The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
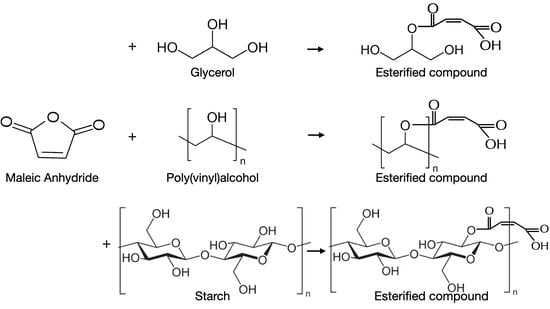
2.2. Production of the Biodegradable Composite Films
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Near-Infrared Hyperspectral Imaging Analysis (NIR-HSI)
2.4.1. NIR-HSI Acquisition
2.4.2. Software
2.4.3. Image Preprocessing
2.4.4. Image Resolution by Multivariate Curve Resolution—Alternating Least Squares (MCR-ALS)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Thermogravimetric Analysis (TGA)
2.7. Physical-Mechanical Properties
2.8. Antimicrobial Activity
2.9. Statistical Analysis
3. Results
3.1. FTIR and NIR Spectra
3.2. NIR-HSI-MCR-ALS Analysis
3.3. Differential Scanning Calorimetry (DSC) and Thermogravimetry (TGA)
3.4. Physical-Mechanical Properties of the Composite Films
3.5. Antimicrobial Effect of the Composite Films over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Cho, S.Y.; Rhee, C.C. Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultrafiltration. LWT Food Sci. Technol. 2004, 37, 833–839. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbargadeh, B.; Entegami, A.A. Physicochemical properties of starch-CMC nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Manson, J.A.; Sperling, L.H. Polymer Blends and Composites; Springer Science & Business Media: New York, NY, USA, 1977; p. 513. [Google Scholar]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable starch-clay nano composite films for food packaging applications. Food Chem. 2005, 3, 467–474. [Google Scholar] [CrossRef]
- Imam, S.H.; Gordon, S.H.; Burgess-Cassler, A.; Greene, R.V. Accessibility of starch to enzymatic degradation in injection-injection-moulded starch-plastic composites. J. Environ. Polym. Degrad. 1995, 3, 107–113. [Google Scholar] [CrossRef]
- Pegoretti, A.; Dorigato, A.; Penati, A. Tensile mechanical response of polyethylene—Clay nanocomposites. Express Polym. Lett. 2007, 1, 123–131. [Google Scholar] [CrossRef]
- Siregar, J.P.; Jaafar, J.; Cionita, F.; Jie, C.C.; Bachtiar, D.; Rejab, M.R.M.; Asmara, Y.P. The effect of maleic anhydride polyethylene on mechanical properties pineapple leaf fibre reinforced polylactic acid composites. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 101–112. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystal: Chemistry, Self-assembly and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Lim, W.S.; Ock, S.Y.; Park, G.D.; Lee, W.; Lee, M.H.; Park, H.J. Heat-sealing property of cassava starch film plasticized with glycerol and sorbitol. Food Packag. Shelf Life 2020, 26, 100556. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, D.M.; Willians, L. Antimicrobial-loaded nano carriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Mulders, J.W.M.; Boerrigter, I.J.; Rollema, H.S.; Siezen, R.J.; Vos, W.M. Identification and characterisation of the lanbiotic Nisin Z, a natural Nisin variant. Eur. J. Biochem. 1991, 201, 581–584. [Google Scholar] [CrossRef]
- Lee, J.M.; Jang, W.J.; Lee, E.-W.; Kong, I.-S. B-glucooligosaccharides derived from barley B-glucan promote growth of lactic acid bacteria and enhance nisin Z secretion by Lactococcus lactic. LWT 2020, 122, 109014. [Google Scholar] [CrossRef]
