Microbiome–Gut Dissociation: Investigating the Origins of Obesity
Abstract
:1. Introduction
2. The Observations of Denis Burkitt
- People living away from the modern world and free from certain non-communicable diseases also consumed substantial amounts of dietary fibre, exhibited low intestinal transit times, and produced copious faecal matter,
- That those same people, transferred to a modern environment, developed modern diseases, thus confirming a primarily environmental cause, and,
- That individuals in the modern environment often suffer from multiple apparently different diseases, a finding backed up by a recent multimorbidity study [18], but,
- That the Maasai (Masai in his day), who were not in a position to consume significant amounts of fibre, still remained healthy. In addition,
- To his credit, Burkitt mentioned those immune system complaints present in the modern world that were not adequately explained by his dietary fibre hypothesis, specifically thyrotoxicosis, pernicious anaemia, rheumatoid arthritis, multiple sclerosis, and coeliac disease [6]. Finally,
- Sadly, but in keeping with the mores of his time, he did not compare rates of poor mental health between peoples living in traditional v. modern societies.
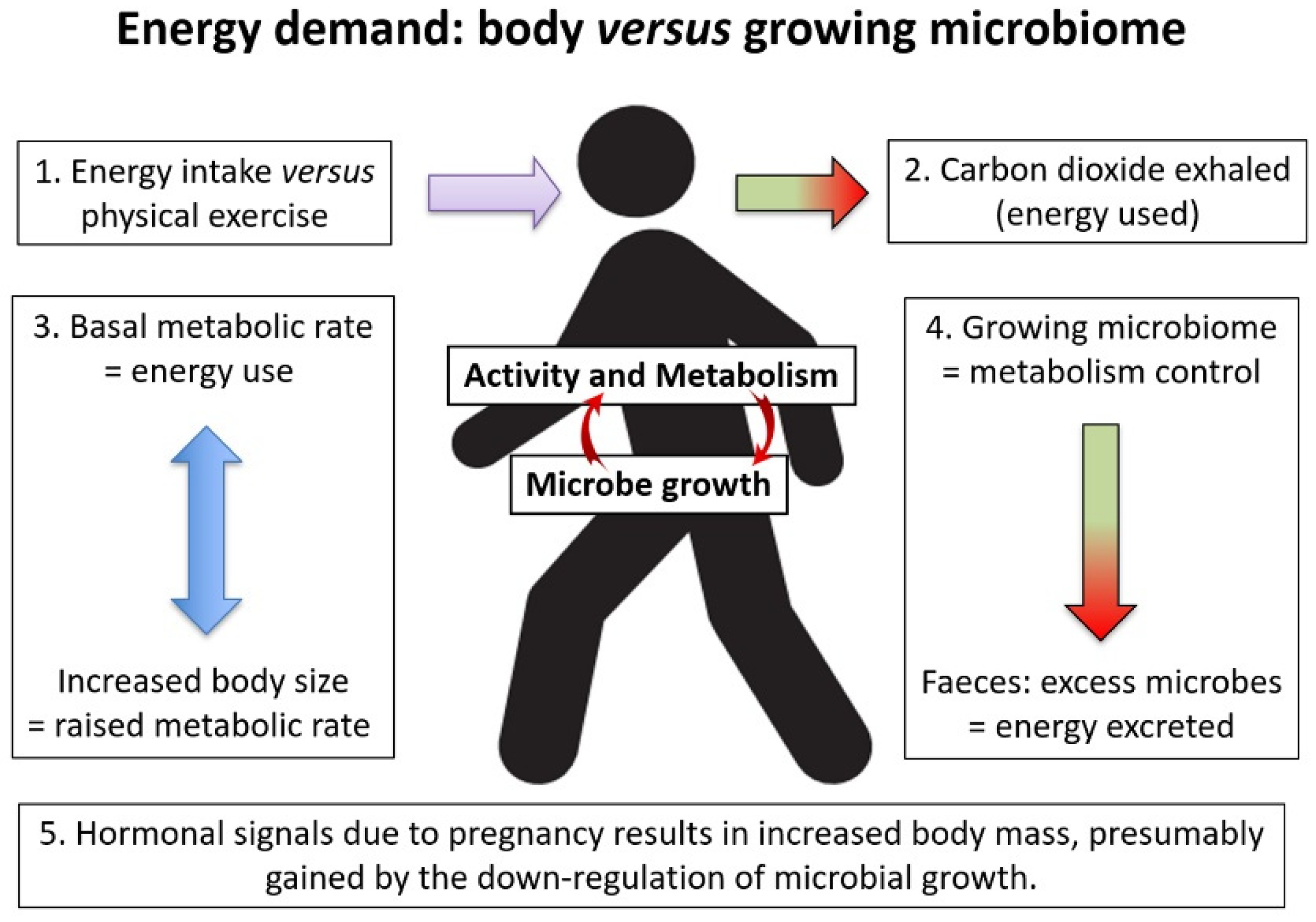
3. The Energy Balance within the Animal/Microbiome Combination
4. The Microbiome as a Cofactor of Evolution
- Maternal microbiota drives the innate immune system [32]
