Advances and Perspectives in Biohydrogen Production from Palm Oil Mill Effluent
Abstract
:1. Introduction
2. Physicochemical Properties of POME
| Parameter | Raw POME | ||||
|---|---|---|---|---|---|
| Reference | [19] | [12] | [27] | [20] | [21] |
| pH | 4.3 ± 0.3 | 4.40 ± 0.07 | 4.63 | 4.5 ± 0.5 | 4.67 |
| Color | - | - | - | brown | |
| Turbidity (NTU) | 67,500 ± 1910 | - | 690 ± 5 | 7560 | |
| Chemical oxygen demand (COD) (mg/L) | 84,450 ± 19,500 | 50,000 ± 1081 | 89,591 | 52,000 ± 2000 | 42,482 |
| Biochemical oxygen demand (BOD) (mg/L) | - | - | 34,771 | 26,000 ± 1000 | 20,838 |
| Total solids (TS) (mg/L) | - | 38,793 ± 395 | 47,050 | 41,000 ± 1000 | 32,157 |
| Dissolved solids (DS) (mg/L) | - | - | 9310 | - | 17,436 |
| Total suspended solids (TSS) (mg/L) | 19,610 ± 7.900 | 5000 ± 438 | 36,560 | 19,500 ± 500 | 14,721 |
| Total nitrogen (TN) (mg/L) | 650 ± 300 | 750 ± 0.00 | - | 720 ± 5 | 340 |
| Ammonical nitrogen (NH3–N) (mg/L) | - | - | - | 32 ± 1 | 133 |
| Phosphorus (PO4–P) (mg/L) | - | - | - | - | 210 |
| Total reducing sugars (mg/L) | - | - | 228 | - | - |
| Oils and greases (mg/L) | 4000 ± 0.13 | 37,883 | 4050 ± 20 | 1927 | |
| Fe (mg/L) | 70.7 ± 1.65 | - | - | - | - |
| Zn (mg/L) | 7.53 ± 1.07 | - | - | - | - |
| Mn (mg/L) | 6.47 ± 1.43 | - | - | - | - |
| Mg (mg/L) | 1144 ± 7.00 | - | 326.89 | - | - |
| Al (mg/L) | 334 ± 22.65 | - | - | - | - |
| Cellulose (% dry basis) | - | 14.34 ± 0.07 | - | - | - |
| Hemicellulose (% dry basis) | - | 10.76 ± 0.28 | - | - | - |
| Lignin (% dry basis) | - | 13.58 ± 0.10 | - | - | - |
| Glucose (mg/L) | - | 2070 ± 14 | - | - | - |
| Xylose (mg/L) | - | 640 ± 0.00 | - | - | - |
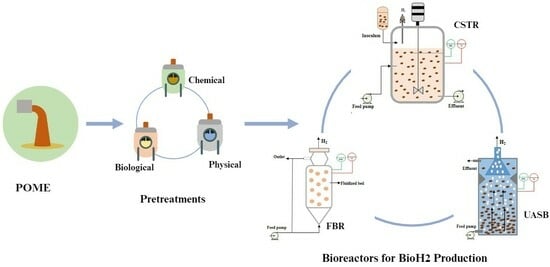
3. POME Pretreatments
3.1. Physical Pretreatment
3.1.1. Ultrasonication Pretreatment
3.1.2. Microwave Pretreatment
3.1.3. Heat Pretreatment
3.2. Chemical Pretreatment
3.2.1. Acid Hydrolysis Pretreatment
3.2.2. Alkaline Hydrolysis Pretreatment
3.2.3. Ozonation Pretreatment
3.3. Biological Pretreatment
Enzymatic Hydrolysis
| Pretreatment | Process | POME | pH | TSS (mg/L) | COD Removal % | H2 Yield | Increase in H2 Production | Reference |
|---|---|---|---|---|---|---|---|---|
| Ultrasonication | Dark fermentation | Raw | 4.63 | 17.53 | - | 0.52 mmol H2/g COD | 16% to 86% | [41] |
| Pretreated | 7.00 | - | 62.24 | 0.68 mmol H2/g COD | ||||
| Photofermentation | Raw | 4.3 ± 0.3 | 19,610 ± 7900 | 4.67 mL H2/mL POME | [19] | |||
| Pretreated | - | - | 36.9 | 8.72 mL H2/mL POME POMEH2/mL POME | ||||
| Ultrasonication and microwaves | Dark fermentation | Raw | 5.2 ± 0.2 | - | - | 3360 mL H2/L-POME | [40] | |
| Pretreated | 3.9 ± 0.4 | - | 75.56 | 4080 mL H2/L-POME | ||||
| Surfactant | Dark fermentation | Raw | 4.63 | 60.46 | - | 1.13 mol H2/g COD | 334% | [2] |
| Pretreated | 7.00 | - | 58 | 4.91 mol H2/g COD | ||||
| Acid hydrolysis | Dark fermentation | Raw | 4.5 | 37,750 | - | 0.14 mol H2/mol of total carbohydrates | 45% to 75% | [55] |
| Pretreated | 2.00 | 26,100 | - | 1.87 mol H2/mol of total carbohydrates | ||||
| Dark fermentation | Raw | 4.2 | 12,000 | - | 1.5 L H2/L-POME | [54] | ||
| Pretreated | 4.1 | 9200 | - | 2.56 L H2/L-POME | ||||
| Dark fermentation | Raw | 4.5 | 52,650 | - | 0.63 mol H2/mol of glucose | [10] | ||
| Pretreated | 2.46 | 39,200 | - | 1.24 mol H2/mol of glucose | ||||
| Dark fermentation | Raw | 4.24 ± 0.6 | 48,560 | - | 0.72 mol H2/mol of total carbohydrates | [5] | ||
| Pretreated | 5.3 | - | - | 1.69 mol H2/mol of total carbohydrates | ||||
| Alkaline hydrolysis | Dark fermentation | Raw | 4.5 | 37,750 | - | 0.14 mol H2/mol of total carbohydrates | Until 90% | [55] |
| Pretreated | 7.7 | 25,050 | - | 2.18 mol H2/mol of total carbohydrates | ||||
| Dark fermentation | Raw | 4.2 | 12,000 | - | 1.5 L H2/L-POME | [54] | ||
| Pretreated | 5.4 | 8500 | 45 | 4.6 L H2/L-POME | ||||
| Dark fermentation | Raw | 4.24 ± 0.6 | 48,560 | - | 0.72 mol H2/mol of total carbohydrates | [5] | ||
| Pretreated | 5.14 ± 0.43 | - | - | 1.08 mol H2/mol of total carbohydrates | ||||
| Ozonation | Dark fermentation | Raw | 4.4–4.7 | 15,000–20,000 | - | 51.5 ± 5.0 mL H2/g COD | 25 to 49% | [56] |
| Pretreated | 6 | 8000–12,000 | - | 77.1 ± 8.1 mL H2/g COD | ||||
| Ozonation | Dark fermentation | Raw | 4.4–4.7 | - | - | 122.0 ± 1.4 mL H2/g COD | [57] | |
| Pretreated | 6 | - | - | 182.3 mL H2/g COD | ||||
| Ozonation | Dark fermentation | Raw | 4.3–4.5 | 15,000–20,000 | - | 122 mL H2/g COD | [58] | |
| Pretreated | 4.4–4.5 | 8000–12,000 | - | 182 mL H2/g COD | ||||
| Enzymatic hydrolysis | Dark fermentation | Raw | 4.63 | 60.46 | 30 | 1.12 mol H2/g COD | 13 to 137% | [60] |
| Pretreated | 7.00 | - | 52 | 1.88 mol H2/g COD | ||||
| Enzymatic hydrolysis | Dark fermentation | Raw | - | - | - | 873.6 mL H2/L POME | [61] | |
| Pretreated | 6.2 | - | - | 2075 mL H2/L POME | ||||
| Enzymatic hydrolysis | Dark fermentation | Raw | 6.5 | - | - | 2.26 ± 0.05 mmol H2/g COD | [33] | |
| Pretreated | 6.5 | - | - | 2.56 ± 0.05 mmol H2/g COD |
4. Processes and Parameters for Green Hydrogen Production
4.1. Steam Reforming of POME
4.2. Microbial Electrolysis Cell
4.3. Dark Fermentation and Photofermentation of POME
5. Bioreactor Types and Operational Conditions
5.1. Bioreactors for Dark Fermentation
5.1.1. Suspension Cell Bioreactors
Continuous Stirred Tank Reactor (CSTR)
Anaerobic Membrane Bioreactor (AnMBR)
Anaerobic Sequencing Batch Reactor (ASBR)
5.1.2. Immobilized Bioreactors
Packed/Fixed-Bed Bioreactor (PBR)
Fluidized Bed Reactor (FBR)
Upflow Anaerobic Sludge Blanket Reactor (UASB)
6. Nanoparticles and Other Technologies to Increase Biohydrogen Production
6.1. Nanoparticles (NPs)
6.2. Cell Immobilization
| Immobilization Technique | Immobilization Media | Inoculum | Operation Mode | Conditions | H2 Yield | H2 Yield Increase | Reference |
|---|---|---|---|---|---|---|---|
| Imprisonment | Polyethylene glycol (PEG) gel—10% w/v | Clostridium sp. | Continuous—working volume of 5000 mL | 37 °C 100 rpm pH 5.5 36 h (HRT) | 0.31 L H2/g COD | - | [142] |