- Oliveira, T.V.; de Freitas, P.A.D.; de Pola, C.C.; da Silva, J.O.R.; Diaz, L.D.A.; Ferreira, S.O.; Soares, N.d.F.F. Development and optimization of antimicrobial active films produced with a reinforced and compatibilizem biodegradable polymers. Food Packag. Shelf Life 2020, 24, 100459. [Google Scholar] [CrossRef]
- Asensio, R.C.; Moya, M.S.A.; de la Roja, J.M.; Gómez, M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef]
- Terra, L.R.; Roque, J.V.; Pola, C.C.; Gonçalves, I.M.; Soares, N.d.F.F.; Teófilo, R.F. Study of chemical compound spatial distribution in biodegradable active films using NIR hyper spectral imaging and multivariate curve resolution. J. Chemom. 2019, 34, 1–15. [Google Scholar]
- Vidal, M.; Amigo, J.M. Pre-processing of hyperspectral images. Essential steps before image analysis. Chemom. Intell. Lab. Syst. 2012, 117, 138–148. [Google Scholar] [CrossRef]
- Moraes, A.R.F.; Pola, C.C.; Bilk, A.P.; Yamashita, F.; Tronto, J.; Medeiros, E.A.A.; Soares, N.d.F.F. Starch, cellulose acetate and polyester biodegradable sheets: Effect of composition and processing conditions. Mater. Sci. Eng. C 2017, 78, 932–941. [Google Scholar] [CrossRef]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving action of citric acid as compatibilizer in starch/polyester blown films. Ind. Crop Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- ASTM D882. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- CLSI. Antimicrobial Susceptibility Testing Standards; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2012. [Google Scholar]
- Olivato, J.B.; Grossmann, M.V.E.; Yamashita, F.; Nobrega, M.M.; Scapin, M.R.S.; EIiras, D.; Pessan, L.A. Compatibilisation of starch/poly(butylene adipate coterephthalate) blends unblown films. Int. J. Food Sci. Technol. 2011, 46, 1934–1939. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V.; Sharma, M. Formulation and Characterization of biodegradable packaging film derived from potato starch & LPDE grafted with maleic anhydride-LDPE composition. J. Polym. Environ. 2010, 18, 489–491. [Google Scholar]
- Tedeschi, G.; Guzman-Puyol, S.; Ceseracciu, L.; Benitez, J.J.; Cataldi, P.; Bisset, M.; Heredia, A.; Athanassiou, A.; Heredia-Guerrero, J.A. Sustainable, high-barrier polyaleuritate/nanocellulose biocomposites. ACS Sustain. Chem. Eng. 2020, 29, 10682–10690. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly(vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Rahman, W.A.; Sina, L.T.; Rahmat, A.R.; Samad, A.A. Thermal behavior and interactions of cassava starch filled with glycerol plasticized polyvinyl alcohol blends. Carbohydr. Polym. 2010, 81, 805–810. [Google Scholar] [CrossRef]
- Pola, C.C.; Moraes, A.R.F.; Silva, C.R.; Menezes, E.G.T.; Mendes, F.Q.; Guimarães, I.C.; Oliveira, I.R.N.; Dores, M.T.; Monteiro, P.S. Ciência de tecnologia de alimentos: sustentabilidade, desafio e inovação. Rio Paranaíba 2017, 1, 205–228. [Google Scholar]
- Kuwano, K.; Tanaka, N.; Shimizu, T.; Nagatoshi, K.; Nou, S.; Sonomoto, K. Dual antibacterial mechanism of Nisin Z against Gram-positive and Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 26, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Canevarolo, S.V. Ciência de Polímeros: Texto Básico Para Tecnolólogos e Engenheiros, 2nd ed.; Artliber Editora: Sao Paulo, Brazil, 2006. [Google Scholar]
- Zhang, J.F.; Sun, X. Mechanical properties of poly(lactic acid)/starch composites compatibilized by maleic anhydride. Biomacromolecules 2004, 5, 1446–1451. [Google Scholar] [CrossRef]
