A Protein Assembly Hypothesis for Population-Specific Decrease in Dementia with Time
Abstract
:1. Introduction
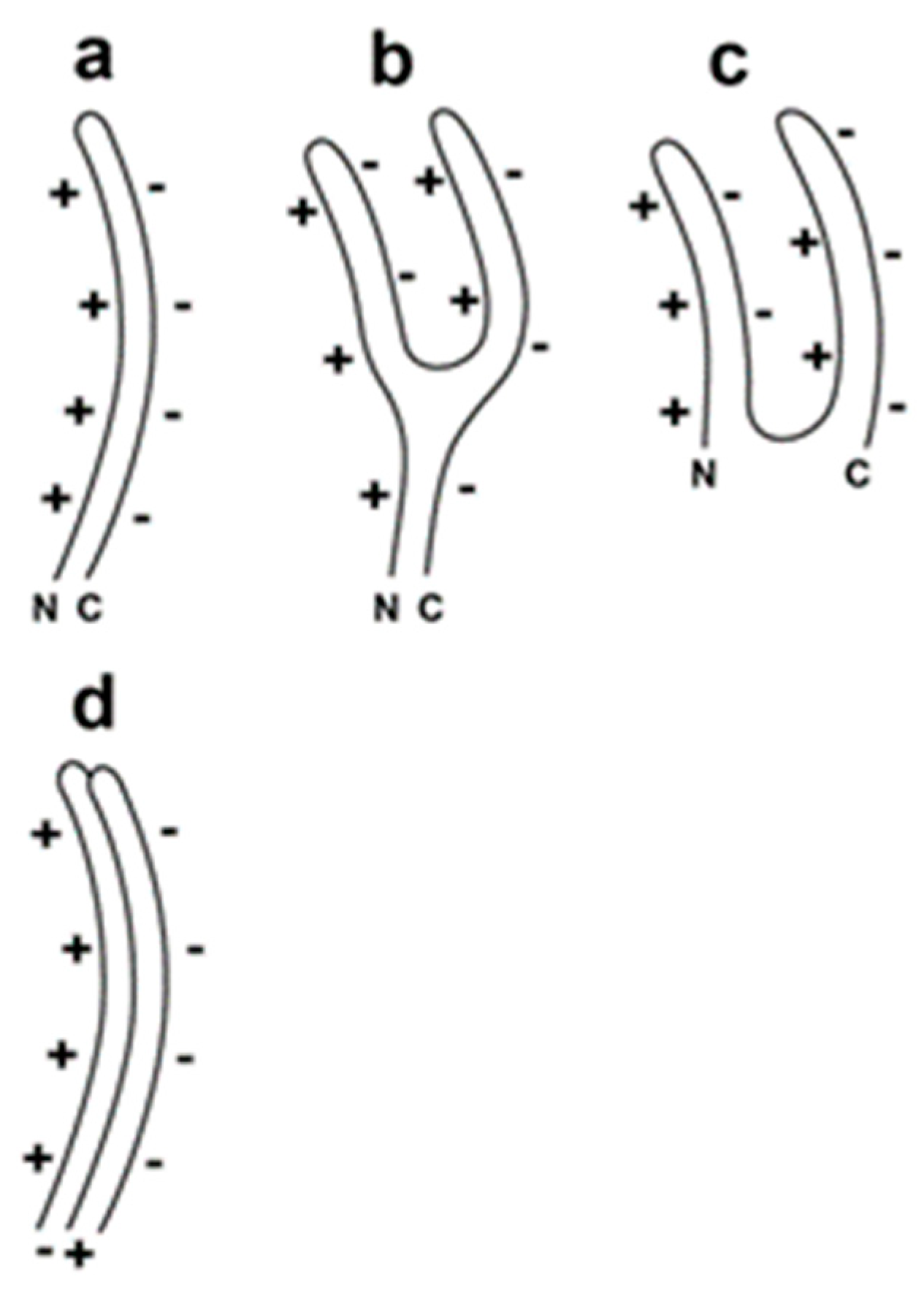
1.1. Foundation for an Explanation: Basics
1.2. Foundation for an Explanation: Details
2. Materials and Methods
3. Results and Discussion
3.1. Outer Shell-Thinning of Hyper-Expanded T3 Capsids
3.2. Other Features of Hyper-Expanded T3 Capsids
3.3. The Hypothesis and Its Explanation of the Data on the Incidence of Alzheimer’s Disease
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolters, F.J.; Chibnik, L.B.; Waziry, R.; Anderson, R.; Berr, C.; Beiser, A.; Bis, J.C.; Blacker, D.; Bos, D.; Brayne, C.; et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020, 95, e519–e531. [Google Scholar] [CrossRef]
- Anonymous. Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Li, S.; Yan, F.; Li, G.; Chen, C.; Zhang, W.; Liu, J.; Jia, X.; Shen, Y. Is the dementia rate increasing in Beijing? Prevalence and incidence of dementia 10 years later in an urban elderly population. Acta Psychiatr. Scand. 2007, 115, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Iwaki, T.; Kitazono, T.; Kanba, S.; Kiyohara, Y.; Ninomiya, T. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 2017, 88, 1925–1932. [Google Scholar] [CrossRef]
- Dobson, C.B.; Itzhaki, R.F. Herpes simplex virus type 1 and Alzheimer’s disease. Neurobiol. Aging 1999, 20, 457–465. [Google Scholar] [CrossRef]
- Itzhaki, R.F. Corroboration of a major role for herpes simplex virus type 1 in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 2018, 99, 64–82.e7. [Google Scholar] [CrossRef] [Green Version]
- Itzhaki, R.F.; Golde, T.E.; Heneka, M.T.; Readhead, B. Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 2020, 16, 193–197. [Google Scholar] [CrossRef]
- Bandea, C.I. Aβ, tau, α-synuclein, Huntingtin, TDP-43, PrP and AA are Members of the Innate Immune System: A Unifying Hypothesis on the Etiology of AD, PD, HD, ALS, CJD and RSA as Innate Immunity Disorders. bioRxiv 2013. Available online: http://biorxiv.org/content/early/2013/11/18/000604 (accessed on 29 October 2020). [CrossRef] [Green Version]
- Eimer, W.A.; Vijaya Kumar, D.K.; Navalpur Shanmugam, N.K.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; György, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 2018, 99, 56–63, Erratum in: Neuron 2018, 100, 1527–1532. [Google Scholar] [CrossRef] [Green Version]
- Serwer, P.; Wright, E.T. Nanomedicine and phage capsids. Viruses 2018, 10, 307. [Google Scholar] [CrossRef] [Green Version]
