VEGF Expression in Colorectal Cancer Metastatic Lymph Nodes: Clinicopathological Correlation and Prognostic Significance
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Sample
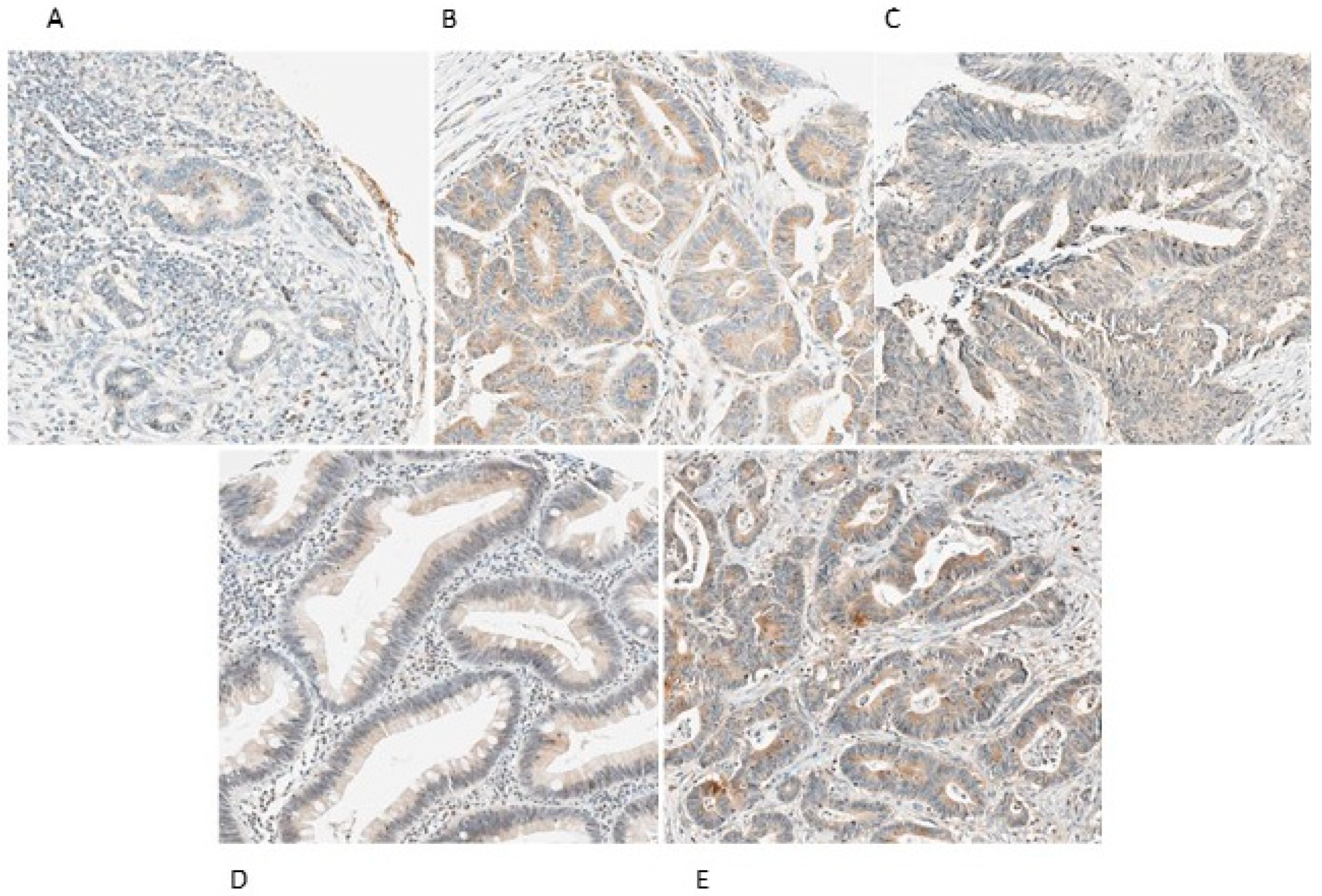
2.2. VEGF-A, VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 Expression in CRC Lymph Nodes Metastasis
2.3. Associations between VEGF-A, VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 Expression in Lymph Nodes Metastasis of CRC and Clinicopathological Characteristics
2.4. Associations Between VEGF-A, VEGF-C, VEGF-D and VEGFR-2, VEGFR-3 Expression in Lymph Nodes of CRC
2.5. Association between VEGF-A, VEGF-C, VEGF-D and VEGFR-2, VEGFR-3 Expression in CRC Primary Tumor and Respective Lymph Nodes Metastasis
2.6. Lack of Relationship between Overall CRC Survival and Expression of VEGF-A, VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 in CRC Lymph Nodes Metastasis
3. Discussion
4. Materials and Methods
4.1. CRC Primary Tumor and Metastasis Lymph Node Human Samples
4.2. Immunohistochemistry
Immunohistochemical Evaluation
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Des, G.D.; Uzzan, B.; Nicolas, P.; Cucherat, M.; Morere, J.F.; Benamouzig, R.; Breau, J.-L.; Perret, G.-Y. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br. J. Cancer 2006, 19, 1823–1832. [Google Scholar]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Rodrigues, O. Colon cancer: ESMO Clinical Recommendations for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 2008, 19, ii29. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.; Henson, D.E.; Hutter, R.V.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [PubMed]
- Svagzdys, S.; Lesauskaite, V.; Pavalkis, D.; Nedzelskienė, I.; Pranys, D.; Tamelis, A. Microvessel density as new prognostic marker after radiotherapy in rectal cancer. BMC Cancer 2009, 9, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, S.F.; Reis, R.M.; Rodrigues, A.M.; Baltazar, F.; Longatto, A. Role of endoglin and VEGF family expression in colorectal cancer prognosis and anti-angiogenic therapies. WJC 2011, 10, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.W.; Rood, P. Clinical implications of angiogenesis in cancers. Vasc. Health Risk Manag. 2006, 2, 97–108. [Google Scholar] [CrossRef]
- Myśliwiec, P.; Pawlak, K.; Kukliński, A.; Kedra, B. Combined perioperative plasma endoglin and VEGF-a assessment in colorectal cancer patients. Folia Histochem. Cytobiol. 2009, 47, 231–236. [Google Scholar] [CrossRef]
