Nitrogen-Doped Flower-Like Hybrid Structure Based on Three-Dimensional Graphene
Abstract
:1. Introduction
2. Materials and Methods
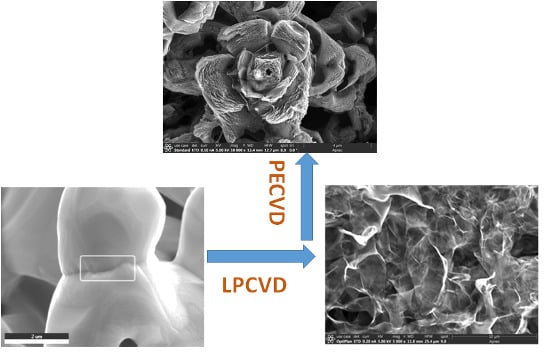
2.1. Synthesis of the 3D Graphene Hybrid Structure
2.2. Characterization of the 3D Graphene Hybrid Structure
2.3. Electrochemical Studies
3. Results and Discussion
3.1. XPS Analysis of the 3D Graphene Hybrid Structures
3.2. Contact Angle Measurements
3.3. Electrical Conductivity
3.4. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; DeArmond, D.; Alvarez, N.T.; Zhao, D.; Wang, T.; Hou, G.; Malik, R.; Heineman, W.R.; Shanov, V. Beyond graphene foam, a new form of three-dimensional graphene for supercapacitor electrodes. J. Mater. Chem. A 2016, 4, 1876–1886. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Yu, Y.; Lin, D.; Fisher, T.S.; Xu, X.; Li, H.; Xiong, G. Hyperbolically Patterned 3D Graphene Metamaterial with Negative Poisson’s Ratio and Superelasticity. Adv. Mater. 2016, 28, 2229–2237. [Google Scholar] [CrossRef]
- Alvarez, N.; DeArmond, D.; Malik, R.; Shanov, V.N.; Zhang, L. Graphene Paper and a Process for Making Graphene Paper and a Graphene Electrode. U.S. Patent Application No. 15/619, 388, 9 June 2017. [Google Scholar]
- Yu, X.; Lu, B.; Xu, Z. Super long-life supercapacitors based on the construction of nanohoneycomb-like strongly coupled CoMoO4–3D graphene hybrid electrodes. Adv. Mater. 2014, 26, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef]
- Dong, X.; Wang, X.; Wang, J.; Song, H.; Li, X.; Wang, L.; Chan-Park, M.B.; Li, C.M.; Chen, P. Synthesis of a MnO2–graphene foam hybrid with controlled MnO2 particle shape and its use as a supercapacitor electrode. Carbon N. Y. 2012, 50, 4865–4870. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, W.; Wang, X.; Zhang, H.; Cui, X.; Wang, H. Ultrahigh capacitive performance from both Co (OH)2/graphene electrode and K3 Fe (CN)6 electrolyte. Sci. Rep. 2013, 3, 2986. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Shi, W.; Lu, G.; Huang, X.; Yan, Q.; Zhang, Q.; Zhang, H. Preparation of novel 3D graphene networks for supercapacitor applications. Small 2011, 7, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, Z.; Ren, W.; Li, F.; Cheng, H.-M. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates. Proc. Natl. Acad. Sci. USA 2012, 109, 17360–17365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Li, L.; Ma, C.; Wang, S.; Shi, Y.; Koratkar, N.; Ren, W.; Li, F.; Cheng, H.-M. A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries. Nano Energy 2015, 11, 356–365. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, J.; Ang, H.; Zeng, Y.; Xiao, N.; Gao, Y.; Liu, W.; Hng, H.H.; Yan, Q. Binder-free graphene foams for O2 electrodes of Li–O2 batteries. Nanoscale 2013, 5, 9651–9658. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Chen, S.; Li, Y.; Tao, Z.; Ye, J.; Ni, K.; Yu, H.; Xiang, B.; Ren, Y.; Qin, F. 3D graphene films enable simultaneously high sensitivity and large stretchability for strain sensors. Adv. Funct. Mater. 2018, 28, 1803221. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, Z.; Liu, Y.; Wang, Y.; Su, F.; Li, S.; Zhang, Y.; Li, H.; Chen, H.; Zhao, Z. Three-dimensional graphene field-effect transistors as high-performance photodetectors. Nano Lett. 2019, 19, 1494–1503. [Google Scholar] [CrossRef] [Green Version]
- Idowu, A.; Boesl, B.; Agarwal, A. 3D graphene foam-reinforced polymer composites—A review. Carbon N. Y. 2018, 135, 52–71. [Google Scholar] [CrossRef]
- Qiu, H.; Du, P.; Hu, K.; Gao, J.; Li, H.; Liu, P.; Ina, T.; Ohara, K.; Ito, Y.; Chen, M. Metal and Nonmetal Codoped 3D Nanoporous Graphene for Efficient Bifunctional Electrocatalysis and Rechargeable Zn–Air Batteries. Adv. Mater. 2019, 31, 1900843. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Maksudov, T.; Konios, D.; Paradisanos, I.; Kioseoglou, G.; Stratakis, E.; Kymakis, E. Efficient and highly air stable planar inverted perovskite solar cells with reduced graphene oxide doped PCBM electron transporting layer. Adv. Energy Mater. 2017, 7, 1602120. [Google Scholar] [CrossRef]
