Recognition and Management of Antipsychotic-Induced Parkinsonism in Older Adults: A Narrative Review
Abstract
:1. Introduction
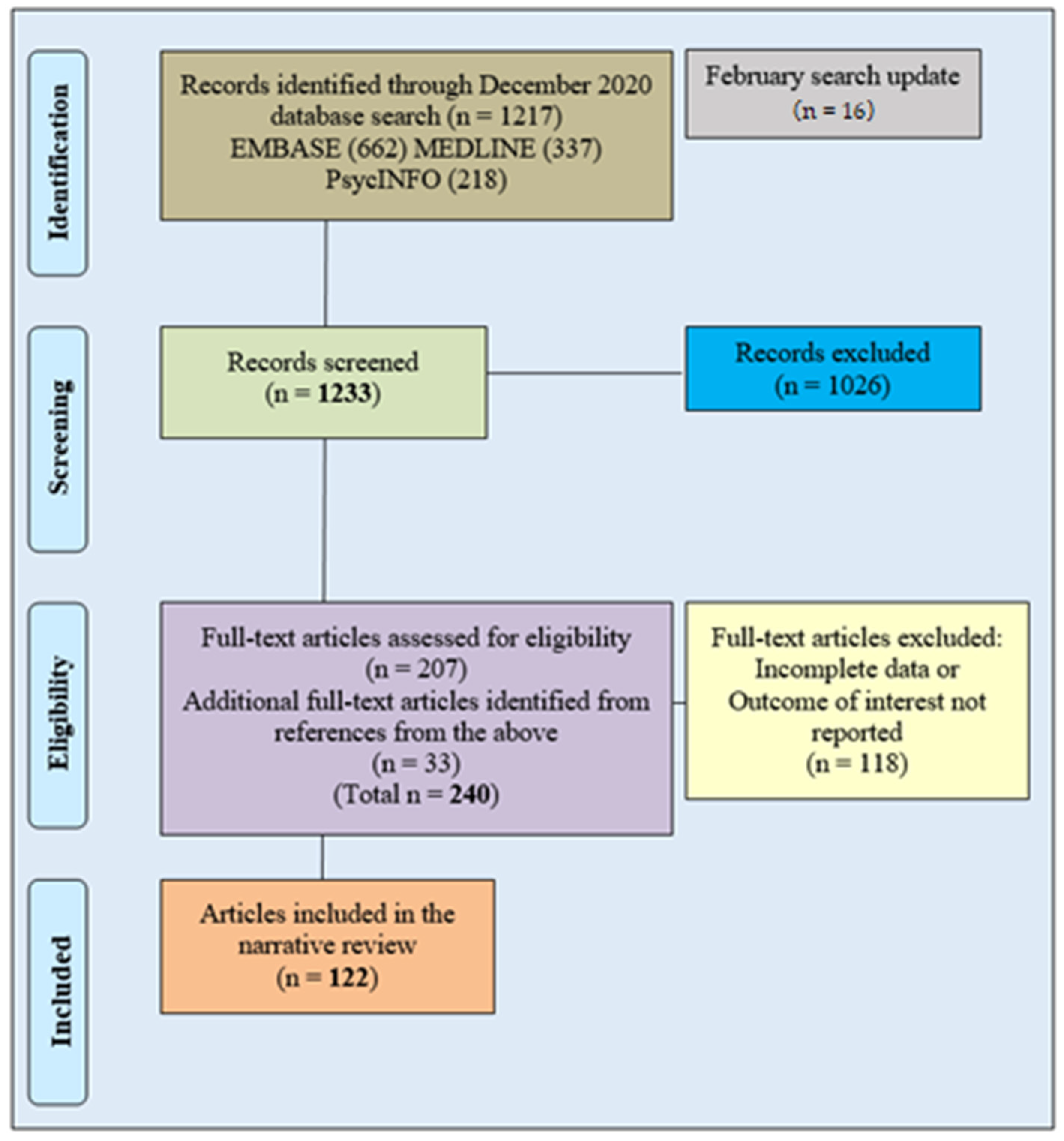
2. Methods
Database Search Methodology
3. Results with Discussion
3.1. Epidemiology and Risk Factors
3.1.1. Drugs
3.1.2. Risk Factors
3.2. Pathophysiology
3.3. Clinical Characteristics
3.3.1. Motor Signs
3.3.2. Non-Motor Signs
3.4. Investigations
3.4.1. Dopamine Transporter Scanning
3.4.2. Cardiac 123I-MIBG (Iodine-123-Meta-Iodobenzylguanidine) Scintigraphy
3.4.3. Substantia Nigra Ultrasonography
3.4.4. Others
4. Management of AIP
4.1. Reduce the Antipsychotic Dose
4.2. Stop the Antipsychotic
4.3. Switch Antipsychotic
4.4. Add Anticholinergic
4.5. Add Amantadine
4.6. Add L-Dopa or Dopamine Agonist
4.7. Electroconvulsive Therapy
4.8. Physiotherapy
5. Prognosis of AIP
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsden, C.D.; Jenner, P. The pathophysiology of extrapyramidal side-effects of neuroleptic drugs. Psychol. Med. 1980, 10, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.L.; Friedman, J.H. Drug-induced parkinsonism in the elderly. Future Neurol. 2007, 2, 307–316. [Google Scholar] [CrossRef]
- Druschky, K.; Bleich, S.; Grohmann, R.; Engel, R.; Toto, S.; Neyazi, A.; Däubl, B.; Stübner, S. Severe parkinsonism under treatment with antipsychotic drugs. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 270, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Youn, J.; Shin, H.; Cho, J.W. Nonmotor symptoms in drug-induced parkinsonism and drug-naive Parkinson disease. Can. J. Neurol. Sci. 2013, 40, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Caligiuri, M.P.; Lacro, J.P.; Jeste, D.V. Incidence and Predictors of Drug-Induced Parkinsonism in Older Psychiatric Patients Treated With Very Low Doses of Neuroleptics. J. Clin. Psychopharmacol. 1999, 19, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.F.; Pawlowski, S.M.; Kesari, A.; Maina, I.; Pantelyat, A.; Duda, J.E. Motor and non-motor features of Parkinson’s disease that predict persistent drug-induced Parkinsonism. Park. Relat. Disord. 2014, 20, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sendon, J.L.; Mena, M.A.; de Yebenes, J.G. Drug-Induced Parkinsonism in the Elderly; Incidence, Management and Prevention. Drugs Aging 2012, 29, 105–118. [Google Scholar] [CrossRef]
- Estevez-Fraga, C.; Zeun, P.; Moreno, J.L.L.-S. Current Methods for the Treatment and Prevention of Drug-Induced Parkinsonism and Tardive Dyskinesia in the Elderly. Drugs Aging 2018, 35, 959–971. [Google Scholar] [CrossRef]
- Savica, R.; Grossardt, B.; Bower, J.H.; Ahlskog, J.E.; Mielke, M.M.; Rocca, W.A. Incidence and time trends of drug-induced parkinsonism: A 30-year population-based study. Mov. Disord. 2017, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-H.; Cho, H.; Kim, Y.J.; Kim, J.-S.; Baik, J.S.; Jang, S.; Ma, H.-I. Trends in the Prevalence of Drug-Induced Parkinsonism in Korea. Yonsei Med. J. 2019, 60, 760–767. [Google Scholar] [CrossRef]
- Hoffman, W.F.; Sharon, M.L.; Daniel, E.C. Neuroleptic-Induced Parkinsonism in Older Schizophrenics. Biol. Psychiatry 1987, 22, 427–439. [Google Scholar] [CrossRef]
- Rajput, A.H.; Offord, K.P.; Beard, C.M.; Kurland, L.T. Epidemiology of parkinsonism: Incidence, classification, and mortality. Ann. Neurol. 1984, 16, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Morgante, L.; Rosa, A.E.; Savettieri, G.; Reggio, A.; Patti, F.; Salemi, G.; Lorenzo, G.; Epifanio, A.; Perri, R. Drug-induced parkinsonism: Prevalence, clinical features and follow-up study in three Sicilian communities. J. Neurol. 1996, 243, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Rocca, W.A. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology 1999, 52, 1214–1220. [Google Scholar] [CrossRef]
- Baldereschi, M.; Di Carlo, A.; Rocca, W.A.; Vanni, P.; Maggi, S.; Perissinotto, E.; Grigoletto, F.; Amaducci, L.; Inzitari, D. Parkinson’s disease and parkinsonism in a longitudinal study. Neurology 2000, 55, 1358–1363. [Google Scholar] [CrossRef]
- Rocca, W.; Bower, J.; McDonnell, S.; Peterson, B.; Maraganore, D. Time trends in the incidence of parkinsonism in Olmsted County, Minnesota. Neurology 2001, 57, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Benito-León, J.; Bermejo-Pareja, F.; Morales-González, J.M.; Porta-Etessam, J.; Trincado, M.; Vega, S.; Louis, E.D. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 2004, 62, 734–741. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.; Giesbergen, P.; De Rijk, M.; Hofman, A.; Koudstaal, P.; Breteler, M. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology 2004, 63, 1240–1244. [Google Scholar] [CrossRef]
- Barbosa, M.T.; Caramelli, P.; Maia, D.P.; Cunningham, M.C.; Guerra, H.L.; Lima-Costa, M.F.; Cardoso, F. Parkinsonism and Parkinson’s disease in the elderly: A community-based survey in Brazil (the Bambui study). Mov. Disord. 2006, 21, 800–808. [Google Scholar] [CrossRef]
