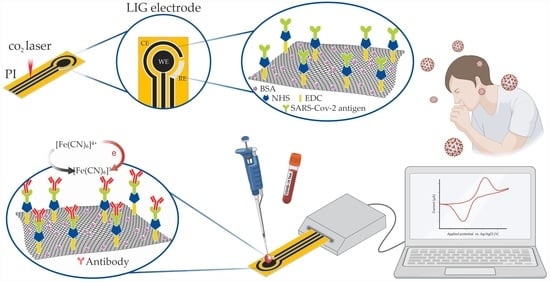
Electrochemical Biosensor Based on Laser-Induced Graphene for COVID-19 Diagnosing: Rapid and Low-Cost Detection of SARS-CoV-2 Biomarker Antibodies
Abstract
:1. Introduction
2. Experimental Section
2.1. Biosensor Preparation
2.1.1. Electrode Fabrication
2.1.2. Biofunctionalization of the Working Electrode
2.2. Detection of SARS-CoV-2 Antibodies in Human Serums
2.3. Characterization
2.3.1. Morphological and Structural Characterizations
2.3.2. Electrochemical Characterizations
2.4. Fabrication of Portable Microcontrolled Potentiostat for SARS-CoV-2 Antibodies Diagnosing
3. Results and Discussion
3.1. Structural and Morphological Results
3.2. Electrochemical Results
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Research on Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov (accessed on 20 February 2021).
- Hallal, P.C. Worldwide differences in COVID-19-related mortality. Cienc Saude Coletiva 2020, 1, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Datta, B.; Ashish, A.; Dutta, G. A comprehensive review on current COVID-19 detection methods: From lab care to point of care diagnosis. Sens. Int. 2021, 2, 100119. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Barros, F.C.; Silveira, M.F.; Barros, A.J.D.; Dellagostin, O.A.; Pellanda, L.C.; Struchiner, C.J.; Burattini, M.N.; Hartwig, F.P.; Menezes, A.M.B.; et al. EPI COVID-19 protocol: Repeated serological surveys on SARS-CoV-2 antibodies in Brazil. Cienc Saude Coletiva 2020, 9, 3573–3578. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.-J.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coron.avirus-2019 (accessed on 12 January 2022).
- Mahase, E. Coronavirus: COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 2020, 368, 641. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Middle East Respiratory Syndrome Coronavirus (MERS-CoV)—United Arab Emirates. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/middle-east-respiratory-syndrome-coronavirus-(mers-cov)-united-arab-emirates (accessed on 12 January 2022).
- Iravani, S. Nano- and biosensors for the detection of SARS-CoV-2: Challenges and opportunities. Mater. Adv. 2020, 1, 3092–3103. [Google Scholar] [CrossRef]
- Traugott, M.; Aberle, S.W.; Aberle, J.H.; Griebler, H.; Karolyi, M.; Pawelka, E.; Puchhammer-Stöckl, E.; Zoufaly, A.; Weseslindtner, L. Performance of Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Assays in Different Stages of Infection: Comparison of Commercial Enzyme-Linked Immunosorbent Assays and Rapid Tests. J. Infect. Dis. 2020, 222, 362–366. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Xu, X.; Liao, G.; Chen, Y.; Hu, C.H. Patterns of IgG and IgM antibody response in COVID-19 patients 2020. Emerg. Microbes Infect. 2020, 9, 1269–1274. [Google Scholar] [CrossRef]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic Immunoassays for Sensitive and Simultaneous Detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioeletronics 2021, 171, 112912. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Dincer, C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelétron. 2020, 163, 112274. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Behbahan, N.G.G.; Bahrani, S.; Mousavi, S.M.; Gholami, A.; Ramakrishna, S.; Firoozsani, M.; Moghadami, M.; Lankarani, K.B.; Omidifar, N. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens. Bioelectron. 2021, 171, 112731. [Google Scholar] [CrossRef] [PubMed]