- Shao, X.; Khursheed, A. A Review Paper on “Graphene Field Emission for Electron Microscopy. ” Appl. Sci. 2018, 8, 868. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls: Synthesis and Emerging Applications; Springer Science & Business Media: Wien, London, 2010; ISBN 3211997180. [Google Scholar]
- Chen, J.; Bo, Z.; Lu, G. Vertically-Oriented Graphene; Springer International Publishing: Cham, Switzerland, 2015; Volume 10, ISBN 978-3-319-15301-8. [Google Scholar]
- Shang, N.G.; Papakonstantinou, P.; McMullan, M.; Chu, M.; Stamboulis, A.; Potenza, A.; Dhesi, S.S.; Marchetto, H. Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv. Funct. Mater. 2008, 18, 3506–3514. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, C.; Zhang, W. Vertically aligned graphene nanosheet arrays: Synthesis, properties and applications in electrochemical energy conversion and storage. Adv. Energy Mater. 2017, 7, 1700678. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.H.; Kumar, S.; Ostrikov, K. Control of morphology and electrical properties of self-organized graphenes in a plasma. Carbon N. Y. 2011, 49, 4331–4339. [Google Scholar] [CrossRef]
- Thomas, R.; Rao, G.M. Synthesis of 3-dimensional porous graphene nanosheets using electron cyclotron resonance plasma enhanced chemical vapour deposition. RSC Adv. 2015, 5, 84927–84935. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganesan, K.; Polaki, S.R.; Mathews, T.; Dhara, S.; Kamruddin, M.; Tyagi, A.K. Influence of substrate on nucleation and growth of vertical graphene nanosheets. Appl. Surf. Sci. 2015, 349, 576–581. [Google Scholar] [CrossRef]
- Davami, K.; Shaygan, M.; Kheirabi, N.; Zhao, J.; Kovalenko, D.A.; Rümmeli, M.H.; Opitz, J.; Cuniberti, G.; Lee, J.-S.; Meyyappan, M. Synthesis and characterization of carbon nanowalls on different substrates by radio frequency plasma enhanced chemical vapor deposition. Carbon N. Y. 2014, 72, 372–380. [Google Scholar] [CrossRef]
- Choi, J.W.; Youn, S.K.; Park, H.G. Carbon micronymphaea: Graphene on vertically aligned carbon nanotubes. J. Nanomater. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Zhang, M.; Alvarez, N.T.; Zhao, D.; Zhang, L.; Haase, M.R.; Malik, R.; Katuscak, C.; Wang, T.; Shanov, V. A corrugated graphene–carbon nanotube composite as electrode material. Nano Life 2014, 4, 1441019. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, B.; Yang, H.; Pan, Q.; Li, Z.; Liu, Z. Growth of carbon nanotubes on graphene by chemical vapor deposition. New Carbon Mater. 2016, 31, 31–36. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kumar, K.; Fisher, F.T.; Yang, E.-H. Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 2011, 23, 15301. [Google Scholar] [CrossRef]
- Wu, S.; Shi, E.; Yang, Y.; Xu, W.; Li, X.; Cao, A. Direct fabrication of carbon nanotube-graphene hybrid films by a blown bubble method. Nano Res. 2015, 8, 1746–1754. [Google Scholar] [CrossRef]
- Harris, P.J.F.; Suarez-Martinez, I.; Marks, N.A. The structure of junctions between carbon nanotubes and graphene shells. Nanoscale 2016, 8, 18849–18854. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Li, P.; Wang, Z.; Zhang, W.; Qin, W.; He, W. Three-dimensional hierarchical graphene-CNT@ Se: A highly efficient freestanding cathode for Li–Se batteries. ACS Energy Lett. 2016, 1, 16–20. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon N. Y. 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Faisal, S.N.; Haque, E.; Noorbehesht, N.; Zhang, W.; Harris, A.T.; Church, T.L.; Minett, A.I. Pyridinic and graphitic nitrogen-rich graphene for high-performance supercapacitors and metal-free bifunctional electrocatalysts for ORR and OER. RSC Adv. 2017, 7, 17950–17958. [Google Scholar] [CrossRef] [Green Version]