- Munhoz, R.P.; Werneck, L.C.; Teive, H.A. The differential diagnoses of parkinsonism: Findings from a cohort of 1528 patients and a 10 years comparison in tertiary movement disorders clinics. Clin. Neurol. Neurosurg. 2010, 112, 431–435. [Google Scholar] [CrossRef]
- Seijo-Martinez, M.; del Rio, M.C.; Alvarez, J.R.; Prado, R.S.; Salgado, E.T.; Esquete, J.P.; Sobrido, M.J. Prevalence of parkinsonism and Parkinson’s disease in the Arosa Island (Spain): A community-based door-to-door survey. J. Neurol. Sci. 2011, 304, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Incidence and Pathology of Synucleinopathies and Tauopathies Related to Parkinsonism. JAMA Neurol. 2013, 70, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savica, R.; Grossardt, B.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016, 73, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Vale, T.C.; Barbosa, M.T.; Resende, E.D.P.F.; Maia, D.P.; Cunningham, M.C.Q.; Guimarães, H.C.; Machado, J.C.B.; Teixeira, A.L.; Cardoso, F.; Caramelli, P. Parkinsonism in a population-based study of individuals aged 75+ years: The Pietà study. Park. Relat. Disord. 2018, 56, 76–81. [Google Scholar] [CrossRef]
- Fleury, V.; Brindel, P.; Nicastro, N.; Burkhard, P.R. Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland. Park. Relat. Disord. 2018, 54, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bondon-Guitton, E.; Perez-Lloret, S.; Bagheri, H.; Brefel, C.; Rascol, O.; Montastruc, J.-L. Drug-induced parkinsonism: A review of 17 years’ experience in a regional pharmacovigilance center in France. Mov. Disord. 2011, 26, 2226–2231. [Google Scholar] [CrossRef]
- De Germay, S.; Montastruc, F.; Carvajal, A.; Lapeyre-Mestre, M.; Montastruc, J.L. Drug-induced parkinsonism: Revisiting the epidemiology using the WHO pharmacovigilance database. Parkinsonism Relat. Disord. 2020, 70, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Kim, S.; Kim, H.; Shin, H.W.; Na, K.S.; Suh, H.S. Prevalence and incidence of Parkinson’s disease and drug-induced parkinsonism in Korea. BMC Public Health 2019, 19, 1328. [Google Scholar] [CrossRef]
- Khedr, E.M.; Fawi, G.; Abbas MA, A.; Mohammed, T.A.; El-Fetoh, N.A.; Attar, G.A.; Zaki, A.F. Prevalence of Parkinsonism and Parkinson’s disease in Qena governorate/Egypt: A cross-sectional community-based survey. Neurol. Res. 2015, 37, 607–618. [Google Scholar] [CrossRef]
- Benito-León, J.; Bermejo-Pareja, F.; Rodríguez, J.; Molina, J.-A.; Gabriel, R.; Morales, J.-M.; For the Neurological Disorders in Central Spain (NEDICES) Study Group. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov. Disord. 2002, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-Y.; Tzeng, I.-S.; Lin, M.-C.; Yeh, Y.-H.; Hsu, C.Y.; Kung, W.-M. Risks of Sulpiride-Induced Parkinsonism in Peptic Ulcer and Gastroesophageal Reflux Disease Patients in Taiwan: A Nationwide Population-Based Study. Front. Pharmacol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.P.; Rockwell, E.; Jeste, D.V. Extrapyramidal Side Effects in Patients with Alzheimer’s Disease Treated with Low-Dose Neuroleptic Medication. Am. J. Geriatr. Psychiatry 1998, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Writers, A.M. Minimize exposure to antidopaminergic drugs whenever possible to reduce the risk of drug-induced parkinsonism and tardive dyskinesia. Drugs Ther. Perspect. 2019, 35, 326–331. [Google Scholar] [CrossRef]
- Friedman, J.H.; Fernandez, H.H.; Trieschmann, M.M. Parkinsonism in a Nursing Home: Underrecognition. J. Geriatr. Psychiatry Neurol. 2004, 17, 39–41. [Google Scholar] [CrossRef]
- Tse, W.; Libow, L.S.; Neufeld, R.; Lesser, G.; Frank, J.; Dolan, S.; Tarshish, C.; Gracies, J.-M.; Olanow, C.W.; Koller, W.C.; et al. Prevalence of movement disorders in an elderly nursing home population. Arch. Gerontol. Geriatr. 2008, 46, 359–366. [Google Scholar] [CrossRef]
- Moghal, S.; Rajput, A.; Meleth, R.; D’Arcy, C.; Rajput, R. Prevalence of Movement Disorders in Institutionalized Elderly. Neuroepidemiology 1995, 14, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Rochon, P.A.; Stukel, T.A.; Sykora, K.; Gill, S.S.; Garfinkel, S.; Anderson, G.M.; Normand, S.-L.T.; Mamdani, M.M.; Lee, P.E.; Li, P.; et al. Atypical Antipsychotics and Parkinsonism. Arch. Intern. Med. 2005, 165, 1882–1888. [Google Scholar] [CrossRef] [Green Version]
- Munhoz, R.P.; Filho, D.B.; Teive, H.A.G. Not all drug-induced parkinsonism are the same: The effect of drug class on motor phenotype. Neurol. Sci. 2017, 38, 319–324. [Google Scholar] [CrossRef]
- Shiraiwa, N.; Tamaoka, A.; Ohkoshi, N. Clinical Features of Drug-induced Parkinsonism. Neurol. Int. 2018, 10, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.-S.; Kwon, Y.; Kim, J.-S.; Park, M.-H.; Berg, D. Transcranial sonographic findings may predict prognosis of gastroprokinetic drug-induced parkinsonism. Park. Relat. Disord. 2018, 46, 36–40. [Google Scholar] [CrossRef]