- Suleman, S.; Shukla, S.K.; Malhotra, N.; Bukkitgar, S.D.; Shetti, N.P.; Pilloton, R.; Narang, J.; Tan, Y.N.; Aminabhavi, T.M. Point of care detection of COVID-19: Advancement in biosensing and diagnostic methods. Chem. Eng. J. 2021, 414, 128759. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, R.P.; Goud, K.Y.; Mohamed, M.A.; Kummari, S.; Tiwari, S.; Li, Z.; Naravan, R.; Stanciu, L.A.; Marty, J.L. Optical Biosensors for Diagnostics of Infectious Viral Disease: A Recent Update. Diagnostics 2021, 11, 2083. [Google Scholar] [CrossRef]
- Zavyalova, E.; Ambartsumyan, O.; Zhdanov, G.; Gribanyov, D.; Gushchin, V.; Tkachuk, A.; Rudakova, E.; Nikiforova, M.; Kuznetsova, N.; Popova, L.; et al. SERS-Based Aptasensor for Rapid Quantitative Detection of SARS-CoV-2. Nanomaterials 2021, 11, 1394. [Google Scholar] [CrossRef]
- Tyagi, D.; Wang, H.; Huang, W.; HU, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent Advances in Two-Dimensional Materials based Sensing Technology towards Health and Environmental Applications. Nanoescale 2020, 12, 3535–3559. [Google Scholar] [CrossRef]
- Krishinan, S.K.; Singh, E.; Singuh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Graphene-Based Sensors for the Detection of Bioactive Compounds: A Review. Int. J. Mol. Sci. 2021, 22, 3316. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.Y.E.R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Huang, L.; Su, J.; Song, Y.; Ye, R. Laser-Induced Graphene: En Route to Smart Sensing. Nano-Micro Lett. 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Hawes, G.F.; Ylman, D.; Noremberg, B.S.; Pope, M.A. Supercapacitors Fabricated via Laser-Induced Carbonization of Biomass-Derived Poly (furfuryl alcohol)/Graphene Oxide Composites. ACS Appl. Nano Mater. 2019, 2, 6312–6324. [Google Scholar] [CrossRef]
- Segura, B.; Jimenez, F.N.; Giraldo, L.R. Prototipo de potenciostato con aplicaciones en procesos electroquímicos. Entre Cienc. E Ing. 2016, 10, 61–69. [Google Scholar]
- Lathi, B.P. Signals and Linear Systems, 2rd ed.; Bookman: Taibei, Taiwan, 2007. [Google Scholar]
- Song, W.; Zhu, J.; Gan, B.; Zhao, S.; Wang, H.; Li, C.; Wang, J. Flexible, Stretchable, and Transparent Planar Microsupercapacitors Based on 3D Porous Laser-Induced Graphene. Small 2017, 14, 1702249. [Google Scholar] [CrossRef]
- Wang, W.; Lu, L.; Xie, Y.; Mei, X.; Tang, Y.; Wu, W.; Liang, R. Tailoring the surface morphology and nanoparticle distribution of laser-induced graphene/CO3O4 for high-performance flexible microsupercapacitors. Appl. Surf. Sci. 2020, 504, 144487. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. CS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Tesing, A.; Loguercio, L.F.; Noremberg, B.S.; Alano, J.H.; Silva, R.M.; Orlandi, M.O.; Marin, G.; Santos, J.F.L.; Carreño, N.L.V. Tunable graphene oxide inter-sheet distance to obtain graphene oxide–silver nanoparticle hybrids. New J. Chem. 2019, 43, 1285–1290. [Google Scholar] [CrossRef]
- Dosi, M.; Lau, I.; Zhuang, Y.; Simakov, D.S.A.; Fowler, M.W.; Pope, M.A. Ultrasensitive Electrochemical Methane Sensors Based on Solid Polymer Electrolyte-Infused Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2019, 11, 6166–6173. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrent-Rodrigres, R.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.R.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Hermanson, G. Bioconjugate Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 259–273. [Google Scholar]