- De Vita, F.; Orditura, M.; Lieto, E.; Infusino, S.; Morgillo, F.; Martinelli, E.; Castellano, P.; Romano, C.; Ciardiello, F.; Catalano, G.; et al. Elevated perioperative serum vascular endothelial growth factor levels in patients with colon carcinoma. Cancer 2004, 100, 270–278. [Google Scholar] [CrossRef]
- Zheng, S.; Han, M.Y.; Xiao, Z.X.; Peng, J.P.; Dong, Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J. Gastroenterol. 2003, 9, 1227–1230. [Google Scholar] [CrossRef]
- Kannarkatt, J.; Joseph, J.; Kurniali, P.C.; Al-Janadi, A.; Hrinczenko, B. Adjuvant chemotherapy for stage II colon cancer: A clinical dilemma. J. Oncol. Pract. 2017, 13, 233–241. [Google Scholar] [CrossRef]
- Hanrahan, V.; Currie, M.J.; Gunningham, S.P.; Morrin, H.R.; Scott, P.A.; Robinson, B.; Fox, S.B. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J. Pathol. 2003, 100, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.F.; García, E.A.; Luz, M.A.M.; Pardal, F.; Rodrigues, M.; Filho, A.L.L. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genom. Proteom. 2013, 10, 55–67. [Google Scholar]
- Liang, J.-F.; Wang, H.-K.; Xiao, H.; Li, N.; Cheng, C.-X.; Zhao, Y.-Z.; Ma, Y.-B.; Gao, J.-Z.; Bai, R.-B.; Zheng, H.-X. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J. Exp. Clin. Cancer Res. 2010, 29, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurzu, S.; Jung, J.; Azamfirei, L.; Mezei, T.; Cîmpean, A.M.; Szentirmay, Z. The angiogenesis in colorectal carcinomas with and without lymph node metastases. Rom. J. Morphol. Embryol. 2008, 49, 149–152. [Google Scholar] [PubMed]
- Rodrigo, J.P.; Cabanillas, R.; Chiara, M.D.; Pedrero, J.G.; Astudillo, A.; Nieto, C.S. Prognostic significance of angiogenesis in surgically treated supraglottic squamous cell carcinomas of the larynx. Acta Otorrinolaringol. Esp. 2009, 60, 272–277. [Google Scholar] [CrossRef]
- Cascinu, S.; Staccioli, M.P.; Gasparini, G.; Giordani, P.; Catalano, V.; Ghiselli, R.; Rossi, C.; Baldelli, A.M.; Graziano, F.; Saba, V.; et al. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin. Cancer Res. 2000, 6, 2803–2807. [Google Scholar] [PubMed]
- Saad, R.S.; Liu, Y.L.; Nathan, G.; Celebrezze, J.; Celebrezze, J.; Silverman, J.F. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod. Pathol. 2004, 17, 197–203. [Google Scholar] [CrossRef]
- Secker, G.A.; Harvey, N.H. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev. Dyn. 2015, 244, 323–331. [Google Scholar] [CrossRef]