| Adsorption | Granular activated carbon (GAC)—10% | Caldicellulosiruptor saccharolyticus | Batch—250 mL working volume | Medium enriched with 10% POME Initial pH 7, 100 rpm, 1:1%wv (inoculum: GAC), and thermophilic temperature of 70 °C | 2.6 mol H2/mol of substrate | 1.94 fold | [143] |
| Imprisonment | Alginate (1:1) | Bacillus anthracis 15% v/v | Batch—Scott bottle with 350 mL working volume | POME and FW sterilized (1:1 v/v ratio), pH 5.0, 35 °C | Maximum hydrogen production rate of 47 mL/h | - | [144] |
| Adsorption | Glass beads (3 mm) | POME anaerobic sludge | Fed-batch—100 mL bottles | pH 6, 37 °C, 72 h | 479.3 ppm | 1.34 fold | [145] |
| Adsorption | Moringa oleifera Seeds (MOS)—0.7–1.3 cm (addition of 5% w/v) | Anaerobic sludge | Batch—100 mL bottles | 37 °C, pH 6 | 560 ppm | 25.5 fold | [146] |
6.3. Genetic Tools and Metabolic Engineering
7. Economic Evaluation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xin, Y.; Sun, L.; Hansen, M.C. Oil Palm Reconciliation in Indonesia: Balancing Rising Demand and Environmental Conservation towards 2050. J. Clean. Prod. 2022, 380, 135087. [Google Scholar] [CrossRef]
- Absalome, M.A.; Massara, C.C.; Alexandre, A.A.; Gervais, K.; Chantal, G.G.A.; Ferdinand, D.; Rhedoor, A.J.; Coulibaly, I.; George, T.G.; Brigitte, T.; et al. Biochemical Properties, Nutritional Values, Health Benefits and Sustainability of Palm Oil. Biochimie 2020, 178, 81–95. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Martinez-Hernandez, E.; Murphy, R.J.; Ng, D.K.S.; Hassim, M.H.; Siew Ng, K.; Yoke Kin, W.; Jaye, I.F.M.; Leung Pah Hang, M.Y.; Andiappan, V. Role of Bioenergy, Biorefinery and Bioeconomy in Sustainable Development: Strategic Pathways for Malaysia. Renew. Sustain. Energy Rev. 2018, 81, 1966–1987. [Google Scholar] [CrossRef]
- Statista. 2024. Production Volume of Palm Oil Worldwide from 2012/13 to 2022/23. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/613471/palm-oil-production-volume-worldwide// (accessed on 20 February 2024).
- Arisht, S.N.; Mahmod, S.S.; Abdul, P.M.; Indera Lutfi, A.A.; Takriff, M.S.; Lay, C.H.; Silvamany, H.; Sittijunda, S.; Jahim, J.M. Enhancing Biohydrogen Gas Production in Anaerobic System via Comparative Chemical Pre-Treatment on Palm Oil Mill Effluent (POME). J. Environ. Manag. 2022, 321, 115892. [Google Scholar] [CrossRef]
- Haryati, Z.; Subramaniam, V.; Noor, Z.Z.; Hashim, Z.; Loh, S.K.; Aziz, A.A. Social Life Cycle Assessment of Crude Palm Oil Production in Malaysia. Sustain. Prod. Consum. 2022, 29, 90–99. [Google Scholar] [CrossRef]
- Shigetomi, Y.; Ishimura, Y.; Yamamoto, Y. Trends in Global Dependency on the Indonesian Palm Oil and Resultant Environmental Impacts. Sci. Rep. 2020, 10, 20624. [Google Scholar] [CrossRef]
- Meijaard, E.; Brooks, T.M.; Carlson, K.M.; Slade, E.M.; Garcia-Ulloa, J.; Gaveau, D.L.A.; Lee, J.S.H.; Santika, T.; Juffe-Bignoli, D.; Struebig, M.J.; et al. The Environmental Impacts of Palm Oil in Context. Nat. Plants 2020, 6, 1418–1426. [Google Scholar] [CrossRef]
- Nelson, P.N.; Gabriel, J.; Filer, C.; Banabas, M.; Sayer, J.A.; Curry, G.N.; Koczberski, G.; Venter, O. Oil Palm and Deforestation in Papua New Guinea. Conserv. Lett. 2014, 7, 188–195. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Jahim, J.M.; Abdul, P.M. Pretreatment Conditions of Palm Oil Mill Effluent (POME) for Thermophilic Biohydrogen Production by Mixed Culture. Int. J. Hydrogen Energy 2017, 42, 27512–27522. [Google Scholar] [CrossRef]
- Khadaroo, S.N.B.A.; Grassia, P.; Gouwanda, D.; He, J.; Poh, P.E. Enhancing the Biogas Production and the Treated Effluent Quality via an Alternative Palm Oil Mill Effluent (POME) Treatment Process: Integration of Thermal Pretreatment and Dewatering. Biomass Bioenergy 2021, 151, 106167. [Google Scholar] [CrossRef]
- Prasertsan, P.; Khangkhachit, W.; Duangsuwan, W.; Mamimin, C.; O-Thong, S. Direct Hydrolysis of Palm Oil Mill Effluent by Xylanase Enzyme to Enhance Biogas Production Using Two-Steps Thermophilic Fermentation under Non-Sterile Condition. Int. J. Hydrogen Energy 2017, 42, 27759–27766. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen Production by Dark Fermentation of Wheat Powder Solution: Effects of C/N and C/P Ratio on Hydrogen Yield and Formation Rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Baykara, S.Z. Hydrogen: A Brief Overview on Its Sources, Production and Environmental Impact. Int. J. Hydrogen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Kumar, G.; Park, J.H.; Sivagurunathan, P.; Lee, S.H.; Park, H.D.; Kim, S.H. Microbial Responses to Various Process Disturbances in a Continuous Hydrogen Reactor Fed with Galactose. J. Biosci. Bioeng. 2017, 123, 216–222. [Google Scholar] [CrossRef]
- Córdova-Lizama, A.; Carrera-Figueiras, C.; Palacios, A.; Castro-Olivera, P.M.; Ruiz-Espinoza, J. Improving Hydrogen Production from the Anaerobic Digestion of Waste Activated Sludge: Effects of Cobalt and Iron Zero Valent Nanoparticles. Int. J. Hydrogen Energy 2022, 47, 30074–30084. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Pérez, M. Effect of the Hydraulic Retention Time on the Acidogenic Fermentation of Sewage Sludge, Wine Vinasse and Poultry Manure for Biohydrogen Production. Biomass Bioenergy 2022, 167, 106643. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, L.; Ab Wahid, Z.; Krishnan, S.; Rana, S.; Amirul Islam, M.; Sakinah, M.; Ameen, F.; Syed, A. Photohydrogen Production from Dark-Fermented Palm Oil Mill Effluent (DPOME) and Statistical Optimization: Renewable Substrate for Hydrogen. J. Clean. Prod. 2018, 199, 11–17. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Ultrasonication Pre-Treatment of Combined Effluents from Palm Oil, Pulp and Paper Mills for Improving Photofermentative Biohydrogen Production. Energy Convers. Manag. 2016, 119, 142–150. [Google Scholar] [CrossRef]
- Akhbari, A.; Ibrahim, S.; Ahmad, M.S. Optimization of Up-Flow Velocity and Feed Flow Rate in up-Flow Anaerobic Sludge Blanket Fixed-Film Reactor on Bio-Hydrogen Production from Palm Oil Mill Effluent. Energy 2022, 266, 126435. [Google Scholar] [CrossRef]