- Liu, E.Y.; Jung, S.; Weitz, D.A.; Yi, H.; Choi, C.-H. High-throughput double emulsion-based microfluidic production of hydrogel microspheres with tunable chemical functionalities toward biomolecular conjugation. Lab Chip 2018, 18, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.M.; Mazon, T. Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor. Talanta 2019, 203, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fleta, P.; Alfranca, A.; González-Álvaro, I.; Casasnovas, J.M.; Fernández-Soto, D.; Esteso, G.; Cáceres-Martell, Y.; Gardeta, S.; López-Sanz, C.; Prat, S.; et al. SARS-CoV-2 cysteine-like protease (Mpro) is immunogenic and can be detected in serum and saliva of COVID-19-seropositive individuals. J. Immunol. 2020, 205, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.C.H.; Alvesa, L.M.; Siquierolia, A.C.S.; Madurrob, J.M.; Brito-Madurroa, A.G. Label-free electrochemical immunosensor for detection of oncomarker CA125 in serum. Microchem. J. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Mikrochim. Acta 2019, 186, 224. [Google Scholar] [CrossRef] [Green Version]
- Beduk, T.; Beduk, B.; Filho, J.I.O.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; Salama, K.N.; et al. Rapid Point-of-Care COVID-19 Diagnosis with a Gold-Nanoarchitecture-Assisted Laser-Scribed Graphene Biosensor. Anal. Chem. 2021, 93, 8585–8594. [Google Scholar] [CrossRef]
- Salman, N.; Khan, M.W.; Lim, M.; Khan, A.; Kemp, A.H.; Noakes, C.J. Use of Multiple LowCost Carbon Dioxide Sensors to Measure Exhaled Breath Distribution with Face Mask Type and Wearing Behaviour. Sensors 2021, 21, 6204. [Google Scholar] [CrossRef]








| Sample Code | Condition | ELISA-LIA Diagnosis (DO ¹/Results) | qRT-PCR Diagnosis | IP 2 (mA) | ||
|---|---|---|---|---|---|---|
| IgG | IgM | IgA | ||||
| NR302 | pre-pandemic | >0.3 (NR) | >0.2 (NR) | >0.2 (NR) | 8.3390 | |
| NR303 | pre-pandemic | >0.7 (NR) | >0.5 (NR) | >0.2 (NR) | - | 7.6060 |
| NR324 | pre-pandemic | >0.2 (NR) | >0.2 (NR) | <0.1 (NR) | - | 9.0160 |
| NR 327 | pre-pandemic | >1.0 (FP) | <0.5 (NR) | >1.0 (FP) | - | 8.4171 |
| NR 328 | pre-pandemic | >2.0 (FP) | <0.5 (NR) | <0.1 (NR) | - | 8.5651 |
| NR 336 | pre-pandemic | >1.0 (FP) | <0.5 (NR) | <0.1 (NR) | - | 7.3086 |
| NR 394 | pre-pandemic | <0.1 (NR) | <0.5 (NR) | <0.1 (NR) | - | 7.2891 |
| R 106 | - | >2.4 (R) | - | - | positive | 9.4739 |
| R 109 | - | >2.8 (R) | - | - | positive | 9.8447 |
| R907B | - | >2.9 (R) | 1.9 (R) | >3.5 (R) | Positive | 10.0650 |
| R908B | - | >2.0 (R) | 1.8 (R) | >2.9 (R) | Positive | 10.0300 |
| R909 | - | >2.9 (R) | >0.6 (FP) | >1.4 (R) | Positive | 9.2733 |
| R 911 | - | >3.5 (R) | >3.0 (R) | >3.5 (R) | positive | 9.8346 |
| R 123 | - | >3.1 (R) | - | - | positive | 9.6368 |
| R1039 | - | >3.5 (R) | >1.7 (R) | >2.9 (R) | positive | 9.3750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.E.; Lopes, B.V.; Rossato, J.H.H.; Maron, G.K.; Gallo, B.B.; La Rosa, A.B.; Balboni, R.D.C.; Alves, M.L.F.; Ferreira, M.R.A.; da Silva Pinto, L.; et al. Electrochemical Biosensor Based on Laser-Induced Graphene for COVID-19 Diagnosing: Rapid and Low-Cost Detection of SARS-CoV-2 Biomarker Antibodies. Surfaces 2022, 5, 187-201. https://0-doi-org.brum.beds.ac.uk/10.3390/surfaces5010012
Oliveira ME, Lopes BV, Rossato JHH, Maron GK, Gallo BB, La Rosa AB, Balboni RDC, Alves MLF, Ferreira MRA, da Silva Pinto L, et al. Electrochemical Biosensor Based on Laser-Induced Graphene for COVID-19 Diagnosing: Rapid and Low-Cost Detection of SARS-CoV-2 Biomarker Antibodies. Surfaces. 2022; 5(1):187-201. https://0-doi-org.brum.beds.ac.uk/10.3390/surfaces5010012
Chicago/Turabian StyleOliveira, Marcely Echeverria, Bruno Vasconcellos Lopes, Jéssica Helisa Hautrive Rossato, Guilherme Kurz Maron, Betty Braga Gallo, Andrei Borges La Rosa, Raphael Dorneles Caldeira Balboni, Mariliana Luiza Ferreira Alves, Marcos Roberto Alves Ferreira, Luciano da Silva Pinto, and et al. 2022. "Electrochemical Biosensor Based on Laser-Induced Graphene for COVID-19 Diagnosing: Rapid and Low-Cost Detection of SARS-CoV-2 Biomarker Antibodies" Surfaces 5, no. 1: 187-201. https://0-doi-org.brum.beds.ac.uk/10.3390/surfaces5010012