A Gluten Free Diet in the Management of Epilepsy in People with Coeliac Disease or Gluten Sensitivity
Abstract
:1. Introduction
1.1. Epilepsy
1.2. Ketogenic Diet in Management of Epilepsy
1.3. Gluten and Coeliac Disease
1.4. Non Coeliac Gluten Sensitivity
1.5. Association between Epilepsy and Coeliac Disease
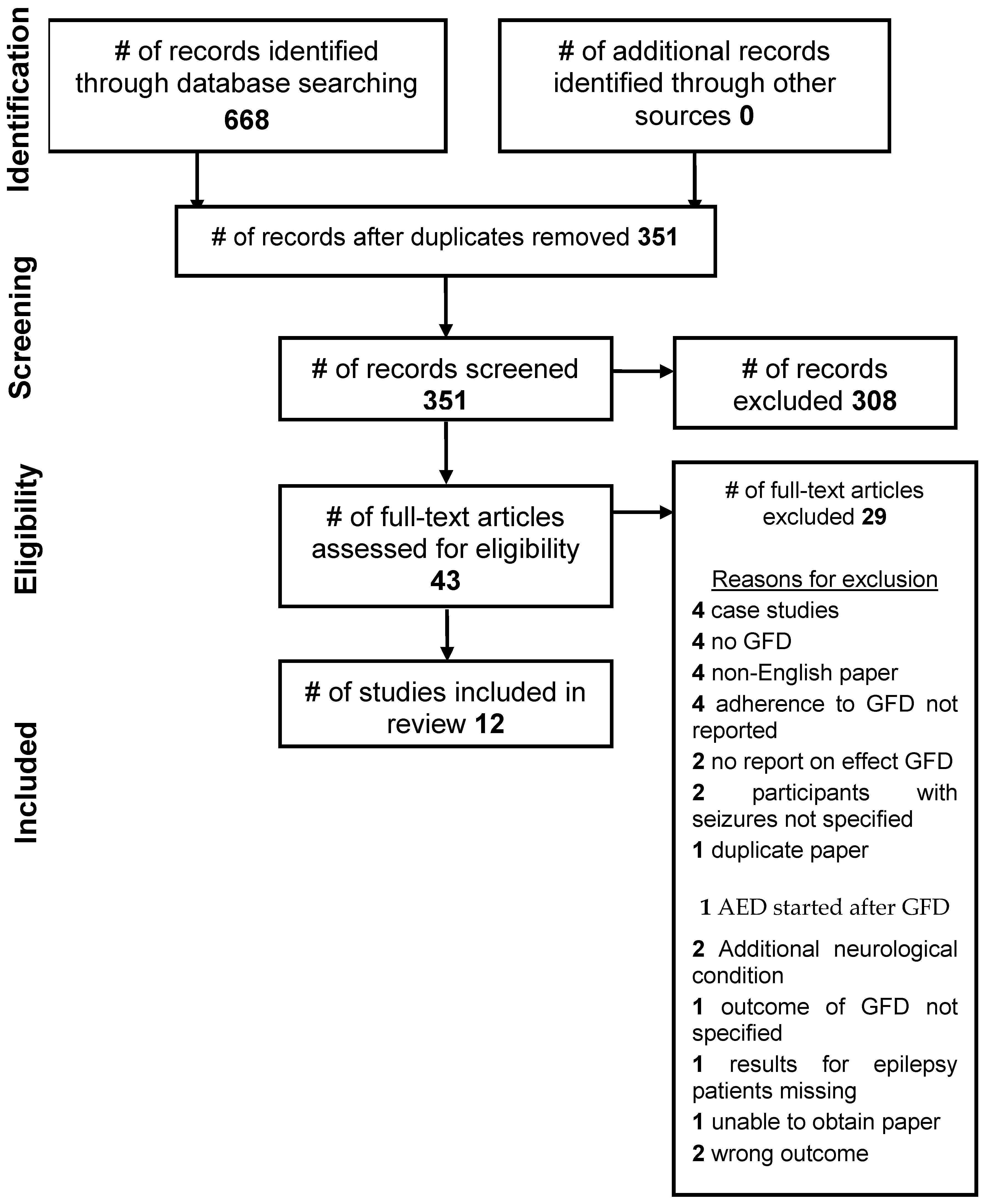
2. Methods
3. Data Collection
- Non-English language papers
- Include participants with other neurological conditions
- Include other known antiepileptic intervention
- Participants with known cause epilepsy
- Non-reporting of compliance to GFD
- Participant characteristics: age, sex, number of participants
- Study characteristics: country, study design, controls, outcomes measured
- Seizure type
- Duration of GFD
- Method of measuring adherence to GFD
- Length of follow up
- Outcome results
4. Results
5. Discussion
5.1. Main Findings
- Gluten mediated toxicity;
- Gluten involved immune-induced cortical damage; and
- CD related malabsorption of nutrients.