- Kadier, A.; Wang, J.; Chandrasekhar, K.; Abdeshahian, P.; Islam, M.A.; Ghanbari, F.; Bajpai, M.; Katoch, S.S.; Bhagawati, P.B.; Li, H.; et al. Performance Optimization of Microbial Electrolysis Cell (MEC) for Palm Oil Mill Effluent (POME) Wastewater Treatment and Sustainable Bio-H2 Production Using Response Surface Methodology (RSM). Int. J. Hydrogen Energy 2022, 47, 15464–15479. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Bittencourt Sydney, E.; Bianchi Pedroni Medeiros, A.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Junior Letti, L.A.; Thomaz Soccol, V.; de Melo Pereira, G.V.; et al. Agro-Industrial Wastewater in a Circular Economy: Characteristics, Impacts and Applications for Bioenergy and Biochemicals. Bioresour. Technol. 2021, 341, 125795. [Google Scholar] [CrossRef]
- Letti, L.A.J.; Woiciechowski, A.L.; Medeiros, A.B.P.; Rodrigues, C.; Carvalho, J.C.; de Vandenberghe, S.; Karp, S.G.; Torres, L.A.Z.; Guarnizo, A.F.C.; Colonia, B.S.O.; et al. Valorization of solid and liquid wastes from palm oil industry. In Waste Biorefinery: Value Addition through Resource Utilization; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–265. [Google Scholar] [CrossRef]
- Poh, P.E.; Yong, W.J.; Chong, M.F. Palm Oil Mill Effluent (POME) Characteristic in High Crop Season and the Applicability of High-Rate Anaerobic Bioreactors for the Treatment of Pome. Ind. Eng. Chem. Res. 2010, 49, 11732–11740. [Google Scholar] [CrossRef]
- Soo, P.L.; Bashir, M.J.K.; Wong, L. Recent Advancements in the Treatment of Palm Oil Mill Effluent ( POME ) Using Anaerobic Biofilm Reactors: Challenges and Future Perspectives. J. Environ. Manag. 2022, 320, 115750. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. A Holistic Approach to Managing Palm Oil Mill Effluent (POME): Biotechnological Advances in the Sustainable Reuse of POME. Biotechnol. Adv. 2009, 27, 40–52. [Google Scholar] [CrossRef]
- Rosa, D.; Medeiros, A.B.P.; Martinez-Burgos, W.J.; do Nascimento, J.R., Jr.; de Carvalho, J.C.; Sydney, E.B.; Soccol, C.R. Biological Hydrogen Production from Palm Oil Mill Effluent (POME) by Anaerobic Consortia and Clostridium Beijerinckii Drielly. J. Biotechnol. 2020, 323, 17–23. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; De Carvalho, J.C.; Soccol, V.T.; Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Biohydrogen Production in Cassava Processing Wastewater Using Microbial Consortia: Process Optimization and Kinetic Analysis of the Microbial Community. Bioresour. Technol. 2020, 309, 123331. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Junior, J.R.; Medeiros, A.B.P.; Herrmann, L.W.; Sydney, E.B.; Soccol, C.R. Biohydrogen Production from Agro-Industrial Wastes Using Clostridium Beijerinckii and Isolated Bacteria as Inoculum. Bioenergy Res. 2021, 15, 987–997. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Vo, D.V.N.; SundarRajan, P.S.; Swathi, M. Co-Hydrothermal Gasification of Scenedesmus sp. with Sewage Sludge for Bio-Hydrogen Production Using Novel Solid Catalyst Derived from Carbon-Zinc Battery Waste. Bioresour. Technol. Rep. 2020, 11, 100459. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Lee, Z.S.; Chong, C.C.; Khan, M.R.; Cheng, C.K.; Ng, K.H.; Hossain, S.S. Hydrogen-Rich Syngas Production via Steam Reforming of Palm Oil Mill Effluent (POME)—A Thermodynamics Analysis. Int. J. Hydrogen Energy 2019, 44, 20711–20724. [Google Scholar] [CrossRef]
- Tee, P.F.; Abdullah, M.O.; Tan, I.A.W.; Mohamed Amin, M.A.; Nolasco-Hipolito, C.; Bujang, K. Performance Evaluation of a Hybrid System for Efficient Palm Oil Mill Effluent Treatment via an Air-Cathode, Tubular Upflow Microbial Fuel Cell Coupled with a Granular Activated Carbon Adsorption. Bioresour. Technol. 2016, 216, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Garritano, A.d.N.; de Sá, L.R.V.; Aguieiras, É.C.G.; Freire, D.M.G.; Ferreira-Leitão, V.S. Efficient Biohydrogen Production via Dark Fermentation from Hydrolized Palm Oil Mill Effluent by Non-Commercial Enzyme Preparation. Int. J. Hydrogen Energy 2017, 42, 29166–29174. [Google Scholar] [CrossRef]
- Yang, H.; Guo, L.; Liu, F. Enhanced Bio-Hydrogen Production from Corncob by a Two-Step Process: Dark- and Photo-Fermentation. Bioresour. Technol. 2010, 101, 2049–2052. [Google Scholar] [CrossRef]
- Sydney, E.B.; Duarte, E.R.; Martinez Burgos, W.J.; de Carvalho, J.C.; Larroche, C.; Soccol, C.R. Development of Short Chain Fatty Acid-Based Artificial Neuron Network Tools Applied to Biohydrogen Production. Int. J. Hydrogen Energy 2020, 45, 5175–5181. [Google Scholar] [CrossRef]
- Basri, M.F.; Yacob, S.; Hassan, M.A.; Shirai, Y.; Wakisaka, M.; Zakaria, M.R.; Phang, L.Y. Improved Biogas Production from Palm Oil Mill Effluent by a Scaled-down Anaerobic Treatment Process. World J. Microbiol. Biotechnol. 2010, 26, 505–514. [Google Scholar] [CrossRef]
- Mamimin, C.; Singkhala, A.; Kongjan, P.; Suraraksa, B.; Prasertsan, P.; Imai, T.; O-Thong, S. Two-Stage Thermophilic Fermentation and Mesophilic Methanogen Process for Biohythane Production from Palm Oil Mill Effluent. Int. J. Hydrogen Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S.; et al. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- del Campo, I.; Alegría, I.; Zazpe, M.; Echeverría, M.; Echeverría, I. Diluted Acid Hydrolysis Pretreatment of Agri-Food Wastes for Bioethanol Production. Ind. Crops Prod. 2006, 24, 214–221. [Google Scholar] [CrossRef]
- Mishra, P.; Wahid, Z.A.; Singh, L.; Zaid, R.M.; Tabassum, S.; Sakinah, M.; Jiang, X. Synergistic Effect of Ultrasonic and Microwave Pretreatment on Improved Biohydrogen Generation from Palm Oil Mill Effluent. Biomass Convers. Biorefinery 2021, 12, 3655–3662. [Google Scholar] [CrossRef]
- Leaño, E.P.; Anceno, A.J.; Babel, S. Ultrasonic Pretreatment of Palm Oil Mill Effluent: Impact on Biohydrogen Production, Bioelectricity Generation, and Underlying Microbial Communities. Int. J. Hydrogen Energy 2012, 37, 12241–12249. [Google Scholar] [CrossRef]
- Isa, M.H.; Wong, L.P.; Bashir, M.J.K.; Shafiq, N.; Kutty, S.R.M.; Farooqi, I.H.; Lee, H.C. Improved Anaerobic Digestion of Palm Oil Mill Effluent and Biogas Production by Ultrasonication Pretreatment. Sci. Total Environ. 2020, 722, 137833. [Google Scholar] [CrossRef]