- Razavi, R.; Tajik, H.; Moradi, M.; Molaei, R.; Ezati, P. Antimicrobial, microscopic and spectroscopic properties of cellulose paper coated with chitoman sol-gel solution formulated by epsilon-poly-L-Lysine and its application in active food packaging. Carbohydr. Res. 2020, 489, 107912. [Google Scholar] [CrossRef] [PubMed]
- Azahari, N.A.; Othman, N.; Ismail, H. Biodegradation studies of polyvinyl alcohol/corn starch blend films in solid and solution media. J. Phys. Sci. 2011, 22, 15–31. [Google Scholar]






| Treatment | Bands (cm−1) | Functional Groups | Reference |
|---|---|---|---|
| T1, T2, T3, T4, T5, T6 | 3320–3300 | ν OH band in bell form present in starch, glycerol, CNC and PVA | Barbosa (2008). |
| 2840–2980 | νas CH3, νs CH3, νas CH2 e νas CH2. | Barbosa (2008). | |
| T2, T3 T4, T5, T6 | 1700 | ν C = O carboxylic acid of nisin present in aminoacids | Barbosa (2008). |
| T1, T2, T3, T4, T5, T6 | 1640 | ν C = C. | Barbosa (2008). |
| T1, T2, T3, T5, T6 | 1540 | δ NH2 amide II band present in nisin Z. | Barbosa (2008). |
| T1, T2, T3, T4, T5, T6 | 1440–1430 | δs CH2. | Barbosa (2008) and Pereira et al. (2015). |
| 1350 | δ CH. | Barbosa (2008). | |
| T2, T3, T4, T5, T6 | 1230 | ν C–O present in anidride ciclic | Barbosa (2008). |
| T2, T3, T4, T5, T6 | 1150 | ν C–O–C from maleic anhydride ring; ν C–O tertiary alcohol present in glycerol, starch and CNC. | Barbosa (2008). |
| T1 | 1140 | ν C–O tertiary alcohol present in starch, glycerol and CNC. | Barbosa (2008). |
| T1, T3, T5 | 1080 | ν C–O primary alcohol present in CH2OH ramification and directly bonding into glucose starch, CNC and glycerol. | Barbosa (2008). |
| T2, T4, T6 | 1070 | ν C–O–C present in maleic anhydride ring; ν C–O glycerol tertiary alcohol; ν C–O primary alcohol present in CH2OH ramification and directly bonding into starch ring and CNC. | Barbosa (2008). |
| T3, T4, T5, T6 | 1040–1022. | ν C–O primary alcohol present in CH2OH ramification and directly bonding into glucose starch, CNC and glycerol. | Barbosa (2008). |
| T2 | 1010 | stretching vibration of C–O in C–O–C bonds, mostly present in the glycosidic linkages | Olivato et al. (2012) |
| T1, T3, T4, T5, T6 | 860 | γNH2 angular deformation of plan of nisin Z amine | Barbosa (2008). |
| Compound | Bands (cm−1) | Functional Groups | Reference |
|---|---|---|---|
| MA | 3080 | νas = CH ring presence | Barbosa (2008). |
| 2975 | νs = CH ring presence | Barbosa (2008). | |
| 1826 | νas C = O ciclic anhydride. | Barbosa (2008). | |
| 1623 | ν C = C ring presence | Barbosa (2008). | |
| 1250 | ν C–O ciclic anhydride | Murillo and López (2015). | |
| 880 | ν C–O–C ring presence | Barbosa (2008). | |
| Starch | 3066–3658 (3340) | ν OH bell-shaped band present in the CH2OH branching and attached directly into the amido ring. | Barbosa (2008). |
| 2930 | νas CH2. | Barbosa (2008). | |
| 1099 | ν C–O secondary alcohol present in ring and ν C–O–C–O–C glycosidic bond (acetal). | Barbosa (2008) and Teixeira et al. (2017). | |
| PVA | 3580–3700 | ν OH bell-shaped band | Barbosa (2008). |
| 2860–2975 | νas and νs CH3 terminal, νas and νs CH2. | Barbosa (2008). | |
| 1470 | δs CH2. | Barbosa (2008), Pereira et al. (2015). | |
| 1380 | δs CH3. | Barbosa (2008). | |
| 1080 | ν C–O secondary alcohol | Barbosa (2008), Xu et al. (2005). | |
| 735 | ρ CH2 deformation | Barbosa (2008). |