- Barsan, M.M.; Prathish, K.P.; Sun, X.; Brett, C.M.A. Nitrogen doped graphene and its derivatives as sensors and efficient direct electron transfer platform for enzyme biosensors. Sens. Actuators B Chem. 2014, 203, 579–587. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Teng, P.-Y.; Yeh, C.-H.; Koshino, M.; Chiu, P.-W.; Suenaga, K. Structural and chemical dynamics of pyridinic-nitrogen defects in graphene. Nano Lett. 2015, 15, 7408–7413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, J.; Holloway, B.C.; Outlaw, R.A.; Zhao, X.; Hou, K.; Shutthanandan, V.; Manos, D.M. A mechanism for carbon nanosheet formation. Carbon N. Y. 2007, 45, 2229–2234. [Google Scholar] [CrossRef]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman spectroscopy of graphene on different substrates and influence of defects. Bull. Mater. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]
- Yanilmaz, A.; Tomak, A.; Akbali, B.; Bacaksiz, C.; Ozceri, E.; Ari, O.; Senger, R.T.; Selamet, Y.; Zareie, H.M. Nitrogen doping for facile and effective modification of graphene surfaces. RSC Adv. 2017, 7, 28383–28392. [Google Scholar] [CrossRef] [Green Version]
- Maddi, C.; Bourquard, F.; Barnier, V.; Avila, J.; Asensio, M.-C.; Tite, T.; Donnet, C.; Garrelie, F. Nano-Architecture of nitrogen-doped graphene films synthesized from a solid CN source. Sci. Rep. 2018, 8, 3247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Lu, Z.; Chen, X.; Chen, Q.; Gao, P.; Yu, T.; Peng, Z.; Ye, J. Efficient and controllable growth of vertically oriented graphene nanosheets by mesoplasma chemical vapor deposition. Carbon N. Y. 2019, 147, 341–347. [Google Scholar] [CrossRef]
- Yang, J.; Qian, X.; Li, H.; Wang, H.; Xue, X.; Cai, L.; Hu, P.; Yu, G. Novel Hollow Graphene Flowers Synthesized by Cu-Assisted Chemical Vapor Deposition. Adv. Mater. Interfaces 2018, 5, 1800347. [Google Scholar] [CrossRef]
- Zhao, J.; Shaygan, M.; Eckert, J.; Meyyappan, M.; Rümmeli, M.H. A growth mechanism for free-standing vertical graphene. Nano Lett. 2014, 14, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ganesan, K.; Polaki, S.R.; Ilango, S.; Amirthapandian, S.; Dhara, S.; Kamruddin, M.; Tyagi, A.K. Flipping growth orientation of nanographitic structures by plasma enhanced chemical vapor deposition. RSC Adv. 2015, 5, 91922–91931. [Google Scholar] [CrossRef] [Green Version]
- Bayer, B.C.; Bosworth, D.A.; Michaelis, F.B.; Blume, R.; Habler, G.; Abart, R.; Weatherup, R.S.; Kidambi, P.R.; Baumberg, J.J.; Knop-Gericke, A. In situ observations of phase transitions in metastable nickel (carbide)/carbon nanocomposites. J. Phys. Chem. C 2016, 120, 22571–22584. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Afaneh, T.; Dharmasena, R.; Jasinski, J.; Sumanasekera, G.; Henner, V. Study of nitrogen doping of graphene via in-situ transport measurements. Phys. B Condens. Matter 2016, 490, 21–24. [Google Scholar] [CrossRef]
- Dementjev, A.P.; De Graaf, A.; Van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-ray photoelectron spectroscopic characterization of chemically modified electrodes used as chemical sensors and biosensors: A review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]














| Sample ID | Growth of N-3DG | Roll Pass | Growth of N-GF | Remarks |
|---|---|---|---|---|
| H1 | Yes | No | No | With 1% CH4 using LPCVD |
| H2 | Yes | No | Yes | With 1% CH4 using LPCVD followed by 20% C2H2 using PECVD |
| H3 | Yes | No | Yes | With 1% CH4 using LPCVD followed by 5% C2H2 using PECVD |
| H4 | Yes | Yes | Yes | N-3DG was compressed between rollers having a gap 0.35mm prior to growth of N-GF with 20% C2H2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, K.; Khosravifar, M.; Sawant, S.; Adusei, P.K.; Kanakaraj, S.N.; Kasik, J.; Shanov, V. Nitrogen-Doped Flower-Like Hybrid Structure Based on Three-Dimensional Graphene. C 2020, 6, 40. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020040
Dasgupta K, Khosravifar M, Sawant S, Adusei PK, Kanakaraj SN, Kasik J, Shanov V. Nitrogen-Doped Flower-Like Hybrid Structure Based on Three-Dimensional Graphene. C. 2020; 6(2):40. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020040
Chicago/Turabian StyleDasgupta, Kinshuk, Mahnoosh Khosravifar, Shrilekha Sawant, Paa Kwasi Adusei, Sathya Narayan Kanakaraj, Jacob Kasik, and Vesselin Shanov. 2020. "Nitrogen-Doped Flower-Like Hybrid Structure Based on Three-Dimensional Graphene" C 6, no. 2: 40. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020040