- Passos, F.; Solé, M.; García, J.; Ferrer, I. Biogas Production from Microalgae Grown in Wastewater: Effect of Microwave Pretreatment. Appl. Energy 2013, 108, 168–175. [Google Scholar] [CrossRef]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The Application of Microwave Heating in Bioenergy: A Review on the Microwave Pre-Treatment and Upgrading Technologies for Biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef]
- Sapci, Z. The Effect of Microwave Pretreatment on Biogas Production from Agricultural Straws. Bioresour. Technol. 2013, 128, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of Microwave––A Brief Review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Eskicioglu, C.; Kennedy, K.J.; Droste, R.L. Characterization of Soluble Organic Matter of Waste Activated Sludge before and after Thermal Pretreatment. Water Res. 2006, 40, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Khadaroo, S.N.B.A.; Poh, P.E.; Gouwanda, D.; Grassia, P. Applicability of Various Pretreatment Techniques to Enhance the Anaerobic Digestion of Palm Oil Mill Effluent (POME): A Review. J. Environ. Chem. Eng. 2019, 7, 103310. [Google Scholar] [CrossRef]
- Khadaroo, S.N.B.A.; Grassia, P.; Gouwanda, D.; Poh, P.E. The Impact of Thermal Pretreatment on Various Solid-Liquid Ratios of Palm Oil Mill Effluent (POME) for Enhanced Thermophilic Anaerobic Digestion Performance. J. Clean. Prod. 2020, 261, 121159. [Google Scholar] [CrossRef]
- Cao, S.; Pu, Y.; Studer, M.; Wyman, C.; Ragauskas, A.J. Chemical Transformations of Populus Trichocarpa during Dilute Acid Pretreatment. RSC Adv. 2012, 2, 10925–10936. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical Conversion of Lignocellulosic Biomass into Reducing Sugars Using Dilute Acid Hydrolysis and Alkaline Pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Arias, J.Z.; Reuter, T.; Sabir, A.; Gilroyed, B.H. Ambient Alkaline Hydrolysis and Anaerobic Digestion as a Mortality Management Strategy for Whole Poultry Carcasses. Waste Manag. 2018, 81, 71–77. [Google Scholar] [CrossRef]
- Ahmed, B.; Tyagi, S.; Rahmani, A.M.; Kazmi, A.A.; Varjani, S.; Tyagi, V.K. Novel Insight on Ferric Ions Addition to Mitigate Recalcitrant Formation during Thermal-Alkali Hydrolysis to Enhance Biomethanation. Sci. Total Environ. 2022, 829, 154621. [Google Scholar] [CrossRef] [PubMed]
- Seengenyoung, J. Biohydrogen Production from Palm Oil Mill Effluent Pretreated by Chemical Methods Using Thermoanaerobacterium-Rich Sludge. Iran. J. Energy Environ. 2013, 4, 312–319. [Google Scholar] [CrossRef]
- Kamal, S.A.; Jahim, J.M.; Anuar, N.; Hassan, O.; Ramli, W.; Daud, W.; Fadzillah Mansor, M.; Rashid, S.S. Pre-Treatment Effect of Palm Oil Mill Effluent (POME) during Hydrogen Production by a Local Isolate Clostridium Butyricum. Int. J. Adv. Sci. Eng. Inf. Technol. 2012, 2, 54–60. [Google Scholar] [CrossRef]
- Tanikkul, P.; Pisutpaisal, N. Biohydrogen Production under Thermophilic Condition from Ozonated Palm Oil Mill Effluent. In Proceedings of the Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 61, pp. 1234–1238. [Google Scholar]
- Pisutpaisal, N.; Tanikkul, P.; Phoochinda, W. Improvement of Mesophilic Biohydrogen Production from Palm Oil Mill Effluent Using Ozonation Process. In Proceedings of the Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 50, pp. 723–728. [Google Scholar]
- Tanikkul, P.; Juntarakod, P.; Pisutpaisal, N. Optimization of Biohydrogen Production of Palm Oil Mill Effluent by Ozone Pretreatment. Int. J. Hydrogen Energy 2019, 44, 5203–5211. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of Chemical Pretreatment for Bioconversion of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Leaño, E.P.; Babel, S. The Influence of Enzyme and Surfactant on Biohydrogen Production and Electricity Generation Using Palm Oil Mill Effluent. J. Clean. Prod. 2012, 31, 91–99. [Google Scholar] [CrossRef]
- Al-Shorgani, N.K.N.; Tibin, E.M.; Ali, E.; Hamid, A.A.; Yusoff, W.M.W.; Kalil, M.S. Biohydrogen Production from Agroindustrial Wastes via Clostridium Saccharoperbutylacetonicum N1-4 (ATCC 13564). Clean Technol. Environ. Policy 2014, 16, 11–21. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Ye, Y.; Bui, X.T.; Hoang, N.B. Advanced Strategies for Enhancing Dark Fermentative Biohydrogen Production from Biowaste towards Sustainable Environment. Bioresour. Technol. 2022, 351, 127045. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Adish Kumar, S.; Bhatia, S.K.; Kumar, G. Integrated Biorefinery Routes of Biohydrogen: Possible Utilization of Acidogenic Fermentative Effluent. Bioresour. Technol. 2021, 319, 124241. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, S.; Lee, B.D.; Kim, S.H. Waste Based Hydrogen Production for Circular Bioeconomy: Current Status and Future Directions. Bioresour. Technol. 2020, 302, 122920. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Manish, S.; Banerjee, R. Comparison of Biohydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Armor, J.N. The Multiple Roles for Catalysis in the Production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Meena, R.A.A.; Rajesh Banu, J.; Yukesh Kannah, R.; Yogalakshmi, K.N.; Kumar, G. Biohythane Production from Food Processing Wastes—Challenges and Perspectives. Bioresour. Technol. 2020, 298, 122449. [Google Scholar] [CrossRef] [PubMed]
- Zuldian, P.; Hastuti, Z.D.; Murti, S.D.S.; Adiarso, A. Biohythane System Using Two Steps of POME Fermentation Process for Supplying Electrical Energi: Economic Evaluation. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Banda Aceh, Indonesia, 2018; Volume 334. [Google Scholar]
- Sekoai, P.T.; Gueguim Kana, E.B. A Two-Stage Modelling and Optimization of Biohydrogen Production from a Mixture of Agro-Municipal Waste. Int. J. Hydrogen Energy 2013, 38, 8657–8663. [Google Scholar] [CrossRef]
