1. Introduction
Immune checkpoint inhibitors (ICIs) belong to a novel cluster of anticancer drugs targeting T-cell proteins implicated in the activation of immune response against malignancies. Their use in clinical practice has been a milestone in current cancer treatment. Nevertheless, the significant advantage of ICIs over classical chemotherapy related to therapeutic efficacy comes with new challenges related to specific side effects. CTLA-4 (cytotoxic T lymphocyte associated antigen 4) and PD-1L (Programmed Death 1 Ligand) play as immune system activation checkpoints that maintain immune tolerance and prevent autoimmunity. However, these pathways are sometimes used by tumor cells to develop immune response evasion mechanisms, generating tolerance over these malignant cells and allowing their survival, favoring tumor progression [
1,
2,
3].
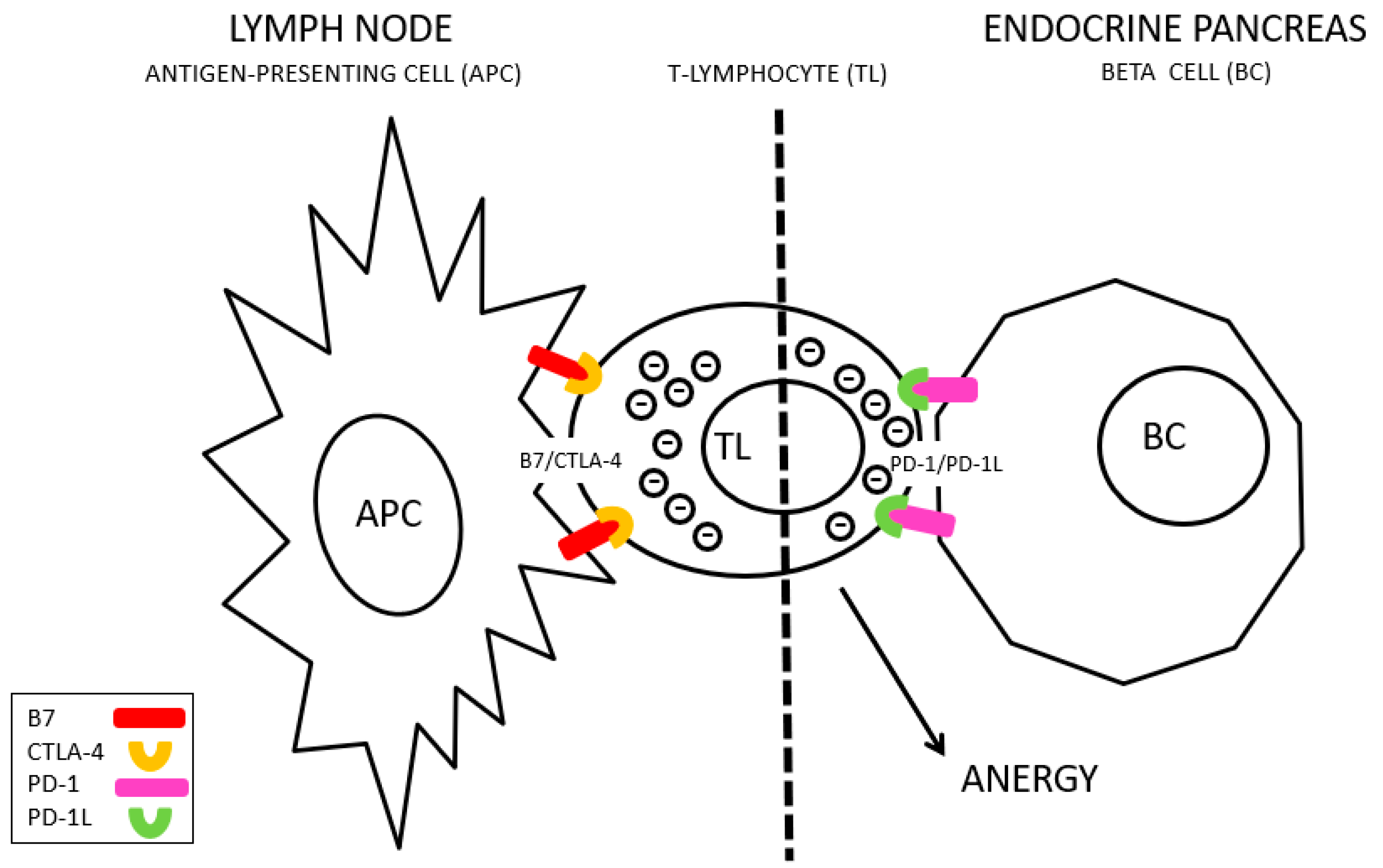
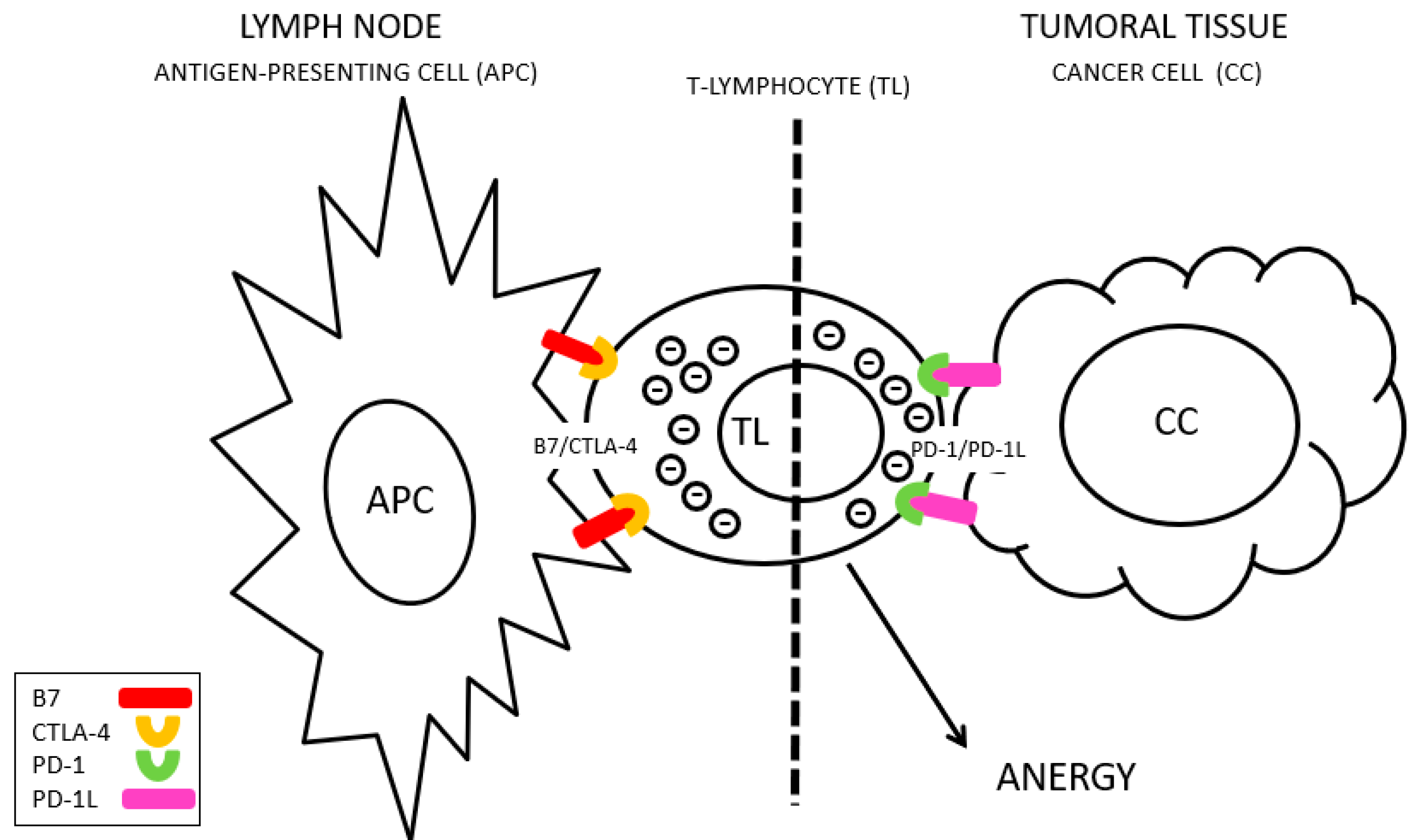
CTLA-4 interacts with non-MHC receptors during antigenic presentation (known as B7) to inhibit the activation of T lymphocytes [
4] (
Figure 1), whereas PD-1L is frequently overexpressed in tumor cells, facilitating their survival after response of the immune system, and favoring tumor progression [
5] (