5.2. Gluten Mediated Toxicity
5.3. Gluten Involved Immune-Mediated Mechanisms
5.4. Malabsorption
5.5. Timing of Commencement of GFD
5.6. GFD and AEDs
5.7. EEG
5.8. GFD and Multidisciplinary Team Management
5.9. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, A.; Trevick, S. The Epidemiology of Global Epilepsy. Neurol. Clin. 2016, 34, 837–847. [Google Scholar] [CrossRef]
- Jones, C.; Reilly, C. Parental anxiety in childhood epilepsy: A systematic review. Epilepsia 2016, 57, 529–537. [Google Scholar] [CrossRef]
- Altintas, E.; Yerdelen, D.; Taskintuna, N. Social anxiety level in adult patients with epilepsy and their first-degree cohabiting relatives. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 339–344. [Google Scholar] [CrossRef]
- What Is Epilepsy? Available online: http://www.epilepsysociety.org.uk (accessed on 18 March 2019).
- Berg, A.; Berkovic, S.; Brodie, M.; Buchhalter, J.; Cross, J.; Van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.; Mathern, G.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- What Is Epilepsy? Epilepsy Research UK: London, UK, 2018.
- Diaz-Arrastia, R.; Agostini, M.A.; Madden, C.J.; Van Ness, P.C. Posttraumatic epilepsy: The endophenotypes of a human model of epileptogenesis. Epilepsia 2009, 50, 14–20. [Google Scholar] [CrossRef]
- Castilla-Guerra, L.; del Carmen Fernandez-Moreno, M.; Lopez-Chozas, M.; Fernandez-Bolanos, R. Electrolytes disturbances and seizures. Epilepsia 2006, 47, 1990–1998. [Google Scholar] [CrossRef]
- Fiest, K.; Sauro, K.; Wiebe, S.; Patten, S.; Kwon, C.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.; Jette, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef]
- Ferlisi, M.; Shorvon, S. Seizure precipitants (triggering factors) in patients with epilepsy. Epilepsy Behav. 2014, 33, 101–105. [Google Scholar] [CrossRef]
- McKee, H.; Privitera, M.D. Stress as a seizure precipitant: Identification, associated factors and treatment options. Seizure 2017, 44, 21–26. [Google Scholar] [CrossRef]
- Chater, S.; Simpson, K. Effect of passive hyperventilation on seizure duration in patients undergoing ECT. Br. J. Anaesth. 1988, 60, 70–73. [Google Scholar] [CrossRef]
- van Koert, R.R.; Bauer, P.R.; Schuitema, I.; Sander, J.W.; Visser, G.H. Caffeine and seizures: Asystematic review and quantitative analysis. Epilepsy Behav. 2018, 80, 37–47. [Google Scholar] [CrossRef]
- Martin, K.; Jackson, C.F.; Levy, R.G.; Cooper, P.N. Ketogenic diet and other dietary treaments for epilepsy. Cochrane Database Syst. Rev. 2016, 2, CD001903. [Google Scholar]
- Neal, E.; Chaffe, H.; Schwartz, R.; Lawson, M.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, K.; Yang, W.; Li, B. The anticonvulsant effects of ketogenic diet on epileptic seizures and potential mechanisms. Curr. Neuropharmacol. 2018, 16, 66–70. [Google Scholar] [CrossRef]
- Bough, K.; Wetherington, J.; Hassel, B.; Pare, J.; Gawryluk, J.; Greene, J.; Shaw, R.; Smith, Y.; Geiger, J.; Dingledine, R. Mitochondrial Biogenesis in the Anticonvulsant Mechanism of the Ketogenic Diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef]
- Epilepsies: Diagnosis and Management. Clinical Guideline [CG137]: NICE; NICE National Institute for Health and Care Excellence: London, UK, 2012.