- Miettinen, M.; Rikala, M.-S.; Rys, J.; Lasota, J.; Wang, Z.-F.; Rysz, J. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: An immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am. J. Surg. Pathol. 2012, 36, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Rivard, A.; Berthou-Soulie, L.; Principe, N.; Kearney, M.; Curry, C.; Branellec, D.; Semenza, G.L.; Isner, J.M. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J. Biol. Chem. 2000, 275, 29643–29647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Betsuyaku, T.; Nagai, K.; Nasuhara, Y.; Nishimura, M. Expression of pulmonary VEGF family declines with age and is further down-regulated in lipopolysaccharide (LPS)-induced lung injury. Exp. Gerontol. 2005, 40, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Pola, R.; Aprahamian, T.; Bosch-Marce, M.; Curry, C.; Gaetani, E.; Flex, A.; Smith, R.C.; Isner, J.M.; Losordo, U.W. Age-dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol. Aging 2004, 25, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Talagas, M.; Uguen, A.; Garlantezec, R.; Fournier, G.; Doucet, L.; Gobin, E.; Marcorelles, P.; Volant, A.; De Braekeleer, M. VEGFR1 and NRP1 endothelial expressions predict distant relapse after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2013, 33, 2065–2075. [Google Scholar]
- Bolander, A.; Wagenius, G.; Larsson, A.; Brattström, D.; Ullenhag, G.; Hesselius, P.; Ekman, S.; Bergqvist, M. The role of circulating angiogenic factors in patients operated on for localized malignant melanoma. Anticancer. Res. 2007, 27, 3211–3217. [Google Scholar]
- Berardi, R.; Torniai, M.; Partelli, S.; Rubini, C.; Pagliaretta, S.; Savini, A.; Polenta, V.; Santoni, M.; Giampieri, R.; Onorati, S.; et al. Impact of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) single nucleotide polymorphisms on outcome in gastroenteropancreatic neuroendocrine neoplasms. PLoS ONE 2018, 13, e0197035. [Google Scholar] [CrossRef]
- Saharinen, P.; Tammela, T.; Karkkainen, M.J.; Alitalo, K. Lymphatic vasculature: Development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004, 25, 387–395. [Google Scholar] [CrossRef]
- Folpe, A.L.; Veikkola, T.; Valtola, R.; Weiss, S.W. Vascular endothelial growth factor receptor-3 (VEGFR-3): A marker of vascular tumors with presumed lymphatic differentiation, including Kaposi’s sarcoma, kaposiform and Dabska-type hemangioendotheliomas, and a subset of angiosarcomas. Mod. Pathol. 2000, 13, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Alessi, C.; Neto, C.S.; Viana, C.R.; Vazquez, V.D.L. PD-1/PD-L1 and VEGF-A/VEGF-C expression in lymph node microenvironment and association with melanoma metastasis and survival. Melanoma Res. 2017, 27, 565–572. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, Y.; Li, W.; Wang, X.; Liu, X.; Chen, Y.; Ouyang, C.; Wang, J. Quantification of STAT3 and VEGF expression for molecular diagnosis of lymph node metastasis in breast cancer. Medicine (Baltimore) 2017, 96, e8488. [Google Scholar] [CrossRef]