- Kim, S.; Cheon, S.-M.; Suh, H.S. Association Between Drug Exposure and Occurrence of Parkinsonism in Korea: A Population-Based Case-Control Study. Ann. Pharmacother. 2019, 53, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Saltz, B.L.; Woerner, M.G.; Kane, J.M.; Lieberman, J.A.; Alvir, J.M.; Bergmann, K.J.; Blank, K.; Koblenzer, J.; Kahaner, K. Prospective study of tardive dyskinesia incidence in the elderly. JAMA Neurol. 1991, 266, 2402–2406. [Google Scholar]
- McManus, D.Q.; Arvanitis, L.A.; Kowalcyk, B.B. Quetiapine, a novel antipsychotic: Experience in elderly patients with psychotic disorders. J. Clin. Psychiatry 1999, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, G.; Beasley, J.C.; Tamura, R. Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am. J. Psychiatry 1997, 154, 1248–1254. [Google Scholar] [PubMed] [Green Version]
- Katz, I.R.; Jeste, D.V.; Mintzer, J.E.; Clyde, C.; Napolitano, J.; Brecher, M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: A randomized, double-blind trial. J. Clin. Psychiatry 1999, 60, 107–115. [Google Scholar] [CrossRef]
- Llau, M.; Nguyen, L.; Senard, J.; Rascol, O.; Montastruc, J. Syndromes parkinsoniens d’origine médicamenteuse: Expérience d’un centre régional de pharmacovigilance sur dix ans [Drug-induced parkinsonian syndromes: A 10-year experience at a regional center of pharmaco-vigilance]. Rev. Neurol. 1994, 150, 757–762. [Google Scholar] [PubMed]
- Thanvi, B.; Treadwell, S. Drug induced parkinsonism: A common cause of parkinsonism in older people. Postgrad. Med. J. 2009, 85, 322–326. [Google Scholar] [CrossRef]
- Lera, G.; Zirulnik, J. Pilot study with clozapine in patients with HIV-associated psychosis and drug-induced parkinsonism. Mov. Disord. 1999, 14, 128–131. [Google Scholar] [CrossRef]
- Hriso, E.; Kuhn, T.; Masdeu, J.C.; Grundman, M. Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. Am. J. Psychiatry 1991, 148, 1558–1561. [Google Scholar] [CrossRef]
- Foubert-Samier, A.; Helmer, C.; Pérez, F.; Le Goff, M.; Auriacombe, S.; Elbaz, A.; Dartigues, J.-F.; Tison, F. Past exposure to neuroleptic drugs and risk of Parkinson disease in an elderly cohort. Neurology 2012, 79, 1615–1621. [Google Scholar] [CrossRef]
- Galoppin, M.; Berroir, P.; Soucy, J.-P.; Suzuki, Y.; Lavigne, G.J.; Gagnon, J.-F.; Montplaisir, J.Y.; Stip, E.; Blanchet, P.J. Chronic Neuroleptic-Induced Parkinsonism Examined with Positron Emission Tomography. Mov. Disord. 2020, 35, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Chabolla, D.R.; Maraganore, D.M.; Ahlskog, J.E.; O’Brien, P.C.; Rocca, W.A. Drug-induced parkinsonism as a risk factor for Parkinson’s disease: A Historical Cohort Study in Olmsted County, Minnesota. Mayo Clin. Proc. 1998, 73, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.; Factor, S.; Hauser, R.; Jimenez-Shahed, J.; Ondo, W.; Jarskog, L.; Meltzer, H.; Woods, S.; Bega, D.; LeDoux, M.; et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: The ARM-TD study. Neurology 2017, 88, 2003–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, S.; Seeman, P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry 2001, 158, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Trosch, R.M. Neuroleptic-Induced Movement Disorders: Deconstructing Extrapyramidal Symptoms. Am. Geriatr. Soc. 2004, 52, S266–S271. [Google Scholar] [CrossRef]
- Seeman, P. Clozapine, a Fast-Off-D2 Antipsychotic. ACS Chem. Neurosci. 2014, 5, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Remington, G.; Seeman, P.; Feingold, A.; Mann, S.; Shammi, C.; Kapur, S. “Extended” antipsychotic dosing in the maintenance treatment of schizophrenia: A double-blind, placebocontrolled trial. J. Clin. Psychiatry 2011, 72, 1042–1048. [Google Scholar] [CrossRef]
- Tarsy, D.; Baldessarini, R.J.; Tarazi, F.I. Effects of Newer Antipsychotics on Extrapyramidal Function. CNS Drugs 2002, 16, 23–45. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Parellada, E.; Bernardo, M.; Lafuente, A. Network analysis of gene expression in peripheral blood identifies mTOR and NF-kappaB pathways involved in antipsychotic-induced extrapyramidal symptoms. Pharm. J. 2015, 15, 452–460. [Google Scholar]
- Mas, S.; Gassó, P.; Lafuente, A. Applicability of gene expression and systems biology to develop pharmacogenetic predictors; antipsychotic-induced extrapyramidal symptoms as an example. Pharm. J. 2015, 16, 1975–1988. [Google Scholar] [CrossRef]