- Serwer, P.; Hunter, B.; Wright, E.T. Electron microscopy of in-plaque phage T3 assembly: Proposed analogs of neurodegenerative disease triggers. Pharmaceuticals 2020, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L.; Corey, R.B. The pleated sheet, a new layer configuration of polypeptide chains. Proc. Natl. Acad. Sci. USA 1951, 37, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Milner-White, E.J.; Russell, M.J. Predicting the conformations of peptides and proteins in early evolution. Biol. Direct 2008, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Milner-White, E.J.; Russell, M.J. Functional capabilities of the earliest peptides and the emergence of life. Genes 2011, 2, 671–688. [Google Scholar] [CrossRef] [Green Version]
- Armen, R.S.; DeMarco, M.L.; Alonso, D.O.; Daggett, V. Pauling and Corey’s alpha-pleated sheet structure may define the prefibrillar amyloidogenic intermediate in amyloid disease. Proc. Natl. Acad. Sci. USA 2004, 101, 11622–11627. [Google Scholar] [CrossRef] [Green Version]
- Serwer, P. Proposed ancestors of phage nucleic acid packaging motors (and cells). Viruses 2011, 3, 1249–1280. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.S.; Sawaya, M.R. Structural studies of amyloid proteins at the molecular level. Annu. Rev. Biochem. 2017, 86, 69–95. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Cao, Q.; Hughes, M.P.; Sawaya, M.R.; Boyer, D.R.; Cascio, D.; Eisenberg, D.S. CryoEM structure of the low-complexity domain of hnRNPA2 and its conversion to pathogenic amyloid. Nat. Commun. 2020, 11, 4090. [Google Scholar] [CrossRef]
- Baker, M.L.; Jiang, W.; Rixon, F.J.; Chiu, W. Common ancestry of herpesviruses and tailed DNA bacteriophages. J. Virol. 2005, 79, 14967–14970. [Google Scholar] [CrossRef] [Green Version]
- Cardone, G.; Winkler, D.C.; Trus, B.L.; Cheng, N.; Heuser, J.E.; Newcomb, W.W.; Brown, J.C.; Steven, A.C. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology 2007, 361, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElwee, M.; Vijayakrishnan, S.; Rixon, F.; Bhella, D. Structure of the herpes simplex virus portal-vertex. PLoS Biol. 2018, 16, e2006191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaljeh, L.F.; Rothschild, J.A.; Naderi, M.; Coghill, L.M.; Brown, J.M.; Brylinski, M. Hinge region in DNA packaging terminase pUL15 of herpes simplex virus: A potential allosteric target for antiviral drugs. Biomolecules 2019, 9, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yang, Q.; Wang, M.; Jia, R.; Chen, S.; Zhu, D.; Liu, M.; Wu, Y.; Zhao, X.; Zhang, S.; et al. Terminase large subunit provides a new drug target for herpesvirus treatment. Viruses 2019, 11, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serwer, P.; Wright, E.T.; Liu, Z.; Jiang, W. Length quantization of DNA partially expelled from heads of a bacteriophage T3 mutant. Virology 2014, 456, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Serwer, P. Internal proteins of bacteriophage T7. J. Mol. Biol. 1976, 107, 271–291. [Google Scholar] [CrossRef]