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.; Mearin, M.; Phillips, A.; Shamir, R. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnoss of coeliac disease. J. Pediatric Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Catassi, C.; Gatti, S.; Fasano, A. The new epidemiology of celiac disease. J. Pediatric Gastroenterol. Nutr. 2014, 59, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Sollid, L.M. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. 2002, 2, 647–655. [Google Scholar] [CrossRef]
- Fasano, A.; Catassi, C. Clinical Practice. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biai, F.; Fasano, A.; Green, P.; Hadjivassilliou, M.; Kaukinen, K.; Kelly, C.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Garsed, K.; Scott, B.B. Can oats be taken in a gluten-free diet? A systematic review. Scand. J. Gastroenterol. 2007, 42, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Silano, M.; Dessi, M.; De Vincenzi, M.; Cornell, H. In vitro tests indicate that certain varieties of oats may be harmful to patients with coeliac disease. J. Gastroenterol. Hepatol. 2007, 22, 528–531. [Google Scholar] [CrossRef]
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M. Gluten-free diet indications, safety, quality, labels and challenges. Nutrients 2017, 9, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sabatino, A.; Corazza, G.R. Clinical Practice. Celiac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef]
- Abdulkarim, A.S.; Burgart, L.J.; See, J.; Murray, J.A. Eitiology of nonresponsive celiac disease: Results of a systematic approach. Am. J. Gastroenterol. 2002, 97, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Spence, D. Bad medicine: Food intolerance. Br. Med. J. 2013, 346. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.; Bonaz, B.; Bouma, G.; Calabro, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-celiac gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Iven, J. Non-coeliac gluten sensitivity: Piecing the puzzle together. United Eur. Gastroenterol. J. 2015, 3, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Sapone, A.; Bai, J.C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; Ullrich, R.; et al. Spectrum of gluten-related disorders: Consensus on new nomeclature and classification. BMC Med. 2012, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; Dieterich, W.; et al. Diagnosis of non-celiac gluten sensitivity (NCGS): The Salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabro, A.; Troncone, R.; Corazza, G.R. Study group for non-coeliac gluten sensitivity. BMC Med. 2014, 12. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Santolaria, S.; Sanders, D.S.; Fernandez-Banares, F. Systematic review: Noncoeliac gluten sensitivity. Aliment. Pharmacol. Ther. 2015, 41, 807–820. [Google Scholar] [CrossRef]
- Tonutti, E.; Bizzaro, N. Diagnosis and classification of celiac disease and gluten sensitivity. Autoimmun. Rev. 2014, 13, 472–476. [Google Scholar] [CrossRef]
- Sapone, A.; Lammers, K.M.; Mazzarella, G.; Mikhailenko, I.; Carten, M.; Casolaro, V.; Fasano, A. Differential mucosal IL-17 expression in two gliadin-induced disorders: Guten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 2010, 152, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Si, Q.; Xiaoyi, Z. Association between epilepsy and systemic autoimmune diseases: A meta-analysis. Seizure 2016, 41, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludvigsson, J.F.; Zingone, F.; Tomson, T.; Ekbom, A.; Ciacci, C. Increased risk of epilepsy in biopsy-verified celiac disease: A population-based cohort study. Neurology 2012, 78, 1401–1407. [Google Scholar] [CrossRef]
- Rana, A.; Musto, A. The role of inflammation in the development of epilepsy. J. Neuroinflamm. 2018, 15, 144. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Sanders, D.; Grunewald, R.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010, 9, 318–330. [Google Scholar] [CrossRef]
- Fois, A.; Vascotto, M.; Di Bartolo, R.M.; Di Marco, V. Celiac disease and epilepsy in pediatric patients. Childs Nerv. Syst. 1994, 10, 450–454. [Google Scholar] [CrossRef]