- Microbes educating the adaptive immune system from birth [33]
- Microbes affecting peripheral dopamine and inhibiting natural killer T cells [34]
- Greater microbiome diversity within pancreatic tumours predicts patient survival [35]
- Parkinson’s disease, but without defining a specific causative agent [36]
- Neuroactive potential of the microbiota in quality of life and depression [37]
5. Microbiome Investigation
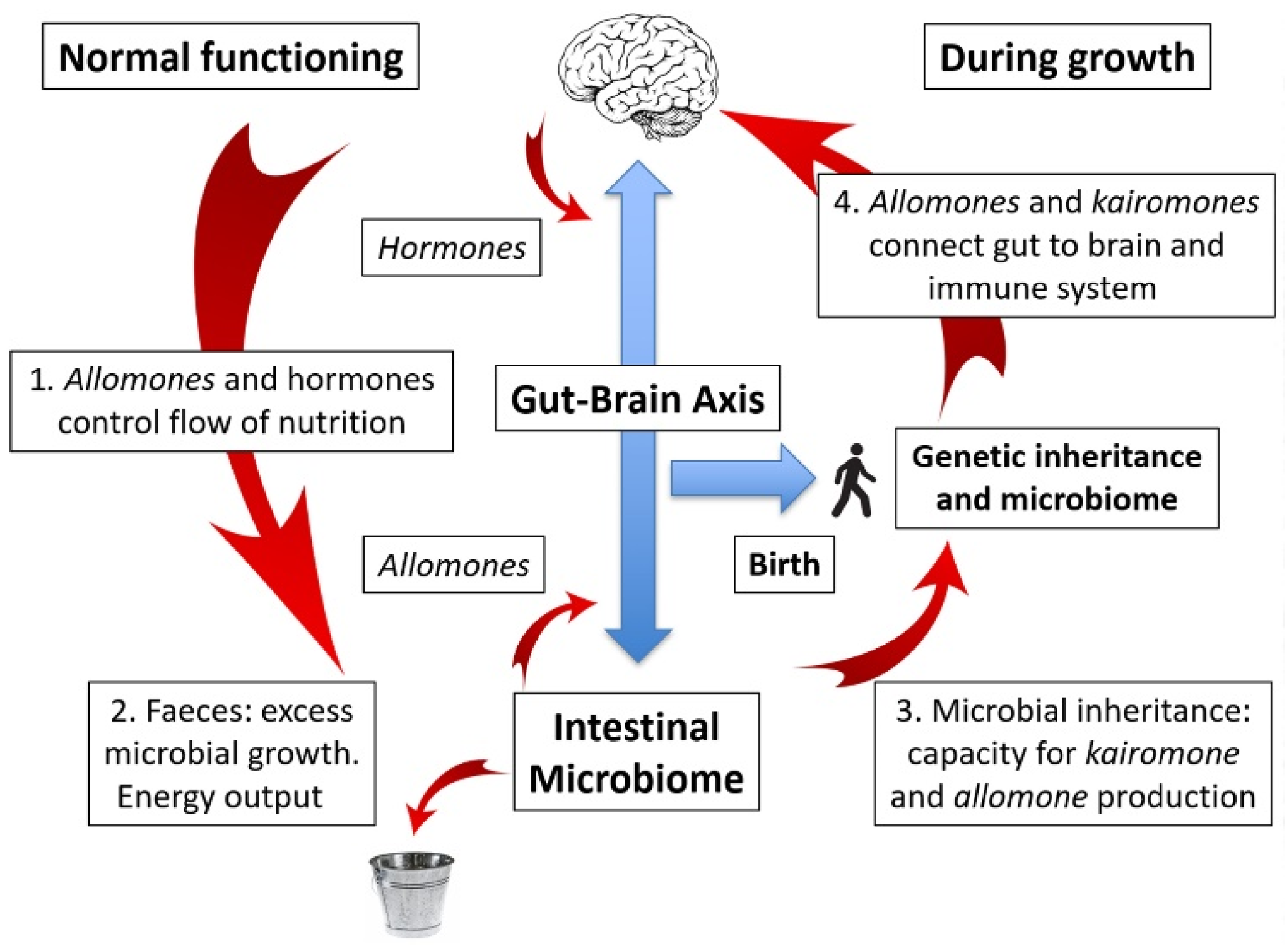
6. The Gut–Brain Axis: Obesity and Varicose Veins
7. Probiotics and Missing Microbes
8. Weight Gain on Reduction of Faecal Energy Output
9. Microbial vs. Genetic Inheritance: A New Angle on an Old Debate
10. The Microbiome as a Mutualistic Entity: Allomones and Kairomones
11. The Road to Disease: Microbiome–Gut Dissociation
12. The Storage of “Excess” Energy: The Personal Fat Threshold and Cancer
- That children will grow larger during their growth period, laying down lean muscle, possibly dependent upon sufficient protein being available, otherwise intramuscular fat [78],
- That adults, and children outside their growth period, will accumulate both subcutaneous and visceral fat dependent on the degree of microbiome–gut dissociation. Excess fat may leach into the circulation, leading to heart disease and stroke, among other conditions, as enumerated by Burkitt [6]; and
- That the growth of cancer cells will accelerate under the availability of excess energy.