- Greene, F.; Page, D.; Fleming, I.; Fritz, A.; Balch, C. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Pinheiro, C.; Longatto-Filho, A.; Cristovam, S.; Ferreira, L.; Martins, S.; Pellerin, L.; Rodrigues, M.; Alves, A.F.V.; Schimtt, F.; Baltazar, F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008, 452, 139–146. [Google Scholar] [CrossRef] [PubMed]
| Clinical Data and Pre-Operative Exams Results | n (%) | |
| Gender | Male | 131 (62.4) |
| Female | 79 (37.6) | |
| Age (years) | ≤45 | 15 (7.1) |
| >45 | 195 (92.9) | |
| Personal history of CRC | Negative | 182 (86.7) |
| Positive | 28 (13.3) | |
| Familiar history of CRC | Negative | 172 (81.9) |
| Positive | 22 (10.5) | |
| Clinical presentation | Asymptomatic | 37 (17.6) |
| Symptomatic | 173 (82.4) | |
| Tumor localization | Right colon | 50 (23.8) |
| Left colon | 111 (52.9) | |
| Rectum | 49 (23.3) | |
| Preoperative value of CEA | ≤10 ng/mL | 134 (63.8) |
| >10 ng/mL | 38 (18.1) | |
| Metastasis at diagnosis | Yes | 70 (33.3) |
| No | 140 (66.7) | |
| Histopathological report | ||
| Tumor size (mm) | ≤45 | 118 (56.2) |
| >45 | 79 (37.6) | |
| Tumor Macroscopic aspect | Polypoid | 91 (43.3) |
| Ulcerative | 53 (25.2) | |
| Infiltrative | 19 (9) | |
| Exophytic | 23 (11) | |
| Vilosous | 1 (0.5) | |
| Tumor Histological type | Adenocarcinoma | 179 (85.2) |
| Mucinous | 27 (12.9) | |
| Signet ring cells and mucinous areas | 4 (1.9) | |
| Tumor differentiation | Well | 74 (35.2) |
| Moderate | 93 (44.3) | |
| Poor | 37 (17.6) | |
| Undifferentiated | 2 (1) | |
| Unknown | 4 (1.9) | |
| Invasion of lymphatic vessels | Absent | 19 (9) |
| Present | 172 (82) | |
| Invasion of venous vessels | Absent | 70 (33.3) |
| Present | 132 (62.9) | |
| Tumor staging | Stage III | 160 (76.2) |
| Stage IV | 50 (23.8) | |
| Follow-up data | ||
| Relapse | Yes | 45 (21.4) |
| No | 165 (78.6) | |
| Death | Alive | 106 (50.5) |
| Dead | 104 (49.5) | |
| Protein Marker | Immunoreaction | |
|---|---|---|
| n | n Positive (%) | |
| VEGF-A | 136 | 124 (59.0%) |
| VEGF-C | 133 | 115 (54.8%) |
| VEGF-D | 113 | 88 (41.9%) |
| VEGFR-2 | 64 | 53 (25.2%) |
| VEGFR-3 | 104 | 83 (39.5%) |
| Protein Marker | VEGF-A | VEGF-C | VEGF-D | VEGFR-2 | VEGFR-3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | |
| Gender | |||||||||||||||
| Male | 83 | 92.8 | 0.412 | 86 | 84.9 | 0.471 | 75 | 76 | 0.5 | 41 | 78 | 0.301 * | 67 | 77.6 | 0.453 |
| Female | 53 | 88.7 | 47 | 89.4 | 38 | 81.6 | 23 | 91.3 | 37 | 83.8 | |||||
| Age, years | |||||||||||||||