- Zelnik, N.; Pacht, A.; Obeid, R.; Lerner, A. Range of neurologic disorders in patients with celiac disease. Pediatrics 2004, 113, 1672–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canales, P.; Mery, V.P.; Larrondo, F.J.; Bravo, F.L.; Godoy, J. Epilepsy and celiac disease: Favourable outcome with a gluten-free diet in a patient refractory to antiepileptic drugs. Neurologist 2006, 12, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Dasappaiah, G.; Grunewald, R.; Aeschlimann, D.; Sarrigiannis, P.; Hoggard, N.; Aeschlimann, P.; Mooney, P.; DAvid, S. Neurological dysfunction in coeliac disease and non-coeliac gluten sensitivity. Am. J. Gastroenterol. 2016, 9, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Peltola, M.; Kaukinen, K.; Dastidar, P.; Haimila, K.; Partanen, J.; Haapala, A.; Maki, M.; Keranen, T.; Peltola, J. Hippocampal sclerosis in refractory temporal lobe epilepsy is associated with gluten sensitivity. J. Neurol. Neurosurg. Psychiatry 2009, 80, 626–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratesi, R.; Modelli, I.C.; Martins, R.C.; Almeida, P.L.; Gandolfi, L. Celiac disease and epilepsy:favourable outcome in a child with difficult to control seizures. Acta Neurol. Scand. 2003, 108, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.; Oliver, S.; Thomas, J. Introducing Systematic Reviews. In An Introduction to Systematic Reviews; Gough, D., Oliver, S., Thomas, J., Eds.; Sage Publications Ltd.: London, UK, 2017; pp. 1–17. [Google Scholar]
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Boland, A.; Cherry, G.; Dickson, R. Doing a Systematic Review; Sage: London, UK, 2017. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ionnidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate health care interventions: Explanation and elaboration. Ann. Int. Med. 2009, 151, 65–94. [Google Scholar] [CrossRef] [Green Version]
- Critical Appraisal Skills Programme (CASP). 2017. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 24 November 2018).
- Gobbi, G.; Bouquet, F.; Greco, L.; Lambertini, A.; Tassinari, C.; Ventura, A.; Zaniboni, M. Coeliac disease, epilepsy, and calcifications. The Italian Working Group on Coeliac Disease and Epilepsy. Lancet 1992, 340, 439–443. [Google Scholar]
- Hernadez, M.A.; Colina, G.; Ortigosa, L. Epilepsy, cerebral calcifications and clinical or subclinical coeliac disease. Course and follow up with gluten-free diet. Seizure 1998, 7, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, H.A.; De Rosa, S.; Ruggieri, V.; de Davila, M.T.G.; Fejerman, N. Epilepsy, occipital calcifications, and oligosymptomatic celiac disease in childhood. J. Child Neurol. 2002, 17, 800–806. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Petrolini, N.; Stanghellini, V.; Barbara, G.; Granito, F.; De Ponti, F. Clinical findings and anti-neuronal antibodies in coeliac disease with neurological disorders. Scand. J. Gastroenterol. 2002, 37, 1276–1281. [Google Scholar] [CrossRef]
- Licchetta, L.; Bisulli, F.; Di Vito, L.; La Morgia, C.; Naldi, I.; Volta, U.; Tinuper, P. Epilepsy in coeliac disease: Not just a matter of calcifications. Neurol. Sci. 2011, 32, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Berio, A.; Mangiate, G.; Mariottini, G.L.; Piazzi, A. Gluten sensitivity and neurological manifestations. J. Biol. Res. 2015, 88, 170–172. [Google Scholar] [CrossRef]
- Casciato, S.; Morano, A.; Albini, M.; Fanella, M.; Lapenta, L.; Fattouch, J.; Carni, M.; Colonnese, C.; Manfredi, M.; Giallonardo, A.T.; et al. Crytogenic focal epilepsy and "hidden" celiac disease in adulthood: A causal or accidental link? Int. J. Neurosci. 2015, 125, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Isikay, S.; Kocamaz, H.; Sezer, S.; Ozkars, M.; Isikay, N.; Filik, B.; San, M.; Kanmaz, A. The frequency of epileptiform discharges in celiac disease. Pediatric Neurol. 2015, 53, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Parisi, P.; Pietropaoli, N.; Ferreti, A.; Nenna, R.; Mastrogiorgio, G.; Del Pozzo, M.; Principessa, L.; Bonamico, M.; Villa, M.P. Role of gluten-free diet on neurological-EEG findings and sleep disordered breathing in children with celiac disease. Seizure 2015, 25, 181–183. [Google Scholar] [CrossRef] [Green Version]