- Serwer, P.; Wright, E.T.; Demeler, B.; Jiang, W. States of phage T3/T7 capsids: Buoyant density centrifugation and cryo-EM. Biophys Rev. 2018, 10, 583–596. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, L.; Sun, X.; Cao, Y.; Wang, Z.; Liu, L.; Xu, Y.; Zhou, M.; Liu, Y. Effectiveness and failure rate of the varicella vaccine in an outbreak in Jiangsu, China: A 1:2 matched case-control study. Hum. Vaccin. Immunother. 2020, 16, 506–512. [Google Scholar] [CrossRef]
- Ozaki, T.; Asano, Y. Development of varicella vaccine in Japan and future prospects. Vaccine 2016, 34, 3427–3433. [Google Scholar] [CrossRef]
- Fleming, D.M.; Cross, K.W.; Cobb, W.A.; Chapman, R.S. Gender difference in the incidence of shingles. Epidemiol. Infect. 2004, 132, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren-Gash, C.; Forbes, H.J.; Williamson, E.; Breuer, J.; Hayward, A.C.; Mavrodaris, A.; Ridha, B.H.; Rossor, M.N.; Thomas, S.L.; Smeeth, L. Human herpesvirus infections and dementia or mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 4743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, W.B.; Eiserling, F.A.; Crowther, R.A. 1994. Long tail fibers: Genes, proteins, structure, and assembly. In Molecular Biology of Bacteriophage T4; Karam, J.D., Ed.; ASM Press: Washington, DC, USA, 1994; pp. 282–290. [Google Scholar]
- Arisaka, F.; Yap, M.L.; Kanamaru, S.; Rossmann, M.G. Molecular assembly and structure of the bacteriophage T4 tail. Biophys. Rev. 2016, 8, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, K.P.; Hook, L.M.; Naughton, A.; Pardi, N.; Awasthi, S.; Cohen, G.H.; Weissman, D.; Friedman, H.M. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLoS Pathog. 2020, 16, e1008795. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwer, P.; Wright, E.T. A Protein Assembly Hypothesis for Population-Specific Decrease in Dementia with Time. Biophysica 2021, 1, 15-21. https://0-doi-org.brum.beds.ac.uk/10.3390/biophysica1010002
Serwer P, Wright ET. A Protein Assembly Hypothesis for Population-Specific Decrease in Dementia with Time. Biophysica. 2021; 1(1):15-21. https://0-doi-org.brum.beds.ac.uk/10.3390/biophysica1010002
Chicago/Turabian StyleSerwer, Philip, and Elena T. Wright. 2021. "A Protein Assembly Hypothesis for Population-Specific Decrease in Dementia with Time" Biophysica 1, no. 1: 15-21. https://0-doi-org.brum.beds.ac.uk/10.3390/biophysica1010002