- Boloc, D.; Gortat, A.; Cheng-Zhang, J.Q.; Garcia-Cerro, S.; Rodriguez, N.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Gassó, P.; Lafuente, A.; et al. Improving pharmacogenetic prediction of extrapyramidal symptoms induced by antipsychotics. Transl. Psychiatry 2018, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Boloc, D.; Rodríguez, N.; Torres, T.; García-Cerro, S.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Bernardo, M.; Gassó, P.; Lafuente, A.; et al. Identifying key transcription factors for pharmacogenetic studies of antipsychotics induced extrapyramidal symptoms. Psychopharmacology 2020, 237, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Erro, R.; Bhatia, K.P.; Tinazzi, M. Parkinsonism following neuroleptic exposure: A double-hit hypothesis? Mov. Disord. 2015, 30, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Yoo, H.S.; Moon, H.; Oh, J.S.; Kim, J.S.; Park, Y.H.; Hong, J.Y.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Early-onset drug-induced parkinsonism after exposure to offenders implies nigrostriatal dopaminergic dysfunction. J. Neurol. Neurosurg. Psychiatry 2018, 89, 169–174. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.H.; Lee, J.J.; Lee, H.S.; Sohn, Y.H.; Lee, J.-M.; Lee, P.H. Microstructural white matter alterations in patients with drug induced parkinsonism. Hum. Brain Mapp. 2017, 38, 6043–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.S.; Bak, Y.; Chung, S.J.; Lee, Y.; Ye, B.S.; Sohn, Y.H.; Shin, N.-Y.; Lee, P.H. Impaired functional connectivity of sensorimotor network predicts recovery in drug-induced parkinsonism. Park. Relat. Disord. 2020, 74, 16–21. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Blanchet, P.J.; Kivenko, V. Drug-induced parkinsonism: Diagnosis and management. J. Park. Restless Legs Syndr. 2016, 6, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Simpson, G.M.; Angus, J.W.S. A Rating Scale for Extrapyramidal Side Effects. Acta Psychiatr. Scand. Suppl. 1970, 212, 11–19. [Google Scholar] [CrossRef]
- Knol, W.; Keijsers, C.J.P.W.; Jansen, P.A.F.; Belitser, S.V.; Schobben, A.F.A.M.; Egberts, A.C.G.; Van Marum, R.J. Validity and reliability of the Simpson-Angus Scale (SAS) in drug induced parkinsonism in the elderly. Int. J. Geriatr. Psychiatry 2009, 24, 183–189. [Google Scholar] [CrossRef]
- Hardie, R.J.; Lees, A.J. Neuroleptic-induced Parkinson’s syndrome: Clinical features and results of treatment with levodopa. J. Neurol. Neurosurg. Psychiatry 1988, 51, 850–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorberboym, M.; Treves, T.; Melamed, E.; Lampl, Y.; Hellmann, M.; Djaldetti, R. [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson’s disease. Mov. Disord. 2006, 21, 510–514. [Google Scholar] [CrossRef]
- Mena, M.; de Yébenes, J. Drug-induced parkinsonism. Expert Opin. Drug Saf. 2006, 5, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Corrales, F.J.; Sanz-Viedma, S.; Garcia-Solis, D.; Escobar-Delgado, T.; Mir, P. Clinical features and 123I-FP-CIT SPECT imaging in drug-induced parkinsonism and Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Hassin-Baer, S.; Sirota, P.; Korczyn, A.D.; Treves, T.A.; Epstein, B.; Shabtai, H.; Martin, T.; Litvinjuk, Y.; Giladi, N. Clinical characteristics of neuroleptic-induced parkinsonism. J. Neural Transm. 2001, 108, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-W.; Kim, J.S.; Oh, M.; You, S.; Kim, Y.J.; Kim, J.; Kim, M.-J.; Chung, S.J. Clinical features of drug-induced parkinsonism based on [18F] FP-CIT positron emission tomography. Neurol. Sci. 2015, 36, 269–274. [Google Scholar] [CrossRef]
- Sethi, K.D.; Zamrini, E.Y. Asymmetry in clinical features of drug-induced parkinsonism. J. Neuropsychiatry Clin. Neurosci. 1990, 2, 64–66. [Google Scholar] [CrossRef]
- Tinazzi, M.; Antonini, A.; Bovi, T.; Pasquin, I.; Steinmayr, M.; Moretto, G.; Fiaschi, A.; Ottaviani, S. Clinical and [123I]FP-CIT SPET imaging follow-up in patients with drug-induced parkinsonism. J. Neurol. 2009, 256, 910–915. [Google Scholar] [CrossRef]
- Ovallath, S.; Sulthana, B. Drug Induced Parkinsonism: An Overview. Open Access J. Neurol. Neurosurg. 2017, 3, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Bruce, L.S.; Margaret, G.W.; Delbert, G.R.; John, M.K. Side effects of Antipsychotic Drugs; Avoiding and minimizing their impact in elderly patients. Postgrad. Med. 2000, 107, 169–178. [Google Scholar]
- Powell, A.; Gallur, L.; Koopowitz, L.; Hayes, M.W. Parkinsonism in the psychiatric setting: An update on clinical differentiation and management. BMJ Neurol. Open 2020, 2, e000034. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Haehner, A.; Thiem, C.; Hummel, T. Neuroleptic-induced parkinsonism is associated with olfactory dysfunction. J. Neurol. 2008, 255, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Yeo, S.H.; Yong, S.W.; Kim, Y.J. Odour identification test and its relation to cardiac 123I-metaiodobenzylguanidine in patients with drug induced parkinsonism. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1250–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovi, T.; Antonini, A.; Ottaviani, S.; Antonioli, A.; Cecchini, M.P.; Di Francesco, V.; Bassetto, M.; Zamboni, M.; Fiaschi, A.; Moretto, G.; et al. The status of olfactory function and the striatal dopaminergic system in drug-induced parkinsonism. J. Neurol. 2010, 257, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Roussakis, A.; Piccini, P.; Politis, M. Clinical utility of DaTscan™ (123I-Ioflupane Injection) in the diagnosis of Parkinsonian Syndromes. Degener. Neurol. Neuromuscul. Dis. 2013, 3, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Brigo, F.; Matinella, A.; Erro, R.; Tinazzi, M. [(1)(2)(3)I]FP-CIT SPECT (DaTSCAN) may be a useful tool to differentiate between Parkinson’s disease and vascular or drug-induced parkinsonisms: A meta-analysis. Eur. J. Neurol. 2014, 21, 1369-e90. [Google Scholar] [CrossRef]