- Hernández, M.A.; Rodríguez Susa, M.; Andres, Y. Use of Coffee Mucilage as a New Substrate for Hydrogen Production in Anaerobic Co-Digestion with Swine Manure. Bioresour. Technol. 2014, 168, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.H.; Brunstermann, R.; Mietzel, T.; Widmann, R. Effect of Pre-Treatment and Hydraulic Retention Time on Biohydrogen Production from Organic Wastes. Int. J. Hydrogen Energy 2018, 43, 4856–4865. [Google Scholar] [CrossRef]
- Rahman, S.N.A.; Masdar, M.S.; Rosli, M.I.; Majlan, E.H.; Husaini, T.; Kamarudin, S.K.; Daud, W.R.W. Overview Biohydrogen Technologies and Application in Fuel Cell Technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.H.; Bhatia, S.K.; Brar, S.K. An Overview on Progress, Advances, and Future Outlook for Biohydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 37264–37281. [Google Scholar] [CrossRef]
- Levalley, T.L.; Richard, A.R.; Fan, M. The Progress in Water Gas Shift and Steam Reforming Hydrogen Production Technologies—A Review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Khan, M.R.; Ng, K.H.; Wongsakulphasatch, S.; Cheng, C.K. Harnessing Renewable Hydrogen-Rich Syngas from Valorization of Palm Oil Mill Effluent (POME) Using Steam Reforming Technique. Renew. Energy 2019, 138, 1114–1126. [Google Scholar] [CrossRef]
- Ng, K.H.; Cheng, Y.W.; Lee, Z.S.; Khan, M.R.; Lam, S.S.; Cheng, C.K. Experimental Evaluation and Empirical Modelling of Palm Oil Mill Effluent Steam Reforming. Int. J. Hydrogen Energy 2018, 43, 15784–15793. [Google Scholar] [CrossRef]
- Wee, A.N.C.H.; Erison, A.E.; Edward Anyek, E.H.; Pakpahan, G.R.; Lim, J.R.; Tiong, A.N.T. Techno-Economic Assessment of Hydrogen Production via Steam Reforming of Palm Oil Mill Effluent. Sustain. Energy Technol. Assess. 2022, 53, 102575. [Google Scholar] [CrossRef]
- Ming, Q.; Healey, T.; Allen, L.; Irving, P. Steam Reforming of Hydrocarbon Fuels. Catal. Today 2002, 77, 51–64. [Google Scholar] [CrossRef]
- Ng, K.H.; Cheng, Y.W.; Lee, Z.S.; Cheng, C.K. A Study into Syngas Production from Catalytic Steam Reforming of Palm Oil Mill Effluent (POME): A New Treatment Approach. Int. J. Hydrogen Energy 2019, 44, 20900–20913. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ng, K.H.; Papadopoulos, A.M.; Le, A.T.; Kumar, S.; Hadiyanto, H.; Pham, V.V. Microbial Fuel Cells for Bioelectricity Production from Waste as Sustainable Prospect of Future Energy Sector. Chemosphere 2022, 287, 132285. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The Role of Microbial Electrolysis Cell in Urban Wastewater Treatment: Integration Options, Challenges, and Prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Nizami, A.S.; Rehan, M.; Ouda, O.K.M.; Sultana, S.; Ismail, I.M.; Shahzad, K. Microbial Electrolysis Cells for Hydrogen Production and Urban Wastewater Treatment: A Case Study of Saudi Arabia. Appl. Energy 2017, 185, 410–420. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.K.; Lee, W. Microbial Fuel Cells for Bioelectricity Generation through Reduction of Hexavalent Chromium in Wastewater: A Review. Int. J. Hydrogen Energy 2021, 46, 11458–11481. [Google Scholar] [CrossRef]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Salihon, J.B. Bioelectricity Generation from Palm Oil Mill Effluent in Microbial Fuel Cell Using Polacrylonitrile Carbon Felt as Electrode. Water Air Soil. Pollut. 2013, 224, 1533. [Google Scholar] [CrossRef]
- Krishnan, S.; Md Din, M.F.; Taib, S.M.; Nasrullah, M.; Sakinah, M.; Wahid, Z.A.; Kamyab, H.; Chelliapan, S.; Rezania, S.; Singh, L. Accelerated Two-Stage Bioprocess for Hydrogen and Methane Production from Palm Oil Mill Effluent Using Continuous Stirred Tank Reactor and Microbial Electrolysis Cell. J. Clean. Prod. 2019, 229, 84–93. [Google Scholar] [CrossRef]
- Tan, S.P.; Kong, H.F.; Bashir, M.J.K.; Lo, P.K.; Ho, C.D.; Ng, C.A. Treatment of Palm Oil Mill Effluent Using Combination System of Microbial Fuel Cell and Anaerobic Membrane Bioreactor. Bioresour. Technol. 2017, 245, 916–924. [Google Scholar] [CrossRef]
- Khongkliang, P.; Jehlee, A.; Kongjan, P.; Reungsang, A.; O-Thong, S. High Efficient Biohydrogen Production from Palm Oil Mill Effluent by Two-Stage Dark Fermentation and Microbial Electrolysis under Thermophilic Condition. Int. J. Hydrogen Energy 2019, 44, 31841–31852. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial Electrolysis Cells and Microbial Fuel Cells for Biohydrogen Production: Current Advances and Emerging Challenges. Biomass Convers. Biorefinery 2020, 13, 8403–8423. [Google Scholar] [CrossRef]
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Lay, C.H.; Chu, C.Y.; Leu, H.J.; Marone, A.; et al. Biohydrogen Production by Dark Fermentation: Scaling-up and Technologies Integration for a Sustainable System. Rev. Environ. Sci. Biotechnol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Nanda, S. Biohydrogen Production Through Dark Fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Potential of Bio-Hydrogen Production from Dark Fermentation of Crop Residues: A Review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; Brar, S.K.; Tanobe, V.O.d.A.; Medeiros, A.B.; De Carvalho, J.; Soccol, C.R. The Effect of Hydrolysis and Sterilization in Biohydrogen Production from Cassava Processing Wastewater Medium Using Anaerobic Bacterial Consortia. Int. J. Hydrogen Energy 2019, 44, 25551–25564. [Google Scholar] [CrossRef]
- García, A.B.; Cammarota, M.C. Biohydrogen Production from Pretreated Sludge and Synthetic and Real Biodiesel Wastewater by Dark Fermentation. Int. J. Energy Res. 2019, 43, 1586–1596. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen Production from Biomass Using Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Garritano, A.N.; Faber, M.d.O.; de Sà, L.R.V.; Ferreira-Leitão, V.S. Palm Oil Mill Effluent (POME) as Raw Material for Biohydrogen and Methane Production via Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 92, 676–684. [Google Scholar] [CrossRef]
- Mona, S.; Kumar, S.S.; Kumar, V.; Parveen, K.; Saini, N.; Deepak, B.; Pugazhendhi, A. Green Technology for Sustainable Biohydrogen Production (Waste to Energy): A Review. Sci. Total Environ. 2020, 728, 138481. [Google Scholar] [CrossRef]