13. Studying the Microbiome–Gut–Brain Axis: A Role for Ingestible Sensors
14. Conclusions: An Unfolding Disaster
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jou, C. The Biology and Genetics of Obesity—A Century of Inquiries. N. Engl. J. Med. 2014, 370, 1874–1877. [Google Scholar] [CrossRef]
- Garner, D.M.; Wooley, S.C. Confronting the failure of behavioral and dietary treatments for obesity. Clin. Psychol. Rev. 1991, 11, 729–780. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Prentice, A.M.; Jebb, A.S. Obesity in Britain: Gluttony or sloth? BMJ 1995, 311, 437–439. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, K.R.; Speakman, J.R. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int. J. Obes. 2008, 32, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkitt, D.P. Some diseases characteristic of modern Western civilization. BMJ 1973, 1, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Jones, D.S.; Podolsky, S.H.; Greene, J.A. The Burden of Disease and the Changing Task of Medicine. N. Engl. J. Med. 2012, 366, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Resongles, E.; Dietze, V.; Green, D.C.; Harrison, R.M.; Ochoa-Gonzalez, R.; Tremper, A.H.; Weiss, D.J. Strong evidence for the continued contribution of lead deposited during the 20th century to the atmospheric environment in London of today. Proc. Natl. Acad. Sci. USA 2021, 118, 2102791118. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Plastics and Health Risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Holman, R.R. Normal weight individuals who develop Type 2 diabetes: The personal fat threshold. Clin. Sci. 2014, 128, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yakov, O.; Lador, D.; Avnit-Sagi, T.; Lo-tan-Pompan, M.; et al. Personalized nutrition by prediction of glycaemic responses. Cell 2016, 163, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Margulis, L. Symbiogenesis and symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92. [Google Scholar]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A. The Human Microbiome and Child Growth–First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Jheeta, S. Evolution from the inside: Do malfunctioning microbiota suggest a role for unicellular eukaryotes? Unpublished work. 2021. [Google Scholar]
- Burkitt, D. A sarcoma involving the jaws in african children. BJS 2005, 46, 218–223. [Google Scholar] [CrossRef]
- Gondek, D.; Bann, D.; Brown, M.; Hamer, M.; Sullivan, A.; Ploubidis, G.B. Prevalence and early-life determinants of mid-life multimorbidity: Evidence from the 1970 British birth cohort. BMC Public Health 2021, 21, 1319. [Google Scholar] [CrossRef]
- Widdowson, E.M. Assessment of the Energy Value of Human Foods. Proc. Nutr. Soc. 1955, 14, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterisation of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the Industrial Revolution. eLife 2020, 9, e49555. [Google Scholar] [CrossRef]
- Tortora, G.J.; Anagnostakos, N.P. Principles of Anatomy and Physiology, 5th ed.; Harper and Row: New York, NY, USA, 1987; p. 624. [Google Scholar]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, N. Biogenic Amines: Signals between Commensal Microbiota and Gut Physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. mBio 2017, 8, e01492-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Ewald, D.R.; Sumner, S.C. Human microbiota, blood group antigens, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1413. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L.; Groisman, E.A. Novel “super-spreader” bacteriophages promote horizontal gene transfer by transformation. mBio 2017, 8, e02115–e02116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Zhao, Q.; Elson, C.O. Adaptive immune education by gut microbiota antigens. Immunol. 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, H.; Pan, J.; Du, Z.; Zhou, W.; Zhang, Z.; Tian, Z.; Zhou, R.; Bai, L. Peripheral Dopamine Controlled by Gut Microbes Inhibits Invariant Natural Killer T Cell-Mediated Hepatitis. Front. Immunol. 2018, 9, 2398. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Miraglia, F.; Colla, E. Microbiome, Parkinson’s Disease and Molecular Mimicry. Cells 2019, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Dominguez, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vina, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Cunningham, A.J.; Sim, K.; Deierl, A.; Kroll, S.; Brannigan, E.; Darby, J. “Vaginal seeding” of infants born by caesarean section. BMJ 2016, 352, i227. [Google Scholar]