- Factor, S.A.; Burkhard, P.R.; Caroff, S.; Friedman, J.H.; Marras, C.; Tinazzi, M.; Comella, C.L. Recent developments in drug-induced movement disorders: A mixed picture. Lancet Neurol. 2019, 18, 880–890. [Google Scholar] [CrossRef]
- Kägi, G.; Klein, C.; Wood, N.W.; Schneider, S.A.; Pramstaller, P.P.; Tadić, V.; Quinn, N.P.; Van De Warrenburg, B.P.; Bhatia, K.P. Nonmotor symptoms in Parkin gene-related parkinsonism. Mov. Disord. 2010, 25, 1279–1284. [Google Scholar] [CrossRef]
- Tinazzi, M.; Cipriani, A.; Matinella, A.; Cannas, A.; Solla, P.; Nicoletti, A.; Zappia, M.; Morgante, L.; Morgante, F.; Pacchetti, C.; et al. [(1)(2)(3)I]FP-CIT single photon emission computed tomography findings in drug-induced Parkinsonism. Schizophr. Res. 2012, 139, 40–45. [Google Scholar] [CrossRef]
- Olivares Romero, J.; Arjona Padillo, A. Diagnostic accuracy of 123 I-FP-CIT SPECT in diagnosing drug-induced parkinsonism: A prospective study. Neurologia 2013, 28, 276–282. [Google Scholar] [CrossRef]
- Jin, S.; Oh, M.; Oh, S.J.; Oh, J.S.; Lee, S.J.; Chung, S.J.; Lee, C.S.; Kim, J.S. Differential Diagnosis of Parkinsonism Using Dual-Phase F-18 FP-CIT PET Imaging. Nucl. Med. Mol. Imaging 2013, 47, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Hwang, Y.M.; Lee, C.-N.; Kim, S.; Oh, S.Y.; Kim, Y.C.; Choe, J.G.; Park, K.W. Differential Diagnosis of Patients with Inconclusive Parkinsonian Features Using [(18)F]FP-CIT PET/CT. Nucl. Med. Mol. Imaging 2014, 48, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Sadasivan, S.; Friedman, J.H. Experience with DaTscan at a tertiary referral center. Park. Relat. Disord. 2015, 21, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Bega, D.; Gonzalez-Latapi, P.; Zadikoff, C.; Spies, W.; Simuni, T. Is There a Role for DAT-SPECT Imaging in a Specialty Movement Disorders Practice? Neurodegener. Dis. 2015, 15, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.Y.; Sunwoo, M.K.; Oh, J.S.; Kim, J.S.; Sohn, Y.H.; Lee, P.H. Persistent Drug-Induced Parkinsonism in Patients with Normal Dopamine Transporter Imaging. PLoS ONE 2016, 11, e0157410. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Chalissery, A.; Barry, T.; O’Sullivan, D.; O’Connell, M.; Lynch, T. Referral practice, reporting standards, and the impact of dopamine transporter scans done in a tertiary hospital. Neurol. India 2017, 65, 1264. [Google Scholar] [CrossRef]
- Shin, H.-W.; Chung, S.J. Drug-Induced Parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rascol, O.; Schelosky, L. 123I-metaiodobenzylguanidine scintigraphy in Parkinson’s disease and related disorders. Mov. Disord. 2009, 24, S732–S741. [Google Scholar] [CrossRef]
- Lee, P.H.; Kim, J.S.; Shin, D.H.; Yoon, S.-N.; Huh, K. Cardiac 123I-MIBG scintigraphy in patients with drug induced parkinsonism. J. Neurol. Neurosurg. Psychiatry 2006, 77, 372–374. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, K.; Healy, D.; Schapira, A. National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Vlaar, A.M.; De Nijs, T.; Kessels, A.G.; Vreeling, F.W.; Winogrodzka, A.; Mess, W.H.; Tromp, S.C.; Van Kroonenburgh, M.J.; Weber, W.E. Diagnostic Value of 123I-Ioflupane and 123I-Iodobenzamide SPECT Scans in 248 Patients with Parkinsonian Syndromes. Eur. Neurol. 2008, 59, 258–266. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón Moreno, J.; Alonso-Cánovas, A.; Buisán Catevilla, J. Substantia nigra echogenicity predicts response to drug withdrawal in suspected drug-induced parkinsonism. Mov. Disord. Clin. Pract. 2016, 3, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Mahlknecht, P.; Stockner, H.; Kiechl, S.; Willeit, J.; Rastner, V.; Gasperi, A.; Rungger, G.; Poewe, W.; Seppi, K. Is transcranial sonography useful to distinguish drug-induced parkinsonism from Parkinson’s disease? Mov. Disord. 2012, 27, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.H.; Noh, Y.; Lee, J.; Kim, E.Y. Drug-induced Parkinsonism versus Idiopathic Parkinson Disease: Utility of Nigrosome 1 with 3-T Imaging. Radiology 2016, 279, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamboa, J.; Jiménez-Jiménez, F.; Nieto, A.; Montojo, J.; Ortí-Pareja, M.; Molina, J.; Cobeta, I. Acoustic voice analysis in patients with Parkinson’s disease treated with dopaminergic drugs. J. Voice 1997, 11, 314–320. [Google Scholar] [CrossRef]