| Treatment | Tmb (°C) | Tmp (°C) | Tme (°C) | ∆Hf (J g−1) | |||||
| T1 | 194.5 | 195.9 | 205.5 | 130.9 | |||||
| T2 | 189.6 | 191.2 | 202.7 | 155.1 | |||||
| T3 | 192.4 | 193.4 | 202.9 | 142.6 | |||||
| T4 | 189.1 | 190.1 | 200.0 | 140.4 | |||||
| T5 | 195.4 | 196.9 | 206.3 | 143.2 | |||||
| T6 | 196.5 | 197.7 | 207.1 | 128.5 | |||||
| Films | Event 1 | Event 2 | Event 3 * | ||||||
| Ti–Tf (°C) | Tp (°C) | ∆w (%) | Ti–Tf (°C) | Tp (°C) | ∆w (%) | Ti–Tf (°C) | Tp (°C) | ∆w (%) | |
| T1 | 30–166 | 78 | 12 | 182–390 | 303 | 72 | 382–534 | 429 | 69 |
| T2 | 30–166 | 78 | 12 | 182–390 | 297 | 65 | 382–534 | 429 | 69 |
| T3 | 30–166 | 78 | 16 | 182–390 | 299 | 71 | 382–534 | 429 | 71 |
| T4 | 30–166 | 78 | 13 | 182–390 | 300 | 70 | - | - | - |
| T5 | 30–166 | 78 | 12 | 182–390 | 300 | 68 | 382–534 | 429 | 73 |
| T6 | 30–166 | 78 | 16 | 182–390 | 299 | 71 | - | - | - |
| Treatment | Thickiness (mm) | TSB (MPa) | EB (mm) | YM (Mpa) |
|---|---|---|---|---|
| T1 | 0.159 | 6.16 | 72.09 | 129.95 |
| T2 | 0.127 | 7.02 | 69.77 | 206.20 |
| T3 | 0.196 | 4.24 | 99.00 | 57.96 |
| T4 | 0.187 | 4.78 | 18.35 | 132.91 |
| T5 | 0.183 | 4.73 | 64.81 | 76.89 |
| T6 | 0.177 | 2.82 | 72.94 | 27.38 |
| Staphylococcus Aureus | ||||
| Storage Days | ||||
| Treatment | 1 | 7 | 14 | 21 |
| T1 | 1.85 ± 0.01 a,x | 1.73 ± 0.16 a,x | 1.8 ± 0.02 a,x | 1.96 ± 0.03 a,x |
| T2 | 1.83 ± 0.11 a,x | 1.93 ± 0.01 a,x | 1.97 ± 0.01 a,x | 2.13 ± 0.07 a,x |
| T3 | 1.86 ± 0.03 a,x | 1.80 ± 0.01 a,x | 1.77 ± 0.21 a,x | 2.02 ± 0.08 a,x |
| T4 | 1.77 ± 0.27 a,x | 1.95 ± 0.02 a,x | 1.54 ± 0.03 a,x | 1.59 ± 0.07 b,x |
| T5 | 1.86 ± 0.05 a,x | 1.83 ± 0.04 a,x | 1.74 ± 0.23 a,x | 1.62 ± 0.08 b,x |
| T6 | 1.94 ± 0.01 a,x | 1.94 ± 0.05 a,x | 1.79 ± 0.01 a,y | 1.80 ± 0.04 a,y |
| Pseudomonas Aeruginosa | ||||
| Storage Days | ||||
| Treatment | 1 | 7 | 14 | 21 |
| T1 | 2.23 ± 0.18 a,x | 2.02 ± 0.54 a,x | 2.30 ± 0.23 a,x | 2.33 ± 0.01 a,x |
| T2 | 2.27 ± 0.18 a,x | 2.44 ± 0.01 a,x | 2.20 ± 0.01 a,x | 2.12 ± 0.17 ab,x |
| T3 | 2.26 ± 0.22 a,x | 2.45 ± 0.07 a,x | 2.00 ± 0.05 a,x | 2.02 ± 0.14 ab,x |
| T4 | 2.12 ± 0.28 a,x | 2.07 ± 0.41 a,x | 2.14 ± 0.14 a,x | 2.36 ± 0.17 a,x |
| T5 | 2.18 ± 0.45 a,x | 2.13 ± 0.21 a,x | 2.16 ± 0.34 a,x | 1.85 ± 0.01 b,x |
| T6 | 2.05 ± 0.24 a,x | 2.09 ± 0.22 a,x | 1.95 ± 0.13 a,x | 2.11 ± 0.12 ab,x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.V.d.; Freitas, P.A.V.d.; Pola, C.C.; Terra, L.R.; Silva, J.O.R.d.; Badaró, A.T.; Junior, N.S.; Oliveira, M.M.d.; Silva, R.R.A.; Soares, N.d.F.F. The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix. Polysaccharides 2021, 2, 661-676. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2030040
Oliveira TVd, Freitas PAVd, Pola CC, Terra LR, Silva JORd, Badaró AT, Junior NS, Oliveira MMd, Silva RRA, Soares NdFF. The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix. Polysaccharides. 2021; 2(3):661-676. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2030040
Chicago/Turabian StyleOliveira, Taíla V. de, Pedro A. V. de Freitas, Cicero C. Pola, Larissa R. Terra, José O. R. da Silva, Amanda T. Badaró, Nelson S. Junior, Marciano M. de Oliveira, Rafael R. A. Silva, and Nilda de F. F. Soares. 2021. "The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix" Polysaccharides 2, no. 3: 661-676. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2030040