- Zainal, B.S.; Gunasegaran, K.; Tan, G.Y.A.; Danaee, M.; Mohd, N.S.; Ibrahim, S.; Chyuan, O.H.; Nghiem, L.D.; Mahlia, T.M.I. Effect of Temperature and Hydraulic Retention Time on Hydrogen Production from Palm Oil Mill Effluent (POME) in an Integrated up-Flow Anaerobic Sludge Fixed-Film (UASFF) Bioreactor. Environ. Technol. Innov. 2022, 28, 102903. [Google Scholar] [CrossRef]
- Maaroff, R.M.; Md Jahim, J.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Abd Nasir, M.A. Biohydrogen Production from Palm Oil Mill Effluent (POME) by Two Stage Anaerobic Sequencing Batch Reactor (ASBR) System for Better Utilization of Carbon Sources in POME. Int. J. Hydrogen Energy 2019, 44, 3395–3406. [Google Scholar] [CrossRef]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of Biohydrogen Production by Photobiological, Fermentation and Electrochemical Processes: A Review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Chookaew, T.; O-Thong, S.; Prasertsan, P. Biohydrogen Production from Crude Glycerol by Two Stage of Dark and Photo Fermentation. Int. J. Hydrogen Energy 2015, 40, 7433–7438. [Google Scholar] [CrossRef]
- Dinesh, G.H.; Nguyen, D.D.; Ravindran, B.; Chang, S.W.; Vo, D.V.N.; Bach, Q.V.; Tran, H.N.; Basu, M.J.; Mohanrasu, K.; Murugan, R.S.; et al. Simultaneous Biohydrogen (H2) and Bioplastic (Poly-β-Hydroxybutyrate-PHB) Productions under Dark, Photo, and Subsequent Dark and Photo Fermentation Utilizing Various Wastes. Int. J. Hydrogen Energy 2020, 45, 5840–5853. [Google Scholar] [CrossRef]
- Syed, Z.; Sogani, M.; Kumar, A.; Rajvanshi, J.; Sharma, G.; Sonu, K. Biodegradation of Synthetic Estrogen Using Bioelectrochemical System and Degradation Pathway Analysis through Quadrupole-Time-of-Flight-Mass Spectrometry. Bioresour. Technol. 2022, 349, 126857. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.S.; Qazi, J.I.; Liang, Y.; Chen, S. Growth Characteristics and Photofermentative Biohydrogen Production Potential of Purple Non Sulfur Bacteria from Sugar Cane Bagasse. Fuel 2019, 255, 115805. [Google Scholar] [CrossRef]
- Verma, D.; Ram Kumar, N.; Subudhi, S. Isolation and Characterization of a Novel Photoheterotrophic Strain ‘Rubrivivax Benzoatilyticus TERI-CHL1′: Photo Fermentative Hydrogen Production from Spent Effluent. Int. J. Hydrogen Energy 2020, 45, 14245–14254. [Google Scholar] [CrossRef]
- Tiang, M.F.; Fitri Hanipa, M.A.; Abdul, P.M.; de Jahim, J.M.; Mahmod, S.S.; Takriff, M.S.; Lay, C.H.; Reungsang, A.; Wu, S.Y. Recent Advanced Biotechnological Strategies to Enhance Photo-Fermentative Biohydrogen Production by Purple Non-Sulphur Bacteria: An Overview. Int. J. Hydrogen Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Zhang, Z.; Zhang, H.; Xia, C. Enhancement Strategies for Photo-Fermentative Biohydrogen Production: A Review. Bioresour. Technol. 2021, 340, 125601. [Google Scholar] [CrossRef]
- Rai, P.K.; Asthana, R.K.; Singh, S.P. Optimization of Photo-Hydrogen Production Based on Cheese Whey Spent Medium. Int. J. Hydrogen Energy 2014, 39, 7597–7603. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; De Souza Candeo, E.; Pedroni Medeiros, A.B.; De Carvalho, J.; Oliveira de Andrade Tanobe, V.; Soccol, C.R.; Sydney, E.B. Hydrogen: Current Advances and Patented Technologies of Its Renewable Production. J. Clean. Prod. 2021, 286, 124970. [Google Scholar] [CrossRef]
- Carolin, F.; Kumar, P.S.; Vo, D.N.; Joshiba, G.J. A Review on Critical Assessment of Advanced Bioreactor Options for Sustainable Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 7113–7136. [Google Scholar] [CrossRef]
- Barca, C.; Soric, A.; Ranava, D.; Giudici-Orticoni, M.T.; Ferrasse, J.H. Anaerobic Biofilm Reactors for Dark Fermentative Hydrogen Production from Wastewater: A Review. Bioresour. Technol. 2015, 185, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Jia, Q.; Wei, L.; Shen, J. Influence of Cu2+ Concentration on the Biohydrogen Production of Continuous Stirred Tank Reactor. Int. J. Hydrogen Energy 2014, 39, 13437–13442. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Effect of Hydraulic Retention Time (HRT) on the Anaerobic Co-Digestion of Agro-Industrial Wastes in a Two-Stage CSTR System. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Seengenyoung, J.; O-Thong, S.; Prasertsan, P. Comparison of ASBR and CSTR Reactor for Hydrogen Production from Palm Oil Mill Effluent under Thermophilic Condition. Adv. Biosci. Biotechnol. 2014, 05, 177–183. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater: A Critical Review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.E.; Iyer, P.; Bruns, M.A.; Logan, B.E. Biological Hydrogen Production Using a Membrane Bioreactor. Biotechnol. Bioeng. 2004, 87, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zaiat, M.; Rodrigues, J.A.D.; Ratusznei, S.M.; De Camargo, E.F.M.; Borzani, W. Anaerobic Sequencing Batch Reactors for Wastewater Treatment: A Developing Technology. Appl. Microbiol. Biotechnol. 2001, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Santiago, S.G.; Morgan-Sagastume, J.M.; Monroy, O.; Moreno-Andrade, I. Biohydrogen Production from Organic Solid Waste in a Sequencing Batch Reactor: An Optimization of the Hydraulic and Solids Retention Time. Int. J. Hydrogen Energy 2020, 45, 25681–25688. [Google Scholar] [CrossRef]
- Karaosmanoglu Gorgec, F.; Karapinar, I. Biohydrogen Production from Hydrolyzed Waste Wheat by Dark Fermentation in a Continuously Operated Packed Bed Reactor: The Effect of Hydraulic Retention Time. Int. J. Hydrogen Energy 2019, 44, 136–143. [Google Scholar] [CrossRef]
- Karapinar, I.; Gokfiliz Yildiz, P.; Pamuk, R.T.; Karaosmanoglu Gorgec, F. The Effect of Hydraulic Retention Time on Thermophilic Dark Fermentative Biohydrogen Production in the Continuously Operated Packed Bed Bioreactor. Int. J. Hydrogen Energy 2020, 45, 3524–3531. [Google Scholar] [CrossRef]
- Andreani, C.L.; Torres, D.G.B.; Schultz, L.; Carvalho, K.Q.D.E.; Gomes, S.D. Hydrogen Production from Cassava Processing Wastewater in an Anaerobic Fixed Bed Reactor with Bamboo as a Support Material. J. Braz. Assoc. Agric. Eng. 2015, 35, 578–587. [Google Scholar] [CrossRef]
