (20.1) | 90 (22.8) | 25 (19.2) | 0.354 | 251 (20.2) | 54 (24.1) | 18 (19.4) | 0.805 |
| 2011, n (%) | 334 (21.4) | 239 (23.1) | 70 (17.7) | 25 (19.2) | 273 (22.0) | 42 (18.8) | 19 (20.4) | ||
| 2012, n (%) | 318 (20.4) | 217 (21.0) | 71 (18.0) | 30 (23.1) | 259 (20.9) | 38 (17.0) | 21 (22.6) | ||
| 2013, n (%) | 318 (20.4) | 208 (20.1) | 86 (21.8) | 24 (18.5) | 253 (20.4) | 50 (22.3) | 15 (16.1) | ||
| 2016, n (%) | 266 (17.1) | 162 (15.7) | 78 (19.7) | 26 (20.0) | 206 (16.6) | 40 (17.9) | 20 (21.5) | ||
| Blood Hg concentration, mean ± SE, µg/L | 2.1 ± 0 | 2.1 ± 0 | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.005 | 2.1 ± 0 | 2.2 ± 0.1 | 2.5 ± 0.2 | 0.002 |
| n (%) | GM (95% CI) | Min | 10% | 25th | Median | 75th | 90% | Max | |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 1559 | 1.88 (1.82, 1.94) | 0.19 | 1.05 | 1.39 | 1.84 | 2.54 | 3.36 | 11.76 |
| Boys | 806 (51.7) | 1.91 (1.83, 2.00) | 0.19 | 1.03 | 1.40 | 1.87 | 2.59 | 3.48 | 11.76 |
| Girls | 753 (48.3) | 1.84 (1.77, 1.91) | 0.42 | 1.08 | 1.37 | 1.77 | 2.49 | 3.24 | 8.82 |
| Boys (n = 806) | Girls (n = 753) | p a | |
|---|---|---|---|
| Age, mean ± SE, years | 14.2 ± 0.1 | 14.1 ± 0.1 | 0.481 |
| 10–12 years, n (%) | 277 (34.4) | 247 (32.8) | 0.896 |
| 13–15 years, n (%) | 294 (36.5) | 274 (36.4) | |
| 16–18 years, n (%) | 235 (29.2) | 232 (30.8) | |
| BMI, mean ± SE, kg/m2 | 21.1 ± 0.2 | 20.6 ± 0.2 | 0.003 |
| Normal weight, n (%) | 629 (78.0) | 600 (79.7) | 0.677 |
| Overweight and obesity, n (%) | 177 (22.0) | 153 (20.3) | |
| Smoking status | |||
| Never smokers, n (%) | 691 (85.7) | 697 (92.6) | 0.001 |
| Smokers, n (%) | 115 (14.3) | 56 (7.4) | |
| Household income level | |||
| Quintile 1, n (%) | 109 (13.5) | 107 (14.2) | 0.484 |
| Quintile 2, n (%) | 160 (19.9) | 162 (21.5) | |
| Quintile 3, n (%) | 173 (21.5) | 170 (22.6) | |
| Quintile 4, n (%) | 179 (22.2) | 143 (19.0) | |
| Quintile 5, n (%) | 185 (23.0) | 171 (22.7) | |
| Physical activity | |||
| Inactive, n (%) | 310 (38.5) | 393 (52.2) | <0.0001 |
| Minimally active, n (%) | 229 (28.4) | 255 (33.9) | |
| Active, n (%) | 267 (33.1) | 105 (13.9) | |
| Menstruation (for girls) | |||
| Premenarcheal, n (%) | - | 169 (22.4) | - |
| Postmenarcheal, n (%) | - | 584 (77.6) | |
| Total energy intake, mean ± SE, kcal/day | 2435.3 ± 39.6 | 1911.1 ± 30.5 | <0.0001 |
| Survey year | |||
| 2010, n (%) | 164 (20.3) | 159 (21.1) | 0.895 |
| 2011, n (%) | 172 (21.3) | 162 (21.5) | |
| 2012, n (%) | 164 (20.3) | 154 (20.5) | |
| 2013, n (%) | 160 (19.9) | 158 (21.0) | |
| 2016, n (%) | 146 (18.1) | 120 (15.9) | |
| Blood Hg concentration, mean ± SE, µg/L | 2.2 ± 0.1 | 2.0 ± 0 | 0.110 |
| TC, mean ± SE, mg/dL | 154.4 ± 1.2 | 166.0 ± 1.3 | <0.0001 |
| Acceptable, n (%) | 582 (72.2) | 452 (60.0) | <0.0001 |
| Borderline high, n (%) | 171 (21.2) | 224 (29.7) | |
| High, n (%) | 53 (6.6) | 77 (10.2) | |
| LDL-cholesterol, mean ± SE, mg/dL | 88.5 ± 1.0 | 95.6 ± 1.2 | <0.0001 |