| ≤ 45 | 7 | 42.9 | 0.001 * | 9 | 88.9 | 1.000 * | 10 | 70 | 0.689 * | 6 | 83.3 | 1.000 * | 8 | 87.5 | 1.000 * |
| > 45 | 129 | 93.8 | 124 | 86.3 | 103 | 78.6 | 58 | 82.8 | 96 | 79.2 | |||||
| CRC Personal history | |||||||||||||||
| Negative | 133 | 91 | 1.000 * | 130 | 86.2 | 1.000 * | 110 | 77.3 | 1.000 * | 63 | 82.5 | 1.000 * | 101 | 79 | 1.000 * |
| Positive | 3 | 100 | 3 | 100 | 3 | 100 | 1 | 100 | 3 | 100 | |||||
| CRC Familiar history | |||||||||||||||
| Negative | 112 | 90.2 | 0.603 * | 109 | 87.2 | 1.000 * | 91 | 79.1 | 0.218 | 53 | 81.1 | 1.000 * | 82 | 80.5 | 0.504 * |
| Positive | 13 | 100 | 15 | 86.7 | 14 | 64.3 | 9 | 88.9 | 15 | 73.3 | |||||
| Relapse | |||||||||||||||
| No | 110 | 91.8 | 0.699 * | 111 | 86.5 | 1.000 * | 94 | 80.9 | 0.09 | 59 | 86.4 | 0.032 * | 86 | 83.7 | 0.03 |
| Yes | 26 | 88.5 | 22 | 86.4 | 19 | 63.2 | 5 | 40 | 18 | 61.1 | |||||
| Death | |||||||||||||||
| Alive | 92 | 91.3 | 1.000 * | 89 | 86.5 | 0.981 | 75 | 78.7 | 0.776 | 49 | 87.8 | 0.058 | 68 | 80.9 | 0.708 |
| Dead | 44 | 90.9 | 44 | 86.4 | 38 | 76.3 | 15 | 66.7 | 36 | 77.8 | |||||
| Protein Marker | VEGF-A | VEGF-C | VEGF-D | VEGFR-2 | VEGFR-3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | |
| Presentation | |||||||||||||||
| Asymptomatic | 25 | 88 | 0.462 * | 22 | 86.4 | 1.000 * | 15 | 80 | 1.000 * | 14 | 78.6 | 0.693 * | 17 | 88.2 | 0.513 * |
| Symptomatic | 111 | 91.9 | 111 | 86.5 | 98 | 77.6 | 50 | 84 | 87 | 78.2 | |||||
| Localization | |||||||||||||||
| Right Colon | 31 | 93.5 | 0.772 | 35 | 85.7 | 0.152 | 31 | 80.6 | 0.84 | 18 | 88.9 | 0.414 | 32 | 78.1 | 0.213 |
| Left Colon | 70 | 91.4 | 68 | 91.2 | 59 | 78 | 35 | 77.1 | 50 | 86 | |||||
| Rectum | 35 | 88.6 | 30 | 76.7 | 23 | 73.9 | 11 | 90.9 | 22 | 68.2 | |||||
| Metastasis | |||||||||||||||
| Not present | 91 | 91.2 | 1.000 * | 87 | 87.4 | 0.68 | 73 | 79.5 | 0.586 | 39 | 87.2 | 0.247 | 67 | 74.6 | 0.124* |
| Present | 45 | 91.1 | 46 | 84.8 | 40 | 75 | 25 | 76 | 37 | 89.2 | |||||
| Macroscopic Appearance | |||||||||||||||
| Polypoid | 60 | 98.3 | 0.072 | 57 | 86 | 0.737 | 48 | 79.2 | 0.417 | 23 | 95.7 | 0.383 | 40 | 75 | 0.083 |
| Ulcerative | 35 | 82.9 | 30 | 93.3 | 28 | 71.4 | 24 | 75 | 30 | 86.7 | |||||
| Infiltrative | 15 | 86.7 | 13 | 84.6 | 10 | 100 | 5 | 80 | 11 | 90.9 | |||||
| Exophytic | 10 | 80 | 15 | 80 | 14 | 78.6 | 5 | 80 | 12 | 50 | |||||
| Vilosous | 1 | 100 | 1 | 100 | 1 | 100 | 1 | 100 | 1 | 100 | |||||
| CEA ( ng/mL) | |||||||||||||||
| ≤10 | 88 | 93.2 | 0.424 * | 82 | 86.6 | 1.000 * | 68 | 77.9 | 0.546 * | 42 | 81 | 0.665 * | 62 | 79 | 0.543 * |
| >10 | 26 | 88.5 | 25 | 88 | 21 | 85.7 | 12 | 91.7 | 22 | 86.4 | |||||
