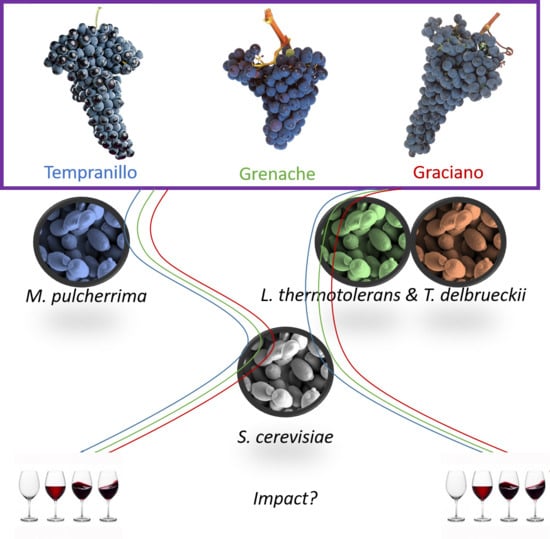
Do Non-Saccharomyces Yeasts Work Equally with Three Different Red Grape Varieties?
Abstract
:1. Introduction
2. Material and Methods
2.1. Grapes and Initial Must Samples of the Three Varieties
2.2. Yeast Species
2.3. Inoculation Procedure and Alcoholic Fermentation
2.4. Analytical Techniques
2.5. Malolactic Fermentation
2.6. Statistical Treatment
3. Results
3.1. Musts Physicochemical Characterization
3.2. Control of Yeast Populations and AF Kinetics in Each Grape Variety
3.2.1. Tempranillo
3.2.2. Grenache
3.2.3. Graciano
3.3. Characterisation of Wines
4. Discussion
4.1. Yeasts Establishment and Fermentation Kinetics
4.2. Discriminant Analysis of Wines after AF
4.2.1. Statistical Analysis of Oenological Parameters
4.2.2. Statistical Analysis of Colour Parameters
4.2.3. Statistical Analysis of Aromatic Profile
4.3. Discriminant Analysis of Wines after MLF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The Genetics of Non-Conventional Wine Yeasts: Current Knowledge and Future Challenges. Front. Microbiol. 2015, 6, 1563. [Google Scholar] [CrossRef] [PubMed]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of Sequential Inoculation (Torulaspora delbrueckii /Saccharomyces cerevisiae) in the First Fermentation on the Foaming Properties of Sparkling Wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Toit, M.D.; Vrhovsek, U. Effect of Non- Saccharomyces Yeasts on the Volatile Chemical Profile of Shiraz Wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Sadineni, V.; Kondapalli, N.; Obulam, V.S.R. Effect of Co-Fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the Aroma and Sensory Properties of Mango Wine. Ann. Microbiol. 2012, 62. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans Yeast Strains as an Alternative to the Traditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [Green Version]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces Cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Commission Regulation. Commission Regulation (EC) No 606/2009 of 10 July 2009 Laying down Certain Detailed Rules for Implementing Council Regulation (EC) No 479/2008 as Regards the Categories of Grapevine Products, Oenological Practices and the Applicable Restrictions. Off. J. Eur. Union 2009, 193, 1–59. [Google Scholar]
- Aerny, J. Composés Azotés Des Moûts et Des Vins. Rev. Suisse Vitic. Arboric. Hortic. 1996, 28, 161–165. [Google Scholar]
- López, I.; Torres, C.; Ruiz-Larrea, F. Genetic Typification by Pulsed-Field Gel Electrophoresis (PFGE) and Randomly Amplified Polymorphic DNA (RAPD) of Wild Lactobacillus plantarum and Oenococcus oeni Wine Strains. Eur. Food Res. Technol. 2008, 227, 547–555. [Google Scholar] [CrossRef]
- Cocolin, L.; Dolci, P.; Rantsiou, K.; Urso, R.; Cantoni, C.; Comi, G. Lactic acid bacteria ecology of three traditional fermented sausages produced in the North of Italy as determined by molecular method. Meat Sci. 2009, 82, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Portu, J.; Garijo, P.; López, R.; Santamaría, P.; López-Alfaro, I.; Gutiérrez, A.R.; González-Arenzana, L. Effect of the Sequential Inoculation of Non-Saccharomyces/Saccharomyces on the Anthocyans and Stilbenes Composition of Tempranillo Wines. Front. Microbiol. 10 APR 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine Aroma Evolution throughout Alcoholic Fermentation Sequentially Inoculated with Non- Saccharomyces/Saccharomyces Yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L. Direct Profiling of the Yeast Dynamics in Wine Fermentations. FEMS Microbiol. Lett. 2000, 189, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lipka, Z.; Tanner, H. Une Nouvelle Méthode de Dosage Rapide de l’acide Tartrique Dans Les Moûts, Les Vins et Autres Boissons (Selon Rebelein). Rev. Suisse de Vitic. Arboric. Hortic. 1974, 6, 5–10. [Google Scholar]
- Somers, T.C.; Evans, E. Wine Quality: Correlations with Colour Density and Anthocyanin Equilibria in a Group of Young Red Wines. J. Sci. Food Agric. 1974, 25, 1369–1379. [Google Scholar] [CrossRef]
- Glories, Y. Recherches Sur La Matière Colorantes Des Vins Rouges. Thesis, Université de Bourdeaux II, Bordeaux, French, 1978. [Google Scholar]
- Ruiz, M. La Cata y El Conocimento de Los Vinos; Madrid Mundi Prensa: Madrid, Spain, 1999. [Google Scholar]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast Analysis of Important Wine Volatile Compounds. Development and Validation of a New Method Based on Gas Chromatographic-Flame Ionisation Detection Analysis of Dichloromethane Microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Portillo, M.C.; Franquès, J.; Araque, I.; Reguant, C.; Bordons, A. Bacterial Diversity of Grenache and Carignan Grape Surface from Different Vineyards at Priorat Wine Region (Catalonia, Spain). Int. J. Food Microbiol. 2016, 219, 56–63. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Revilla, E.; Cabello, F. Anthocyanin Pattern of Several Red Grape Cultivars and Wines Made from Them. Eur. Food Res. Technol. 2002, 215, 32–37. [Google Scholar] [CrossRef]
- Giudici, P.; Zambonelli, C.; Kunkee, R.E. Increased Production of N-Propanol in Wine by Yeast Strains Having an Impaired Ability to Form Hydrogen Sulfide. Am. J. Enol. Vitic. 1993, 44, 17–21. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative Study of Aromatic Compounds in Two Young White Wines Subjected to Pre-Fermentative Cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Etievant, P.X. Wine. In Volatile Compounds in Food; Maarse, Ed.; Food Science and Technology: New York, NY, USA, 1991. [Google Scholar]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escribano-Viana, R.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R.; González-Arenzana, L. Do Non-Saccharomyces Yeasts Work Equally with Three Different Red Grape Varieties? Fermentation 2020, 6, 3. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation6010003
Escribano-Viana R, Garijo P, López-Alfaro I, López R, Santamaría P, Gutiérrez AR, González-Arenzana L. Do Non-Saccharomyces Yeasts Work Equally with Three Different Red Grape Varieties? Fermentation. 2020; 6(1):3. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation6010003
Chicago/Turabian StyleEscribano-Viana, Rocío, Patrocinio Garijo, Isabel López-Alfaro, Rosa López, Pilar Santamaría, Ana Rosa Gutiérrez, and Lucía González-Arenzana. 2020. "Do Non-Saccharomyces Yeasts Work Equally with Three Different Red Grape Varieties?" Fermentation 6, no. 1: 3. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation6010003