- Bashiri, H.; Afshari, D.; Babaei, N.; Ghadami, M. Celiac disease and epilepsy: The effect of gluten-free diet on seizure control. Adv. Clin. Exp. Med. 2016, 25, 751–754. [Google Scholar] [CrossRef] [Green Version]
- Gerace, E.; Resta, F.; Landucci, E.; Renzi, D.; Masi, A.; Pellegrini-Giampietro, D.E.; Calabro, A.; Mannaioni, G. The gliadin peptide 31-43 exacerbateskainate neurotoxicity in epilepsy models. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sel, C.G.; Aksoy, E.; Aksoy, A.; Yuksel, D.; Ozbay, F. Neurological manifestations of atypical celiac disease in childhood. Acta Neurol. Belg. 2017, 117, 719–727. [Google Scholar] [CrossRef]
- Falcon-Moya, R.; Sihra, T.S.; Rodriguez-Moreno, A. Kainate receptors: Role in epilepsy. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef]
- Berio, A.; Badolati, G.; Mangiante, G.; Calcagno, E.; Piazzi, A. Anti-tissue transglutaminase antibodies and EEG pattern in celiac patients on prolonged gluten-free diet. J. Biol. Res. 2013, 86. [Google Scholar] [CrossRef] [Green Version]
- Giovanni, C.; Sanchez, M.; Straface, E.; Scazzocchio, B.; Silano, M.; De Vincenzi, M. Induction of apoptosis in caco-2 cells by wheat gliadin peptides. Toxicology 2000, 145, 63–71. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Balista, P.A.; Lopes-Aguiar, C.; Ruggiero, R.N.; Umeoka, E.H.; Garcia-Cairasco, N.; Bueno-Junior, L.S.; Leite, J.P. Animal models of epilepsy: Use and limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennisi, M.; Bramanti, A.; Cantone, M.; Pennisi, G.; Bella, R.; Lanza, G. Neurophysiology of the "celiac brain": Disentangling gut-brain connections. Front. Neurosci. 2017, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, R.; Gandolfi, L.; Friedman, H.; Farage, L.; De Castro, C.A.M.; Catassi, C. Serum IgA antibodies from patients with coeliac disease strongly react with human brain blood-vessel structures. Scand. J. Gastroenterol. 1998, 33, 817–822. [Google Scholar] [PubMed]
- Pellecchia, M.T.; Scala, R.; Filla, A.; De Michele, G.; Ciacci, C.; Barone, P. Idiopathic cerebellar ataxia associated with celiac disease: Lack of distinctive neurological features. J. Neurol. Neurosurg. Psychiatry 1999, 66, 32–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salur, L.; Uibo, O.; Talvik, I.; Justus, I.; Metskula, K.; Talvik, T.; Uibo, R. The high frequency of coeliac disease among children with neurological disorders. Eur. J. Neurol. 2002, 7, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Labate, A.; Gambardella, A.; Messina, D.; Tammaro, S.; Le Piane, E.; Pirritano, D.; Cosco, C.; Doldo, P.; Mazzei, R.; Oliveri, R.L.; et al. Silent celiac disease in patients with childhood localization-related epilepsies. Epilepsia 2001, 42, 1153–1155. [Google Scholar] [CrossRef] [Green Version]
- Mavroudi, A.; Karatza, E.; Papastravou, T.; Panteliadis, C.; Spiroglou, K. Successful treatment of epilepsy and celiac disease with a gluten-free diet. Pediatric Neurol. 2005, 33, 292–295. [Google Scholar] [CrossRef]
- Emami, M.H.; Taheri, H.; Kohestani, S.; Chitsaz, A.; Etemadifar, M. How frequent is celiac disease among epileptic patients? J. Gastroenterol. Liver Dis. 2008, 17, 379–382. [Google Scholar]
- Geuking, M.B.; Koller, Y.; Rupp, S.; McCoy, K.D. The interplay between the gut microbiota and the immune system. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef]
- Dahlin, M.; Prast-Neilsen, S. The gut and epilepsy. EBioMedicine 2019, 44, 741–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdu, E.; Galipeau, H.; Jabri, B. Novel players in coeliac disease pathogenesis: Role of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac disease and the microbiome. Nutrients 2019, 11, 2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Epps, S.A.; Weinshenker, D. Rhythm and blues: Animal models of epilepsy and depression comorbidity. Biochem. Pharmacol. 2013, 85, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mazcorro, J.F.; Noratto, G.; Remes-Troche, J.M. The effect of gluten-free diet on health and the gut microbiota cannot be extrapolated from one population to others. Nutrients 2018, 10, 1421. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, Y.; Yang, H.; Rao, Y.; Miao, J.; Lu, X. Intestinal Microbiota as an Alternative Therapeutic Target for Epilepsy. Can. J. Infect. Dis. Med Microbiol. 2016, 2016, 9032809. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Chen, G.; Bao, Y.; Hua, Z.; Wang, D.; Bao, Y. Effect of topiramate on interleukin 6 expression in the hippocampus of amygdala-kindled epileptic rats. Exp. Ther. Med. 2014, 7, 223–227. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef]