- Waddington, C.H. Epigenetics and evolution. Symp. Soc. Exp. Biol. 1953, 7, 186–199. [Google Scholar]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curley, J.P.; Mashoodh, R.; Champagne, F.A. Epigenetics and the origins of paternal effects. Horm. Behav. 2011, 59, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.P. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Eriksson, J.G. The fetal origins hypothesis–10 years on. BMJ 2005, 330, 1096–1097. [Google Scholar] [CrossRef]
- Almond, D.; Currie, J. Killing Me Softly: The Fetal Origins Hypothesis. J. Econ. Perspect. 2011, 25, 153–172. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, J.G.; Chain, F.; Martín, R.; Humaran, L.G.B.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypotheses 2019, 125, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Jheeta, S. The epidemiology of the dysfunctional microbiome in animals and in humans: The propensity for the de-velopment of non-communicable disease. EC Gastroenterol. Dig. Syst. 2020, 7, 83–93. [Google Scholar]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, K.; Tomariguchi, N. Occurrence of randomly recombined functional 16S rRNA genes in Thermus thermophilus suggests genetic interoperability and promiscuity of bacterial 16S rRNAs. Sci. Rep. 2019, 9, 11233. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level mi-crobiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [Green Version]
- Jheeta, S. Extremophiles and Horizontal Gene Transfer: Clues to the Emergence of Life; Seckbach, J., Stan-Lotter, H., Eds.; Scrivener Publishing: Beverly, MA, USA, 2020; Chapter 16; pp. 329–358. [Google Scholar]
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the Human Mycobiome over the First Month of Life and across Body Sites. mSystems 2018, 3, e00140-17. [Google Scholar] [CrossRef] [Green Version]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef] [Green Version]
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial Eukaryotes: A Missing Link in Gut Microbiome Studies. mSystems 2018, 3, e00201-17. [Google Scholar] [CrossRef] [Green Version]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Kaplan, H.; Thompson, R.C.; Trumble, B.C.; Wann, L.S.; Allam, A.H.; Beheim, B.; Frohlich, B.; Sutherland, M.L.; Sutherland, J.D.; Stieglitz, J.; et al. Coronary atherosclerosis in indigenous South American Tsimane: A cross-sectional cohort study. Lancet 2017, 389, 1730–1739. [Google Scholar] [CrossRef]
- Ryan, C.R. Towards an ethics of reciprocity: Ethnobotanical knowledge and medicinal plants as cancer therapies. Humanities 2014, 3, 624–644. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Jheeta, S. Measuring Microbiome Effectiveness: A Role for Ingestible Sensors. Gastrointest. Disord. 2020, 2, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Beardslee, L.A.; Banis, G.E.; Chu, S.; Liu, S.; Chapin, A.A.; Stine, J.M.; Pasricha, P.J.; Ghodssi, R. Ingestible Sensors and Sensing Systems for Minimally Invasive Diagnosis and Monitoring: The Next Frontier in Minimally Invasive Screening. ACS Sensors 2020, 5, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Remmers, C.; Michalak, J. Losing your gut feelings. Intuition in depression. Front. Psychol. 2016, 7, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey Jr., G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacte-ria-gut-brain signals. Trend. Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-Rich Fecal Donor May Be a Positive Predictor for Successful Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Digestion 2017, 96, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, C.; Campbell, M.D. The effect of bifidobacterium on reducing symptomatic pain in patients with irritable bowel syn-drome: A systematic review. Probiotics Antimicrob. Proteins 2020, 12, 834–839. [Google Scholar]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.-Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nat. Cell Biol. 2020, 582, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, A.T.; Chadaideh, K.S.; Diggins, C.E.; Schell, L.D.; Beckel, M.; Callahan, P.; Ryan, R.; Thompson, M.E.; Carmody, R.N. Effects of domestication on the gut microbiota parallel those of human industrialization. eLife 2021, 10, e60197. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; De Benedetto, A. Atopic Dermatitis in Animals and People: An Update and Comparative Review. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef]