- Mamo, D.C.; Sweet, R.A.; Keshavan, M.S. Managing Antipsychotic-Induced Parkinsonism. Drug Saf. 1999, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Cort, E.; Bradley, R.; Harper, E.; Kelly, L.; Bentham, P.; Ritchie, C.; Reeves, S.; Fawzi, W.; Livingston, G.; et al. Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): A randomised, controlled, double-blind trial. Lancet Psychiatry 2018, 5, 553–563. [Google Scholar] [CrossRef]
- Reeves, S.; Bertrand, J.; Uchida, H.; Yoshida, K.; Otani, Y.; Ozer, M.; Howard, R. Towards safer risperidone prescribing in Alzheimer’s disease. Br. J. Psychiatry 2020, 218, 268–275. [Google Scholar] [CrossRef]
- Kalish, S.C.; Bohn, R.L.; Mogun, H.; Glynn, R.J.; Gurwitz, J.H.; Avorn, J. Antipsychotic Prescribing Patterns and the Treatment of Extrapyramidal Symptoms in Older People. J. Am. Geriatr. Soc. 1995, 43, 967–973. [Google Scholar] [CrossRef]
- Margolesky, J.; Betté, S.; Singer, C. Management of Urologic and Sexual Dysfunction in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 69–80. [Google Scholar] [CrossRef]
- Keks, N.; Schwartz, D.; Hope, J. Stopping and switching antipsychotic drugs. Aust. Prescr. 2019, 42, 152–157. [Google Scholar] [CrossRef]
- Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N. Engl. J. Med. 1999, 340, 757–763. [Google Scholar] [CrossRef]
- Kirrane, A.; Majumdar, B.; Richman, A. Clozapine use in old age psychiatry. BJPsych Adv. 2018, 24, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J. Viewpoint: Challenges in our understanding of neuroleptic induced parkinsonism. Parkinsonism Relat. Disord. 2014, 20, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.M.; Citrome, L. Antipsychotic-Related Movement Disorders: Drug-Induced Parkinsonism vs. Tardive Dyskinesia—Key Differences in Pathophysiology and Clinical Management. Neurol. Ther. 2018, 7, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Aoki, F.Y.; Sitar, D.S. Clinical Pharmacokinetics of Amantadine Hydrochloride. Clin. Pharmacokinet. 1988, 14, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Fann, W.E.; Lake, C.R. Amantadine versus trihexyphenidyl in the treatment of neuroleptic- induced parkinsonism. Am. J. Psychiatry 1976, 133, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Mindham, R.H.S. Assessment of drug-induced extrapyramidal reactions and of drugs given for their control. Br. J. Clin. Pharmac. 1976, 3, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.A.; Maclennan, W.J. Review: Drug-induced Parkinsonism in Elderly Patients. Age Ageing 1989, 18, 208–210. [Google Scholar] [CrossRef]
- Moellentine, C.; Rummans, T.; Ahlskog, J.E.; Harmsen, W.S.; Suman, V.J.; O’Connor, M.K.; Black, J.L.; Pileggi, T. Effectiveness of ECT in Patients with Parkinsonism. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 187–193. [Google Scholar] [CrossRef]
- Baez, M.A.; Avery, J. Improvement in Drug-Induced Parkinsonism with Electroconvulsive Therapy. Am. J. Geriatr. Pharmacother. 2011, 9, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Sadananda, S.K.; Holla, B.; Viswanath, B.; Narasimha, A.; Sebastian, A.; Math, S.B.; Chandrashekar, C.R. Effectiveness of Electroconvulsive Therapy for Drug-Induced Parkinsonism in the Elderly. J. ECT 2013, 29, e6–e7. [Google Scholar] [CrossRef] [PubMed]
| Study * | Prevalence of DIP |
| Estevez-Fraga et al., 2018 Review article | Globally over 50% of aged 65 and over |
| Hoffman et al., 1987 Cross-sectional study in inpatients and outpatients (n = 21) | 76% |
| Morgante et al., 1996 Prevalence survey in the community population study (n = 24,496) | 32.7/100,000 |
| Benito-Leon et al., 2003 Epidemiological study in the community (n = 5278) | 0.5% |
| Barbosa et al., 2006 Community based survey (n = 1186) | 2.7% |
| Fleury et al., 2008 Cross-sectional prevalence study in the community (n = 2312) | 21.7/100,000 |
| Buyn et al., 2019 Korean National Health Insurance Claims Database (n = 1285) | 4.09/100,000 in 2009 and 7.02/100,000 in 2015 |
| Han et al., 2019 Korean National Health Insurance Review and Assessment Service Database (n = circa 50 million) | 9.78/100,000 |
| Khedr et al., 2015 Cross-sectional community based survey (n = 8027) | 37/100,000 |
| Tse et al., 2008 Cross-sectional study in nursing homes n = 28/397 had parkinsonism, 2 of which had DIP | 0.5% |
| Moghal et al., 1995 Survey in nursing homes (n = 67) | 3% |
| Study * | Incidence of DIP |
| Caligiuri et al., 1999 Longitudinal prospective study in psychiatric outpatients (n = 120) | 28.6% |
| Rajpur et al., 1984 Epidemiological study in the community (n = 138 new cases of parkinsonism) | 7.2% (of 138) |
| Bower et al., 1999 Epidemiological study in the community (n = 364 incident cases of parkinsonism) | 20% (of 364) |