- Pruimbom, L.; de Punder, K. The opioid effects of gluten exorphins: Asymptomatic celiac disease. J. Health Popul. Nutr. 2015, 33, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaedini, A.; Okamoto, H.; Briani, C.; Wollenberg, K.; Shill, H.A.; Bushara, K.O.; Sander, H.W.; Green, P.H.R.; Hallett, M.; Latov, N. Immune cross-reactivity in celiac disease: Anti-gliadin antiboides bind to neuronal synapsin I. J. Immunol. 2007, 178, 6590–6595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronica, E.; Crino, P.B. Inflammation in epilepsy: Clinical observations. Epilepsia 2011, 52, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Gorter, J.A. Gene expression profile in temporal lobe epilepsy. Neuroscientist 2007, 13, 100–108. [Google Scholar] [CrossRef]
- Aziz, I.; Hadjivassiliou, M. Coeliac disease: Noncoeliac gluten sensitivity—Food for thought. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 398–399. [Google Scholar] [CrossRef]
- Johnson, A.M.; Dale, R.C.; Wienholt, L.; Hadjivassiliou, M.; Aeschlimann, D.; Lawson, J.A. Coeliac disease, epilepsy, and cerebral calcifications: Association with TG6 autoantibodies. Dev. Med. Child Neurol. 2012, 55, 90–93. [Google Scholar] [CrossRef]
- Amanat, M.; Thijs, R.D.; Salehi, M.; Sander, J.W. Seizures as a clinical manifestation in somatic autoimmune disorders. Seizure 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Lea, M.E.; Harbord, M.; Sage, M.R. Bilateral occipital calcification associated with celiac disease, folate deficiency, and epilepsy. Am. J. Neuroradiol. 1995, 16, 1498–1500. [Google Scholar]
- Maniar, V.; Yadav, S.; Gokhale, Y. Intractable seizures and metabolic bone disease secondary to celiac disease. J. Assoc. Physicians India 2010, 58, 512–515. [Google Scholar]
- Hadjivassiliou, M.; Gibson, A.; Davies-Jones, G.A.B.; Lobo, A.; Stephenson, T.J.; Millford-Ward, A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet 1996, 347, 369–371. [Google Scholar] [CrossRef]
- Molteni, N.; Bardella, M.T.; Baldassarri, A.R.; Bianchi, P.A. Celiac disease associated with epilepsy and intracranial calcifications: Report of two patients. Am. J. Gastroenterol. 1988, 83, 992–994. [Google Scholar] [PubMed]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac disease symptom resolution: Effectiveness of the gluten-free diet. J. Pediatric Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, R.; Gandolfi, L.; Martins, R.C.; Tauil, P.; Nobrega, Y.N.; Teixeira, W.A. Is the prevalence of celiac disease increased among epiletic patients? Arq. Neuro-Psiquiatr. 2003, 61, 330–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lionetti, E.; Francavilla, R.; Pavone, P.; Pavone, L.; Francavilla, T.; Pulvirenti, A. The neurology of coeliac disease in childhood: What is the evidence? A systematic review and meta-analysis. Dev. Med. Child Neurol. 2010, 52, 700–707. [Google Scholar] [CrossRef]
- Benbadis, S.R.; Tatum, W.O. Overinterpretation of EEGs and misdiagnosis of epilepsy. J. Clin. Neurophysiol. 2003, 20, 42–44. [Google Scholar] [CrossRef]
- Smith, S.J.M. EEG in neurological conditions other than epilepsy: When does it help, what does it add? J. Neurol. Neurosurg. Psychiatry 2005, 76, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.; Crino, P.B. Systemic and neurologic autoimmune disorders assoicated with seizures or epilepsy. Epilepsia 2011, 52, 12–17. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Mariani, P.; Viti, M.G.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The gluten-free diet: A nutritional risk factor for adolescents with celiac disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef]