Figure 2). Tremelimumab is a monoclonal antibody against CTLA-4 and Durvalumab is an anti-PD-L1 antibody. Blocking these molecules improves the immune response against cancer cells, increasing the time of clinical remission and the survival of these patients [
6] (
Figure 3). However, adverse events related to this abnormal activation of the immune system have been described, including autoimmunity against endocrine glands such as lymphocytic hypophysitis, thyroiditis and adrenalitis [
7] (
Figure 4).
2. Case Presentation
This is the case of a 45-year-old woman with a previous diagnosis of lung cancer (carcinoid) and metastatic liver progression. As data of interest, this patient suffered a self-limited process of autoimmune hyperthyroidism (Graves-Basedow disease) after a regular treatment, 24 months earlier. She had no history of another autoimmune disease or recognized hyperglycemia. Random baseline blood glucose levels during previous months were 92, 100, 87, and 99 mg/dL. Previous Thyroid-stimulating hormone (TSH) was within normal limits (1.06 and 1.17 mUI/L). HLA typing, TPOs, basal cortisol, and ACTH were not verified before Tremelimumab/Durvalumab administration. Her body mass index (BMI) was 27 kg/m2 and she had no family history of autoimmune disease.
In relation to her cancer disease, during previous years she received surgical treatment (left hepatectomy and segment VII nodulectomy) but also medical. She initially received somatostatin analogues and everolimus for several months, with a slow clinical-radiological progression. Later, after confirmed disease progression, she was recruited for a clinical trial with Tremelimumab/Durvalumab, as a third-line treatment, which was suspended due to tumor progression after 2 cycles. Four weeks after the last dose of Tremelimumab/Durvalumab, symptoms of asthenia, polyuria, and polydipsia began, and a baseline glycemia of 384 mg/dL, pH 7.02, corresponded to the diagnosis of diabetic debut in ketoacidosis.
After studies, a C peptide of 0.59 corresponding to an insufficient pancreatic reserve was observed, and the presence of an intense autoimmunity against beta islets (antibodies against glutamic acid decarboxylase, GAD65 > 2000 WHO U/mL). These data were compatible with autoimmune diabetes. Insulin needs according to this diagnosis were observed (0.5 IU/kg/day).
During this hospital admission, other studies were carried out, including thyroid function tests. Elevated TSH, low FT4 and anti-peroxidase antibodies > 600 compatible with autoimmune hypothyroidism were identified. During the following 2 months, it was necessary to adjust the replacement hormone treatment to a final dose of 125 µg/24 h FT4 in a single morning intake corresponding to the full needs of thyroid hormone by the patient.