| Baldereschi et al., 2000 Longitudinal study in the community (n = 3084 of which n = 68 had parkinsonism) | 10% (of 68 cases) |
| Rocca et al., 2001 Epidemiological study in the community (n = 2739 of which n = 364 with parkinsonism) | 20% (of 364) |
| Benito-Leon et al., 2004 Epidemiological study in community (n = 3813, of which n = 68 parkinsonism) | 32.3% (of 68) |
| De Lau et al., 2004 Prospective community population cohort (n = 6839) | 12% |
| Munhoz et al., 2010 Cohort study in outpatient service (n = 1528) | 7.9% |
| Seijo-Martinez et al., 2011 Community-based survey (n = 41) | 31.7% |
| Bondon-Guitton et al., 2011 French Pharmacovigilance Database (n = 20,855) | 0.7% |
| Savica et al., 2013 Cohort Study in community population (n = 542) | 6.6% |
| Savica et al., 2017 Epidemiological study in community population (n = 906) | 11.9% |
| Vale et al., 2018 Cross-sectional study in community population (n = 610) | 12.3% |
| Druschky et al., 2020 German Pharmacovigilance Database (n = 340,099) | 0.08% |
| De Germay et al., 2020 WHO Pharmacovigilance Database (n = 9,009,107) | 0.05% |
| Han et al., 2019 Korean Health Insurance Review and Assessment Service Database (n = circa 50 million) | 8.69/100,000 |
| Fleury et al., 2018 Retrospective Incidence study in community population (n = 2312) | 2.5/100,000 |
| First Generation APs | Second Generation APs | |
|---|---|---|
| Phenothiazines | Chlorpromazine Promazine Levomepromazine Triflupromazine Mesoridazine Thioridazine Fluphenazine (HP) Perphenazine Prochlorperazine (HP) Trifluoperazine (HP) | |
| Butyrophenones (HP) | Haloperidol Benperidol Droperidol | |
| Thioxanthenes | Chlorprothixene Clopenthixol (HP) Flupenthixol (HP) Thiothixene Zuclopenthixol (HP) | |
| Benzamides | Sulpiride Tiapride Veralipride Levosulpiride Metoclopramide Mosapride Lisepride Clebopride | Amisulpiride (FD) Remoxipride (FD) Sultopride Itopride |
| Indole derivatives | Oxypertine Molindone | Ziprasidone Lurasidone |
| Diphenylbutylpiperidines | Pimozide (HP) | |
| Other | Loxapine Clozapine (FD) Olanzapine Quetiapine (FD) Asenapide Clotiapine Zotepine Paliperidone Sertindole Aripiprazole |
| Study (3, 20, 26, 27, 38, 40, 41) | Drug Class | % and Number of Subjects |
|---|---|---|
| Munhoz et al., 2010 | Antipsychotics | 52.9% n = 74 |
| Calcium Channel Blockers | 35.7% n = 50 | |
| Other drug classes | 11.4% n = 16 | |
| Bondon-Guitton et al., 2011 | Central dopaminergic antagonists | 49% n = 128 |
| Antidepressants | 8% n = 21 | |
| Calcium Channel Blockers | 5% n = 13 | |
| Peripheral dopaminergic antagonists | 4.6% n = 12 | |
| H1 antihistamines | 4.6% n = 12 | |
| Miscellaneous drugs | 28.7% n = 75 | |
| Druschky et al., 2020 | Antipsychotic Drugs: | |
| -First Generation—Low Potency | 0.024% n = 17 | |
| -First Generation—High Potency | 0.159% n = 78 | |
| -Second Generation | 0.073% n = 139 | |
| Munhoz et al., 2017 | Classic neuroleptics | n = 78 |
| -Haloperidol | 48.7% n = 38 | |
| -Levomepromazine | 24.4% n = 19 | |
| -Chlorpromazine | 17.9% n = 14 | |
| -Thioridazine | 9% n = 7 | |
| Second-generation neuroleptics | n = 21 | |
| -Risperidone | 81% n = 17 | |
| -Olanzapine | 19% n = 4 | |
| Calcium channel blockers | n = 58 | |
| -Flunarizine | 65.5% n = 38 | |
| -Cinnarizine | 34.5% n = 20 | |
| De Germay et al., 2020 | Risperidone | 14% n = 637 |
| Haloperidol | 9.4% n = 428 | |
| Aripiprazole | 7.2% n = 330 | |
| Olanzapine | 6.2% n = 283 | |
| Valproic acid | 5.7% n = 262 | |
| Quetiapine | 4.0% n = 184 | |
| Sulpiride | 3.6% n = 164 | |
| Clozapine | 3.5% n = 160 | |
| Metoclopramide | 3.5% n = 160 | |
| Paliperidone | 3.3% n = 151 | |
| Oh et al., 2018 | Levosulpiride | 78.2% n = 54 |
| Metoclopramide | 11.58% n = 8 | |
| Clebopride | 7.24% n = 5 | |
| Itopride | 2.89% n = 2 | |
| Kim S et al., 2019 | Typical Antipsychotics | 0.3% n = 15 |
| Atypical Antipsychotics | 0.8% n = 45 | |
| Gastrokinetic | 22.2% n = 1222 |
| Patient-Related | Drug-Related |
|---|---|
| Age > 60 | High potency first generation antipsychotics |
| Female Gender | High dose of antipsychotics (first and second generation) |
| Organic Brain Damage | Long-term exposure to antipsychotics |
| Intellectual Disability | |
| Dementia | |
| Idiopathic Parkinson’s Disease | |
| Hypertension | |
| Non-European ancestry | |
| HIV infection | |
| HLA-B44 | |
| Schizophrenia, depression |
| AIP | IPD | |
|---|---|---|
| General | ||
| Symptoms | More symmetrical | More asymmetrical |
| Onset | Acute or subacute | Chronic |
| Course | Reversible after withdrawal of drug (*) | Progressive |
| Motor | ||
| Upper Limb Predominance | ↑ | ↑↓ |
| Axial Impairment | ↓ | ↑ |
| Oro-facial dyskinesias | ↑ | ↓ |
| Akathisia | ↑ | ↓ |
| Resting Tremor | ↓ | ↑↑ |
| Postural Tremor | ↑ | ↓ |
| Perioral Tremor | ↑ | ↓ |
| Amimia | ↓ | ↑ |
| Postural instability | ↓ | ↑ |