- Tortora, R.; Capone, P.; De Stefano, G. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2015, 41, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Patsalos, P.N.; Froscher, W.; Pisani, F.; van Rijn, C.M. The importance of drug interations in epilepsy therapy. Epilepsia 2002, 43, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Xhakollari, V.; Canavari, M.; Osman, M. Factors affecting consumers’ adherence to gluten-free diet, a systematic review. Trends Food Sci. Technol. 2019, 85, 23–33. [Google Scholar] [CrossRef]
- Greenhalgh, T. How to Read a Paper. The Basics of Evidence-Based Medicine, 5th ed.; John Wiley and Sons: Chichester, UK, 2014. [Google Scholar]
- Balakireva, A.V.; Zamyatin, J.A. Properties of gluten intolerance: Gluten structure, evolution and detoxification properties. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itzlinger, A.; Branchi, F.; Schumann, M. Gluten-free diet in celiac disease—Forever and for all? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [Green Version]
| Location | Worldwide |
| Language | English |
| Population | No age restriction, people with a diagnosis of idiopathic epilepsy (irrespective of seizure type); people with seizure disorders and CD with seizure-related neurological abnormalities Animal studies/models investigating gluten and seizure/epileptiform activity EEG |
| Intervention | Gluten-free diet |
| Comparisons | No intervention |
| Setting | No restriction |
| Outcomes | Any of the following: Reduction in seizures Reduction in medication Reduction in EEG abnormalities Adverse reactions Cognitive or behavioural outcomes Quality of life outcomes |
| Study design |
|
| Study | Number of Participants on GFD | Method of Measuring Adherence to Gluten-Free Diet | Outcome Seizure Reduction | Outcome AED Reduction | EEG before GFD | EEG after GFD | Adverse Outcome |
|---|---|---|---|---|---|---|---|
| Arroyo 2002 | 24 | Patients described as compliant with diet in group 3 and implied for other patients Authors comment on patients who had not strictly followed the diet which indicates adherence was monitored | 11 patients (45.8%) on AED became seizure free before GFD Nine patients (37.5%) who were on AED became seizure free after introduction of GFD | Not documented | 15/32 normal | 20/32 normal | Four patients on an AED had uncontrolled seizures Three developed epileptic encephalopathy despite GFD |
| Bashiri 2016 | 7 | Intestinal biopsy repeated 3 months after starting GFD | Six patients became seizure free One patient seizures controlled | AEDs discontinued in 6 patients AED reduced by half in 1 patient | n/a | n/a | None reported |
| Berio 2013 | 12 | Groups differentiated good/poor compliance | n/a | n/a | Not measured | 5/6 normal with good compliance to GFD 5/6 abnormal with poor compliance | None reported |
| Casciato 2015 | 10 | Serological and histological testing | Two patients non-compliant GFDOne patient lost at follow-up Of the remaining seven patients: Three patients became seizure free Three reduction in number of seizures One no change | Not reported | 10 abnormal | Not measured | None reported |
| Gobbi 1992 | 29 20 followed up | Adherence assumed. Authors state patients were ‘prescribed’ a GFD | Four patients’ seizures decreased by more than 50% Six patients seizure free; Nine patients no change | Not reported | Four normal 25 abnormal | Not measured | One patient increased seizures >50% |
| Hernandez 1998 | 4 | Monitored and reported Poor compliance noted | Patient 1: seizure frequency decreased by 30% Patient 2: seizure free Patient 3: seizure frequency decreased by 10% Patient 4: seizure frequency decreased by 50% | Not reported | Four abnormal | Not measured | None reported |
| Isikay 2015 | 132 patients 99 controls | Notes ‘effective GFD’ for formerly diagnosed CD patients and ‘ineffective GFD’ for newly diagnosed CD patients | n/a | n/a | Four new patients abnormal 9.3% Two former patients abnormal 1.5% One control case abnormal 1% | All normal, including case control which spontaneously normalised | None reported |