- Jamali, N.S.; Dzul Rashidi, N.F.; Jahim, J.M.; O-Thong, S.; Jehlee, A.; Engliman, N.S. Thermophilic Biohydrogen Production from Palm Oil Mill Effluent: Effect of Immobilized Cells on Granular Activated Carbon in Fluidized Bed Reactor. Food Bioprod. Process. 2019, 117, 231–240. [Google Scholar] [CrossRef]
- Daud, M.K.; Rizvi, H.; Akram, M.F.; Ali, S.; Rizwan, M.; Nafees, M.; Jin, Z.S. Review of Upflow Anaerobic Sludge Blanket Reactor Technology: Effect of Different Parameters and Developments for Domestic Wastewater Treatment. J. Chem. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Sohaili, J. Energy for Sustainable Development Effect of Organic Loading Rate on Hydrogen (H2) and Methane (CH4) Production in Two-Stage Fermentation under Thermophilic Conditions Using Palm Oil Mill Effluent (POME). Energy Sustain. Dev. Eff. 2016, 34, 130–138. [Google Scholar] [CrossRef]
- Senan, S.; Madihah, A.; Ping, J.; Jahim, J.; Mohamed, P.; Shahbudin, M.; Anuar, N.; Faisal, M.; Yunus, M.; Jaril, A.; et al. Operation Performance of Up- Fl Ow Anaerobic Sludge Blanket (UASB) Bioreactor for Biohydrogen Production by Self-Granulated Sludge Using Pre-Treated Palm Oil Mill Ef Fl Uent (POME) as Carbon Source. Renew. Energy 2019, 134, 1262–1272. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Daramola, M.O.; Mogwase, B.; Engelbrecht, N.; Yoro, K.O.; Petrus du Preez, S.; Mhlongo, S.; Ezeokoli, O.T.; Ghimire, A.; Ayeni, A.O.; et al. Revising the Dark Fermentative H2 Research and Development Scenario—An Overview of the Recent Advances and Emerging Technological Approaches. Biomass Bioenergy 2020, 140, 105673. [Google Scholar] [CrossRef]
- Do Nascimento Junior, J.R.; Zevallos Torres, L.A.; Medeiros, A.B.P.; Woiciechowski, A.L.; Martinez-Burgos, W.J.; Soccol, C.R. Enhancement of Biohydrogen Production in Industrial Wastewaters with Vinasse Pond Consortium Using Lignin-Mediated Iron Nanoparticles. Int. J. Hydrogen Energy 2021, 46, 27431–27443. [Google Scholar] [CrossRef]
- Kumar, Y.; Yogeshwar, P.; Bajpai, S.; Jaiswal, P.; Yadav, S.; Pathak, D.P.; Sonker, M.; Tiwary, S.K. Nanomaterials: Stimulants for Biofuels and Renewables, Yield and Energy Optimization. Mater. Adv. 2021, 2, 5318–5343. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Engliman, N.S.; Abdul, P.M.; Wu, S.Y.; Jahim, J.M. Influence of Iron (II) Oxide Nanoparticle on Biohydrogen Production in Thermophilic Mixed Fermentation. Int. J. Hydrogen Energy 2017, 42, 27482–27493. [Google Scholar] [CrossRef]
- Chai, Y.; Lyu, Z.; Du, H.; Li, P.; Ding, S.; Jiang, Y.; Wang, H.; Min, Q.; Du, D.; Lin, Y.; et al. Recent Progress on Rational Design of Catalysts for Fermentative Hydrogen Production. SusMat 2022, 2, 392–410. [Google Scholar] [CrossRef]
- Bosu, S.; Rajamohan, N. Nanotechnology Approach for Enhancement in Biohydrogen Production- Review on Applications of Nanocatalyst and Life Cycle Assessment. Fuel 2022, 323, 124351. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, L.; Amirul Islam, M.; Nasrullah, M.; Mimi Sakinah, A.M.; Wahid, Z.A. NiO and CoO Nanoparticles Mediated Biological Hydrogen Production: Effect of Ni/Co Oxide NPs-Ratio. Bioresour. Technol. Rep. 2019, 5, 364–368. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, L.; Wahid, Z.A. Influence of Nano Nickel Oxide (NNO) Particles on Hydrogen Production in Dark Fermentation of Palm Oil Mill Effluent. In The National Conference for Postgraduate Research; Universiti Malaysia Pahang: Pekan, Malaysia, 2016; pp. 783–787. [Google Scholar]
- Shanmugam, S.; Hari, A.; Pandey, A.; Mathimani, T.; Felix, L.O.; Pugazhendhi, A. Comprehensive Review on the Application of Inorganic and Organic Nanoparticles for Enhancing Biohydrogen Production. Fuel 2020, 270, 117453. [Google Scholar] [CrossRef]
- Baker, S.; Chromy, B.; Henderson, P.; Hoeprich, P. Nanolipoprotein Particles Comprising Hydrogenases and Related Products, Methods and Systems. U.S. Patent 9688718B2, 12 January 2009. [Google Scholar]
- Qin, Z.; Juanjuan, C.; Pei, Z.; Yonggui, Z.; Siyuan, X.; Cheng, Y.J. Nickel Ferrite Nano Particle and Green Synthesis Method and Application Thereof. CN Patent 114524470A, 24 February 2022. [Google Scholar]
- Shì, Z.J.; Zhenmin, L.; Lihua, Z.; Fei, Y.; Long, Z.J. Preparation of Nickel Cobaltate Nanoparticles and Method for Promoting Dark Fermentation to Produce Hydrogen. CN Patent 115215386A, 2022. [Google Scholar]
- Kumar, G.; Mudhoo, A.; Sivagurunathan, P.; Nagarajan, D.; Ghimire, A.; Lay, C.H.; Lin, C.Y.; Lee, D.J.; Chang, J.S. Recent Insights into the Cell Immobilization Technology Applied for Dark Fermentative Hydrogen Production. Bioresour. Technol. 2016, 219, 725–737. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Various Additives for Improving Dark Fermentative Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2018, 95, 130–146. [Google Scholar] [CrossRef]
- Singh, L.; Wahid, Z.A. Enhancement of Hydrogen Production from Palm Oil Mill Effluent via Cell Immobilisation Technique. Int. J. Energy Res. 2015, 39, 215–222. [Google Scholar] [CrossRef]
- Jamali, N.S.; Md Jahim, J.; Mumtaz, T.; Abdul, P.M. Dark Fermentation of Palm Oil Mill Effluent by Caldicellulosiruptor Saccharolyticus Immobilized on Activated Carbon for Thermophilic Biohydrogen Production. Environ. Technol. Innov. 2021, 22, 101477. [Google Scholar] [CrossRef]
- Mishra, P.; Hai, T.; Mohamad Zain, J.; Saini, K.; Manoj Kumar, N.; Ab Wahid, Z. Co-Digestion of Domestic Kitchen Food Waste and Palm Oil Mill Effluent for Biohydrogen Production. Sustain. Energy Technol. Assess. 2023, 55, 102965. [Google Scholar] [CrossRef]
- Tan, J.B.; Lutpi, N.A.; Wong, Y.S.; Rahmat, N.R.; Siripatana, C. Study on the Effect of Headspace on Biohydrogen Production Using Palm Oil Mill Effluent (POME) via Immobilized and Suspended Growth. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Penang, Malaysia, 2021; Volume 920. [Google Scholar]
- Hamid, W.Z.W.A.; Lutpi, N.A.; Wong, Y.S.; Ong, S.A.; Malek, M.A. Biohydrogen Production from Palm Oil Mill Effluent with Moringa Oleifera Seeds as Support Carrier in Attached Growth System. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Penang, Malaysia, 2020; Volume 476. [Google Scholar]