| Non-motor | ||
| Mood Changes | ↑ | ↑ |
| Autonomic Dysfunction | ↑ | ↑↑ |
| Cognitive Deficits | ↑ | ↑↑ |
| Pain | ↑ | ↑ |
| Sleep disturbances | ↑ | ↑↑↑ |
| Olfactory dysfunction | ↓↓↓ | ↑↑↑ |
| Urinary symptoms | ↑ | ↑↑ |
| Concentration Problems | ↑ | ↑↑↑ |
| Sexual dysfunction | ↑ | ↑↑ |
| Investigation | AIP | IPD |
|---|---|---|
| DaT Scan | Normal (*) | Abnormal/Frequently asymmetrical findings |
| Cardiac MIBG Scintigraphy | Normal (*) | Abnormal |
| TCS of Substantia Nigra | Normal (*) | Abnormal |
| Study * | N | Conclusions |
|---|---|---|
| DAT Scanning | ||
| Lorberboym et al., 2006 | 30 | [123I]FP-CIT SPECT can help distinguish whether DIP is drug-induced or an exacerbation of subclinical IPD. |
| Diaz-Corrales 2010 | 79 | DIP and IPD are clinically difficult to differentiate, and can be improved by [123I]FP-CIT SPECT imaging. |
| Shin et al., 2015 | 92 | Symmetrical parkinsonism was more prevalent and duration of drug exposure before the onset of parkinsonism shorter for patients with normal vs. abnormal [18F]FP-CIT PET scans. |
| Tinazzi et al., 2009 | 19 | [123I]FP-CIT SPECT imaging helps identify subjects with DIP secondary to a loss of dopamine nerve terminals in the context of a progressive degenerative parkinsonism. |
| Bovi et al., 2010 | 48 | Patients with DIP and pathological putamen uptake had abnormal olfactory function. Smell deficits in DIP patients may be more associated with dopaminergic loss than drug-mediated dopamine receptor blockade. |
| Tinazzi et al., 2012 | 97 | D2-receptor blockade may accompany a dopamine nigrostriatal terminal defect, as assessed by [123I]FP-CIT SPECT abnormalities, in an applicable proportion of DIP patients. |
| Jin et al., 2013 | 98 | Dual-phase [18F]FP-CIT PET imaging helps demonstrate striatal DAT loss in neurodegenerative parkinsonism. |
| Park et al., 2014 | 33 | [18F]FP-CIT PET imaging useful to differentiate parkinsonism in patients with inconclusive parkinsonian features, except in patients who show atypical features or who eventually progress to PD. |
| Sadasivan et al., 2015 | 65 | [18F]FP-CIT PET can significantly impact patient clinical management in those with clinically uncertain parkinsonian syndromes in a tertiary referral center. |
| Bega et al., 2015 | 83 | [18F]FP-CIT PET had a significant impact on clinical diagnosis and management. |
| Hong et al., 2016 | 50 | Persistent DIP in patients with visually normal [18F]FP-CIT PET DAT imaging may be associated with subtle reduction of DAT activity. |
| Bhattacharjee et al., 2017 | 48 | Compliance of the [123I]FP-CIT SPECT imaging with the existing standard guidelines is good and influences the clinical diagnosis and management in 23% of the patients with parkinsonism. |
| Vlaar et al., 2008 | 248 | [123I]FP-CIT SPECT is accurate to differentiate patients with IPD from those with essential tremor (ET), and IPD from vascular parkinsonism (VP) and DIP. |
| VMAT using PET and radioligand | ||
| Galoppin et al., 2020 | 45 | Striatal VMAT2 binding is abnormal in a fraction of chronic DIP cases and differs in spatial distribution from PD. |
| Cardiac Scintigraphy | ||
| Lee et al., 2006 | 20 | MIBG uptake was not different between the DIP patients and controls. Two DIP patients whose MIBG uptake was significantly reduced showed persistent parkinsonism and responded dramatically to levodopa. |
| Lee et al., 2007 | 15 | An olfactory function test may be useful to detect DIP unrelated to PD and to identify patients with DIP who have subclinical PD. |
| Transcranial Ultrasonography of the Substantia Nigra | ||
| Oh et al., 2018 | 193 | SN echogenicity on TCS could help differentiate PD from DIP in clinical situations. Pure DIP and unmasked PD exhibited different SN echogenicity patterns. Early SN echogenicity findings on TCS could be used as a biomarker to predict clinical prognosis of DIP. |
| López-Sendón Moreno et al., 2016 | 60 | SN hyperechogenicity assessed with TCS is a valid prognostic marker in the setting of suspected DIP. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisidagama, S.; Selladurai, A.; Wu, P.; Isetta, M.; Serra-Mestres, J. Recognition and Management of Antipsychotic-Induced Parkinsonism in Older Adults: A Narrative Review. Medicines 2021, 8, 24. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060024
Wisidagama S, Selladurai A, Wu P, Isetta M, Serra-Mestres J. Recognition and Management of Antipsychotic-Induced Parkinsonism in Older Adults: A Narrative Review. Medicines. 2021; 8(6):24. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060024
Chicago/Turabian StyleWisidagama, Sharadha, Abiram Selladurai, Peter Wu, Marco Isetta, and Jordi Serra-Mestres. 2021. "Recognition and Management of Antipsychotic-Induced Parkinsonism in Older Adults: A Narrative Review" Medicines 8, no. 6: 24. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060024