| Liccheta 2011 | 8 | Control visit interviews in the last 12 months to assess compliance | Five patients no clinical benefit One patient reduced seizure frequency One patient seizure free. One not F/U | Not reported | Eight abnormal | Not reported | None reported |
| Parisi 2014 | 19 | Complete adherence confirmed by parents | n/a | n/a | Nine abnormal | One abnormal One unknown (parents refused follow-up EEG) Seven normal (77.7%) | None reported |
| Sel 2017 | 5 | Adherence implied. Reported non-compliance in 1 patient | Three patients stopped AEDs One due to stop AEDs | Three patients stopped AEDs One due to stop AEDs | Four abnormal One normal | None reported | |
| Volta 2002 | 3 | Monitored, recorded and reported in paper | Two patients improved, one patient not | n/a | n/a | n/a | None reported |
| Study | Mean Age (Years) | Sex f/m | Mean Age at Seizure Onset (Years) | Seizure Type | Seizure Frequency | EEG | AEDs | Gastrointestinal Symptoms | Coeliac Disease |
|---|---|---|---|---|---|---|---|---|---|
| Arroyo 2002 | 11 | 22/10 | 6.13 | 31 partial One generalised tonic-clonic | Most patients: monthly or less Three patients daily | 19 normal 13 abnormal | Yes, all | Absent or rare at time of CD diagnosis but chronic diarrhoea in all cases, appearing at mean age 2.8 years, preceding seizure onset | Yes Biopsy proven |
| Bashiri 2016 | 30.2 | 3/4 | 16.2 | 46% generalised tonic-clonic 38% complex partial | Three patients weekly Four patients monthly | Not measured | Yes, all | No | Yes IgA serum antibodiesBiopsy proven |
| Berio 2013 | 10–44 | Not reported | No seizures | n/a | n/a | Five normal Six abnormal One not measured | n/a | Not reported | Yes anti-tTG serum antibodies and/or biopsy proven |
| Casciato 2015 | 31.5 | 9/1 | 21.4 | Six generalised tonic-clonic Four partial | Two patients daily Three patients weekly Five patients monthly | 10 abnormal | Yes, all | Yes, three | Yes IgA/IgG-Ttg, IgA/IgG-DGP and IgA EMA serum antibodies Biopsy proven |
| Gerace 2017 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Gobbi 1992 | 16.4 | 28/15 | 4.5 | Five generalised tonic-clonic 24 partial | Eight patients >1/day Seven patients >1/week Five patients >1/month | Four normal 25 abnormal | Not reported | Yes, 26 No, three | Yes Biopsy proven |
| Hernandez 1998 | 18 | 1/3 | 7 | Three partial One generalised tonic-clonic | Two patients > 1/day One patient > 1/week One patient = 1/week | Four abnormal | Not reported | Yes, two No, two | Yes Biopsy proven |
| Isikay 2015 | 10.6 | 76/56 | n/a | n/a | n/a | Four new patients abnormal 9.3% Two former patients abnormal 1.5% One control case abnormal 1% | n/a | Yes, 121 No, 11 | Yes Biopsy proven |
| Liccheta 2011 | 25.7 | 7/1 | 12.85 | Eight partial | 2 >daily 3 daily 2 weekly 1 yearly | Eight abnormal | Yes, all | No | Yes Biopsy proven |
| Parisi 2014 | 9.82 | 16/3 | No seizures | n/a | n/a | Nine abnormal After 6 months GFD EEG abnormalities in one patient and one patient unknown as parents refused follow-up EEG | n/a | Not reported | Yes Biopsy proven |
| Sel 2017 | 6.4 | 0/4 | 4.5 | Two generalised tonic-clonic Two partial One no seizures (EEG abnormalities only) | Not documented | All normalised | Four out of five | No | Yes Biopsy proven Anti-tTG serum antibodies |
| Volta 2002 | 32 | 3/0 | 23 | One complex partial temporal lobe One simple partial One generalised absence | Not documented | Not measured | Not documented | Yes, one No, two | Yes Biopsy proven IgaAGA, IgAEMA, IgA h-tTGA serum antibodies |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbey, Z.; Bold, J. A Gluten Free Diet in the Management of Epilepsy in People with Coeliac Disease or Gluten Sensitivity. Gastrointest. Disord. 2020, 2, 281-299. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030026
Gilbey Z, Bold J. A Gluten Free Diet in the Management of Epilepsy in People with Coeliac Disease or Gluten Sensitivity. Gastrointestinal Disorders. 2020; 2(3):281-299. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030026
Chicago/Turabian StyleGilbey, Zoë, and Justine Bold. 2020. "A Gluten Free Diet in the Management of Epilepsy in People with Coeliac Disease or Gluten Sensitivity" Gastrointestinal Disorders 2, no. 3: 281-299. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030026