- Davison, T.F.; Freeman, B.M. Physiological aspects of growth promotion in poultry. Vet. Res. Commun. 1983, 7, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.; Ibrahim, R.; Ahmed, E.-N.G.; El-Bouhy, Z. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, G.R.H.; Cohen, D.D. Temporal trends in muscular fitness of English 10-year-olds 1998–2014: An allometric ap-proach. J. Sci. Med. Sport 2019, 22, 201–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEvoy, B.P.; Visscher, P. Genetics of human height. Econ. Hum. Biol. 2009, 7, 294–306. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Seymour, R.S. Allometric scaling of mammalian metabolism. J. Exp. Biol. 2005, 208, 1611–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, C.; Tremblay, A.; Després, J.-P.; Nadeau, A.; Lupien, P.J.; Thériault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The Response to Long-Term Overfeeding in Identical Twins. N. Engl. J. Med. 1990, 322, 1477–1482. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Liver Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [Green Version]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.; Koonin, E.V. Evolution of cell–cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile Infection with Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [Green Version]
- Walker, S.; Khan-Wasti, S.; Fletcher, M.; Sheikh, A. Prevalence of hayfever symptoms and diagnosis in UK teenagers. Prim. Care Respir. J. 2005, 14, 270. [Google Scholar] [CrossRef] [Green Version]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef]
- Steel, Z.; Marnane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, D.J.; Hicks, L.A.; Pavia, A.T.; Hersh, A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–2009. J. Antimicrob. Chemother. 2014, 69, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Bostock, J. Case of a Periodical Affection of the Eyes and Chest. J. R. Soc. Med. 1819, 10, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Bostock, J. Of the Catarrhus Æstivus, or Summer Catarrh. J. R. Soc. Med. 1828, 14, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Boerma, T.; Ronsmans, C.; Melesse, D.Y.; Barros, A.J.D.; Barros, F.C.; Juan, L.; Moller, A.-B.; Say, L.; Hosseinpoor, A.R.; Yi, M.; et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018, 392, 1341–1348. [Google Scholar] [CrossRef]
- Taylor, R. Calorie restriction for long-term remission of type 2 diabetes. Clin. Med. 2019, 19, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Abid, Z.; Cross, A.J.; Sinha, R. Meat, diary, and cancer. Am. J. Clin. Nutr. 2014, 100, 386S–393S. [Google Scholar] [CrossRef]
- Ter Horst, K.W.; Lammers, N.M.; Trinko, R.; Opland, D.M.; Figee, M.; Ackermans, M.T.; Booij, J.; Munckhof, P.V.D.; Schuurman, P.R.; Fliers, E.; et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 2018, 10, eaar3752. [Google Scholar] [CrossRef] [Green Version]
- Vallgårda, S. Why the concept “lifestyle diseases” should be avoided. Scand. J. Public Health 2011, 39, 773–775. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R.; Rajilic-Stojanovic, M.; Heilig, H.G.H.J.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Noeël, C.; Dufernez, F.; Gerbod, D.; Edgcomb, V.; Delgado-Viscogliosi, P.; Ho, L.-C.; Singh, M.; Wintjens, R.; Sogin, M.L.; Capron, M.; et al. Molecular Phylogenies of Blastocystis Isolates from Different Hosts: Implications for Genetic Diversity, Identification of Species, and Zoonosis. J. Clin. Microbiol. 2005, 43, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Lepczyńska, M.; Białkowska, J.; Dzika, E.; Piskorz-Ogorek, K.; Korycińska, J. Blastocystis: How do specific diets and human gut microbiota affect its development and pathogenicity? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.; Jheeta, S. Microbiome–Gut Dissociation: Investigating the Origins of Obesity. Gastrointest. Disord. 2021, 3, 156-172. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord3040017
Smith D, Jheeta S. Microbiome–Gut Dissociation: Investigating the Origins of Obesity. Gastrointestinal Disorders. 2021; 3(4):156-172. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord3040017
Chicago/Turabian StyleSmith, David, and Sohan Jheeta. 2021. "Microbiome–Gut Dissociation: Investigating the Origins of Obesity" Gastrointestinal Disorders 3, no. 4: 156-172. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord3040017