| Protein Marker | VEGF-A | VEGF-C | VEGF-D | VEGFR-2 | VEGFR-3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | |
| Tumor size (cm) | |||||||||||||||
| ≤ 4.5 | 72 | 91.7 | 0.905 | 73 | 89 | 0.329 | 62 | 83.9 | 0.101 | 38 | 89.5 | 0.096 * | 57 | 86 | 0.145 |
| > 4.5 | 56 | 91.1 | 53 | 83 | 48 | 70.8 | 25 | 72 | 43 | 74.4 | |||||
| Histological Type | |||||||||||||||
| Adenocarcinoma | 119 | 91.6 | 0.741 | 114 | 87.7 | 0.383 | 95 | 77.9 | 0.724 | 52 | 82.7 | 0.898 | 90 | 81.1 | 0.535 |
| Mucinous | 15 | 86.7 | 17 | 76.5 | 16 | 75 | 11 | 81.8 | 13 | 69.2 | |||||
| Signet ring cells and mucinous areas | 2 | 100 | 2 | 100 | 2 | 100 | 1 | 100 | 1 | 100 | |||||
| Differentiation | |||||||||||||||
| Well | 54 | 92.6 | 0.773 | 52 | 88.5 | 0.91 | 42 | 78.6 | 0.283 | 24 | 87.5 | 0.731 | 41 | 82.9 | 0.105 |
| Moderate | 60 | 88.3 | 55 | 87.3 | 49 | 83.7 | 29 | 79.3 | 43 | 83.7 | |||||
| Poor | 19 | 94.7 | 24 | 83.3 | 21 | 66.7 | 11 | 81.8 | 19 | 68.4 | |||||
| Undifferentiated | 1 | 100 | 1 | 100 | 0 | 0 | 0 | 0 | 1 | 0 | |||||
| Lymph invasion | |||||||||||||||
| Absent | 12 | 91.7 | 1.000 * | 11 | 100 | 0.360 * | 7 | 85.7 | 1.000 * | 2 | 100 | 1.000 * | 8 | 87.5 | 1.000 * |
| Present | 109 | 89.9 | 110 | 84.5 | 93 | 78.5 | 58 | 81 | 83 | 79.5 | |||||
| Venous invasion | |||||||||||||||
| Absent | 44 | 95.5 | 0.217* | 39 | 84.6 | 0.677 | 31 | 77.4 | 0.992 | 19 | 84.2 | 1.000 * | 29 | 75.9 | 0.576 |
| Present | 84 | 88.1 | 87 | 87.4 | 75 | 77.3 | 42 | 81 | 68 | 80.9 | |||||
| TNM stage | |||||||||||||||
| Stage III | 104 | 89.4 | 0.293 * | 97 | 85.6 | 0.779* | 80 | 77.5 | 0.881 | 47 | 83 | 1.000 * | 73 | 80.8 | 0.693 |
| Stage IV | 32 | 96.9 | 36 | 88.9 | 33 | 78.8 | 17 | 82.4 | 31 | 77.4 | |||||
| VEGFR-2 | VEGFR-3 | |||||
|---|---|---|---|---|---|---|
| n Positive (%) | n | p-Value | n Positive (%) | n | p-Value | |
| VEGF-A | 39 (81.3) | 48 | 0.649 * | 58 (79.5) | 73 | 1.000 * |
| VEGF-C | 48 (87.3) | 55 | # | 77 (83.7) | 92 | 0.031 * |
| VEGF-D | 45 (97.8) | 46 | 0.000 * | 69 (88.5) | 78 | 0.001 |
| Metastatic Lymph Node | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Tumor | VEGF-A | VEGF-C | VEGF-D | VEGFR-2 | VEGFR-3 | ||||||||||
| n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | n | Positive (%) | p | |
| VEGF-A Positive | 124 | 93.5 | 0.039 | 121 | 87.6 | 0.002 | 103 | 80.6 | 0.000 | 57 | 84.2 | 0.021 | 95 | 78.9 | 0.000 |
| VEGF-C Positive | 122 | 91.8 | 1.000 | 120 | 88.3 | 0.189 | 105 | 78.1 | 0.001 | 62 | 83.9 | 0.012 | 95 | 82.1 | 0.003 |
| VEGFR-2 Positive | 128 | 91.4 | 0.006 | 121 | 87.6 | 0.019 | 104 | 76.9 | 0.000 | 62 | 82.3 | # | 96 | 80.2 | 0.000 |