Three months after the diagnosis of T1D and autoimmune hypothyroidism, polyuria and polydipsia began with up to 5 L of water per day. Having ruled out decompensated T1D as a cause of clinical situation, the laboratory tests showed hypernatremia and plasma hyperosmolality as well as a low urinary osmolality (specific density < 1.005), compatible with diabetes insipidus. A pituitary MRI performed during said admission showed the presence of an evident thickening of the pituitary stalk (5 mm) and the absence of the hyperintense signal of the neurohypophysis compatible with probably lymphocytic hypophysitis with autoimmune etiology. On the other hand, the presence of other lesions in the pituitary that justified the symptoms was not demonstrated. After starting treatment with oral desmopressin (0.2 mg daily), a satisfactory clinical response was achieved, maintaining diuresis of 2–3 L/24 h, and laboratory tests, with normalization of natraemia and plasma and urinary osmolality. A control MRI was performed at 4 months, observing persistence of the pituitary stalk thickening without significant changes.
3. Discussion
The immune system has two complementary functions. It must identify foreign or abnormal elements to the organism to which it belongs (bacteria, viruses, tumor cells), control, and eventually destroy them (immune response). On the other hand, the immune system identifies healthy structures belonging to said organism and respects them (immunological tolerance). This narrow field where the functionality of the immune system is located has two frontiers: the inability to respond adequately to nosological threats, which would imply a certain degree of immunodeficiency, and on the other side, the inability to respect healthy own structures, which would imply the processes of autoimmunity.
There are key points in the regulation of the immune system whose alteration is related to the inability to tolerate adequately. CTLA-4 is a protein expressed in T lymphocytes (CD4+ or helper, CD8+ or cytotoxic and others), and its function is to modulate the activation of the immune system. Moreover, PD-1, which is a membrane protein in T lymphocytes, and its interaction with its PD-L1 (Programmed Death-ligand 1) ligand, overexpressed in tumor cells, transfers an inhibition signal to lymphocytes that reduces its activity, blocking the immune response. The activation of PD-L1 in these malignant cells transmits signals that facilitate cell proliferation and tumor survival [
4].
Immunotherapy with Tremelimumab (anti-CTLA-4 antibodies) and Durvalumab (anti-PD-L1 antibodies) has proven to be effective in cancer, increasing the activation capacity of the immune system and generating a more effective antitumor response and reducing the mechanisms of evasion of the immune response by tumor cells but this implies a potential alteration of immunological tolerance. A large number of autoimmune-related adverse events have been reported in patients receiving anti-CTLA-4 therapy, including colitis, arthritis, hepatitis, skin reactions, uveitis, pancreatitis, and nephritis. From an endocrine perspective, cases of autoimmune diabetes, lymphocytic hypophysitis, thyroiditis, and adrenalitis have been described [
7].
This article describes the correlation over time of two doses of Tremelimumab and Durvalumab in the context of an immunotherapy treatment of advanced cancer and the development of different hypophysis, thyroid and pancreatic autoimmune processes previously absent. CTLA-4 is a gene formerly associated with susceptibility to the development of DM1 as well as with thyroid autoimmunity. As etiopathogenic possibility contemplated that a deficient activity of CTLA-4, mediated by different polymorphisms, affects immunological tolerance and facilitates autoimmunity. In addition, other autoimmune diseases have been related to anti-CTLA-4 treatments, which also relate their CTLA-4 polymorphisms with susceptibility for the development of autoimmunity [
8]. In the same way, polymorphisms associated with the functionality of PD-1 have also been related to an increase in susceptibility for the development of DM1 [
9]. The activation of autoimmunity and associated diseases are situations that are generally polygenic, and the defective function of CTLA-4 and PD-1L (either due to susceptibility polymorphism or mediated by an immunotherapy treatment that blocks their function), possibly de-structure correct mechanisms immunological tolerance that are usually present causing these autoimmune processes (
Figure 5).
4. Conclusions
It is increasing the number of patients who need these immunotherapeutic treatments with an augmented risk of autoimmune endocrine disease. They could benefit from a closer follow-up in collaboration with endocrinologists and oncologists for an optimal approach to this type of pathology.