- Damayanti, A.; Sarto; Sediawan, W.B.; Syamsiah, S. Performance Analysis of Immobilized and Co-Immobilized Enriched-Mixed Culture for Hydrogen Production. J. Mech. Eng. Sci. 2018, 12, 3515–3528. [Google Scholar] [CrossRef]
- Jamali, N.S.; Jahim, J.M.; Isahak, W.N.R.W.; Abdul, P.M. Particle Size Variations of Activated Carbon on Biofilm Formation in Thermophilic Biohydrogen Production from Palm Oil Mill Effluent. Energy Convers. Manag. 2017, 141, 354–366. [Google Scholar] [CrossRef]
- Ashah, M.A.; Lutpi, N.A.; Wong, Y.S.; Ong, S.A.; Malek, M.A. Study on Biohydrogen Production Using Different Type of Carrier Materials in Attached Growth System. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Penang, Malaysia, 2020; Volume 476. [Google Scholar]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the Biological Hydrogen Production Pathway of Dark Fermentation: Insight into the Impact of Strain Improvement. Microb. Cell Fact. 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Y. Clostridium Species for Fermentative Hydrogen Production: An Overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Krishnan, S.; Kamyab, H.; Nasrullah, M.; Wahid, Z.A.; Yadav, K.K.; Reungsang, A.; Chaiprapat, S. Recent Advances in Process Improvement of Dark Fermentative Hydrogen Production through Metabolic Engineering Strategies. Fuel 2023, 343, 127980. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2023, 52, 335–357. [Google Scholar] [CrossRef]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current Strategies and Future Perspectives in Biological Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Rosales-Colunga, L.M.; De León Rodríguez, A. Escherichia Coli and Its Application to Biohydrogen Production. Rev. Environ. Sci. Biotechnol. 2015, 14, 123–135. [Google Scholar] [CrossRef]
- Valle, A.; Cantero, D.; Bolívar, J. Metabolic Engineering for the Optimization of Hydrogen Production in Escherichia Coli: A Review. Biotechnol. Adv. 2019, 37, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Song, W.; Cheng, J.; Liu, M.; Zhang, C.; Cen, K. Improvement of Fermentative Hydrogen Production Using Genetically Modified Enterobacter Aerogenes. Int. J. Hydrogen Energy 2017, 42, 3676–3681. [Google Scholar] [CrossRef]
- Husaini, C.U.N.; Roslan, R.; Ramzi, A.B.; Luthfi, A.A.I.; Tan, J.P.; Lim, S.S.; Ding, G.T.; Jahim, J.M.; Abdul, P.M. The CRISPR Technology: A Promising Strategy for Improving Dark Fermentative Biohydrogen Production Using Clostridium Spp. Int. J. Hydrogen Energy 2023, 48, 23498–23515. [Google Scholar] [CrossRef]
- Taifor, A.F.; Zakaria, M.R.; Mohd Yusoff, M.Z.; Toshinari, M.; Hassan, M.A.; Shirai, Y. Elucidating Substrate Utilization in Biohydrogen Production from Palm Oil Mill Effluent by Escherichia coli. Int. J. Hydrogen Energy 2017, 42, 5812–5819. [Google Scholar] [CrossRef]
- Ganeshan, P.; Vigneswaran, V.S.; Gowd, S.C.; Kondusamy, D.; Sanjay Kumar, C.; Krishnamoorthy, N.; Kumar, D.; Juneja, A.; Paramasivan, B.; Raju, N.N.; et al. How Does Techno-Economic Analysis and Lifecycle Assessment Help in Commercializing the Biohydrogen Supply Chain? Fuel 2023, 341, 127601. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.; Hewage, K.; Siddiqui, O.; Hettiaratchi, P.; Sadiq, R. Waste-to-Hydrogen Technologies: A Critical Review of Techno-Economic and Socio-Environmental Sustainability. Int. J. Hydrogen Energy 2022, 47, 5842–5870. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. A Well to Pump Life Cycle Environmental Impact Assessment of Some Hydrogen Production Routes. Int. J. Hydrogen Energy 2019, 44, 5773–5786. [Google Scholar] [CrossRef]
- Akhbari, A.; Onn, C.C.; Ibrahim, S. Analysis of Biohydrogen Production from Palm Oil Mill Effluent Using a Pilot-Scale up-Flow Anaerobic Sludge Blanket Fixed-Film Reactor in Life Cycle Perspective. Int. J. Hydrogen Energy 2021, 46, 34059–34072. [Google Scholar] [CrossRef]
- Akhbari, A.; Ibrahim, S.; Ahmad, M.S. Feasibility of Semi-Pilot Scale up-Flow Anaerobic Sludge Blanket Fixed-Film Reactor for Fermentative Bio-Hydrogen Production from Palm Oil Mill Effluent. Renew. Energy 2023, 212, 612–620. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Jahim, J.M.; Abdul, P.M.; Luthfi, A.A.I.; Takriff, M.S. Techno-Economic Analysis of Two-Stage Anaerobic System for Biohydrogen and Biomethane Production from Palm Oil Mill Effluent. J. Environ. Chem. Eng. 2021, 9, 105679. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Arisht, S.N.; Jahim, J.M.; Takriff, M.S.; Tan, J.P.; Luthfi, A.A.I.; Abdul, P.M. Enhancement of Biohydrogen Production from Palm Oil Mill Effluent (POME): A Review. Int. J. Hydrogen Energy 2022, 47, 40637–40655. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Takriff, M.S.; AL-Rajabi, M.M.; Abdul, P.M.; Gunny, A.A.N.; Silvamany, H.; Jahim, J.M. Water Reclamation from Palm Oil Mill Effluent (POME): Recent Technologies, by-Product Recovery, and Challenges. J. Water Process Eng. 2023, 52, 103488. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, M.M.; Martinez-Burgos, W.J.; De Bona Sartor, G.; Letti, L.A.J.; De Carvalho, J.C.; Soccol, C.R.; Medeiros, A.B.P. Advances and Perspectives in Biohydrogen Production from Palm Oil Mill Effluent. Fermentation 2024, 10, 141. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation10030141
Albuquerque MM, Martinez-Burgos WJ, De Bona Sartor G, Letti LAJ, De Carvalho JC, Soccol CR, Medeiros ABP. Advances and Perspectives in Biohydrogen Production from Palm Oil Mill Effluent. Fermentation. 2024; 10(3):141. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation10030141
Chicago/Turabian StyleAlbuquerque, Marcela Moreira, Walter Jose Martinez-Burgos, Gabriela De Bona Sartor, Luiz Alberto Junior Letti, Júlio Cesar De Carvalho, Carlos Ricardo Soccol, and Adriane Bianchi Pedroni Medeiros. 2024. "Advances and Perspectives in Biohydrogen Production from Palm Oil Mill Effluent" Fermentation 10, no. 3: 141. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation10030141