| VEGFR-3 Positive | 31 | 90.3 | 0.000 | 33 | 84.8 | 0.000 | 28 | 85.7 | 0.000 | 20 | 75.0 | 0.000 | 23 | 95.7 | 0.000 |
| Protein Marker | N | n of Deaths (%) | Average for Survival Time | p |
|---|---|---|---|---|
| [95% CI] | (Log-Rank Test) | |||
| VEGF-A | ||||
| Positive | 124 | 40 (32.26%) | 65.01 [58.86–71.17] | 0.748 |
| Negative | 12 | 4 (33.33%) | 57.89 [37.15–78.63] | |
| VEGF-C | ||||
| Positive | 115 | 38 (33.04%) | 63.61 [56.74–70.47] | 0.824 |
| Negative | 18 | 6 (33.33%) | 65.31 [49.74–80.97] | |
| VEGF-D | ||||
| Positive | 88 | 29 (32.95%) | 62.21 [54.54–69.87] | 0.688 |
| Negative | 25 | 9 (36.00%) | 53.25 [39.85–66.66] | |
| VEGFR-2 | ||||
| Positive | 53 | 10 (1.89%) | 50.26 [50.22–62.31] | 0.062 |
| Negative | 11 | 5 (4.55%) | 34.03 [22.00–46.06] | |
| VEGFR-3 | ||||
| Positive | 83 | 28 (3.37%) | 62.81 [54.66–70.97] | 0.788 |
| Negative | 21 | 8 (3.81%) | 57.17 [41.84–72.50] |
| Protein Marker | Antigen Retrieval | Peroxidase Inactivation | Detection System | Antibody | ||
|---|---|---|---|---|---|---|
| Company | Dilution | Incubation Period | ||||
| VEGF-A | EDTA Buffer 1X pH = 8.0 | 3% H2O2 in methanol, 10 min | LabVision | Abcam | 1:100 | Overnight 4 °C |
| VEGF-C | EDTA Buffer 1X pH = 8.0 | 3% H2O2 in methanol, 10 min | LabVision | Invitrogen | 1:200 | Overnight 4 °C |
| VEGF-D | EDTA Buffer 1X pH = 8.0 | 3% H2O2 in methanol, 10 min | LabVision | Invitrogen | 1:200 | Overnight 4 °C |
| VEGF-R2 | Citrate Buffer 0.01M pH = 6.0 | 3% H2O2 in methanol, 10 min | LabVision | Abcam | 1:200 | Overnight 4 °C |
| VEGF-R3 | Citrate Buffer 0.01M pH = 6.0 | 3% H2O2 in methanol, 10 min | LabVision | Abcam | 1:100 | Overnight 4 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazeda, I.; Martins, S.F.; Garcia, E.A.; Rodrigues, M.; Longatto, A. VEGF Expression in Colorectal Cancer Metastatic Lymph Nodes: Clinicopathological Correlation and Prognostic Significance. Gastrointest. Disord. 2020, 2, 267-280. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030025
Mazeda I, Martins SF, Garcia EA, Rodrigues M, Longatto A. VEGF Expression in Colorectal Cancer Metastatic Lymph Nodes: Clinicopathological Correlation and Prognostic Significance. Gastrointestinal Disorders. 2020; 2(3):267-280. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030025
Chicago/Turabian StyleMazeda, Inês, Sandra F. Martins, Eduardo A. Garcia, Mesquita Rodrigues, and Adhemar Longatto. 2020. "VEGF Expression in Colorectal Cancer Metastatic Lymph Nodes: Clinicopathological Correlation and Prognostic Significance" Gastrointestinal Disorders 2, no. 3: 267-280. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030025