| Acceptable, n (%) | 658 (81.6) | 584 (77.6) | 0.006 |
| Borderline high, n (%) | 109 (13.5) | 115 (15.3) | |
| High, n (%) | 39 (4.8) | 54 (7.2) | |
| TG, mean ± SE, mg/dL | 81.6 ± 2.0 | 86.3 ± 2.2 | 0.013 |
| Acceptable, n (%) | 546 (67.7) | 479 (63.6) | 0.734 |
| Borderline high, n (%) | 169 (21.0) | 178 (23.6) | |
| High, n (%) | 91 (11.3) | 96 (12.7) | |
| HDL-cholesterol, mean ± SE, mg/dL | 49.5 ± 0.4 | 53.1 ± 0.5 | <0.0001 |
| Acceptable, n (%) | 550 (68.2) | 583 (77.4) | 0.002 |
| Borderline low, n (%) | 136 (16.9) | 104 (13.8) | |
| Low, n (%) | 120 (14.9) | 66 (8.8) |
| Concentration Range (µg/L) | Overall | Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case/Total | OR (95% CI) a | OR (95% CI) b | Case/Total | OR (95% CI) a | OR (95% CI) b | Case/Total | OR (95% CI) a | OR (95% CI) b | ||
| Borderline hypercholesterolemia | ||||||||||
| Continuous | 395/1429 | 1.36 (1.02, 1.80) | 1.48 (1.10, 2.00) | 171/753 | 1.50 (1.03, 2.18) | 1.63 (1.10, 2.41) | 224/676 | 1.31 (0.85, 2.00) | 1.23 (0.77, 1.96) | |
| Tertile 1 | 0.192–1.531 | 120/482 | 1.00 (Reference) | 1.00 (Reference) | 42/240 | 1.00 (Reference) | 1.00 (Reference) | 78/242 | 1.00 (Reference) | 1.00 (Reference) |
| Tertiles 2 and 3 | 1.532–11.761 | 275/947 | 1.27 (0.94, 1.72) | 1.36 (0.99, 1.86) | 129/513 | 1.70 (1.07, 2.71) | 1.92 (1.19, 3.10) | 146/434 | 1.04 (0.69, 1.58) | 0.93 (0.59, 1.46) |
| Borderline hyper-LDL cholesterolemia | ||||||||||
| Continuous | 224/1466 | 1.28 (0.90, 1.83) | 1.19 (0.81, 1.75) | 109/767 | 1.33 (0.86, 2.05) | 1.21 (0.74, 1.97) | 115/699 | 1.28 (0.74, 2.22) | 1.15 (0.62, 2.10) | |
| Tertile 1 | 0.192–1.531 | 70/495 | 1.00 (Reference) | 1.00 (Reference) | 26/245 | 1.00 (Reference) | 1.00 (Reference) | 44/250 | 1.00 (Reference) | 1.00 (Reference) |
| Tertiles 2 and 3 | 1.532–11.761 | 154/971 | 1.19 (0.79, 1.80) | 1.10 (0.72, 1.69) | 83/522 | 1.69 (0.95, 3.00) | 1.63 (0.88, 3.04) | 71/449 | 0.93 (0.55, 1.55) | 0.79 (0.45, 1.38) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.; Park, E.Y.; Kim, B.; Oh, J.-K. Association between Blood Mercury Concentration and Prevalence of Borderline Hypercholesterolemia among Adolescents: The Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2013 and 2016. Toxics 2021, 9, 242. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9100242
Jin T, Park EY, Kim B, Oh J-K. Association between Blood Mercury Concentration and Prevalence of Borderline Hypercholesterolemia among Adolescents: The Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2013 and 2016. Toxics. 2021; 9(10):242. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9100242
Chicago/Turabian StyleJin, Taiyue, Eun Young Park, Byungmi Kim, and Jin-Kyoung Oh. 2021. "Association between Blood Mercury Concentration and Prevalence of Borderline Hypercholesterolemia among Adolescents: The Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2013 and 2016" Toxics 9, no. 10: 242. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9100242