Optimization of the Mechanical Properties of Polyolefin Composites Loaded with Mineral Fillers for Flame Retardant Cables
Abstract
:1. Introduction
2. Materials and Methods
- Poly (ethylene-co-vinyl acetate) EVA28 ELVAX 265A, Du Pont, containing 28 wt.% of vinyl acetate, Melt Flow Index = 3 g/10 min, Density = 0.955 g/cm3.
- ULDPE-g-MAH, Fusabond N525, Dow, Ultra Low Density Polyethylene C2-C8 Copolymer, grafted with Maleic Anhydride (0.7–1.1 wt.%), Melt Flow Index = 3.7 g/10 min, Density = 0.88 g/cm3.
- Masterbatch of PDMS, Silmaprocess AL1142A by Silma Srl (Bologna, Italy), composed by 50 wt.% of high viscosity PMDS and 50 wt.% LLDPE, Silicon MB.
- Fillers used are described in Table 1:
- Grades of poly (ethylene-co-α-olefin) used are described in Table 2:
- Grades of C3-C2 copolymers (propylene-rich) used are described in Table 3:
3. Results
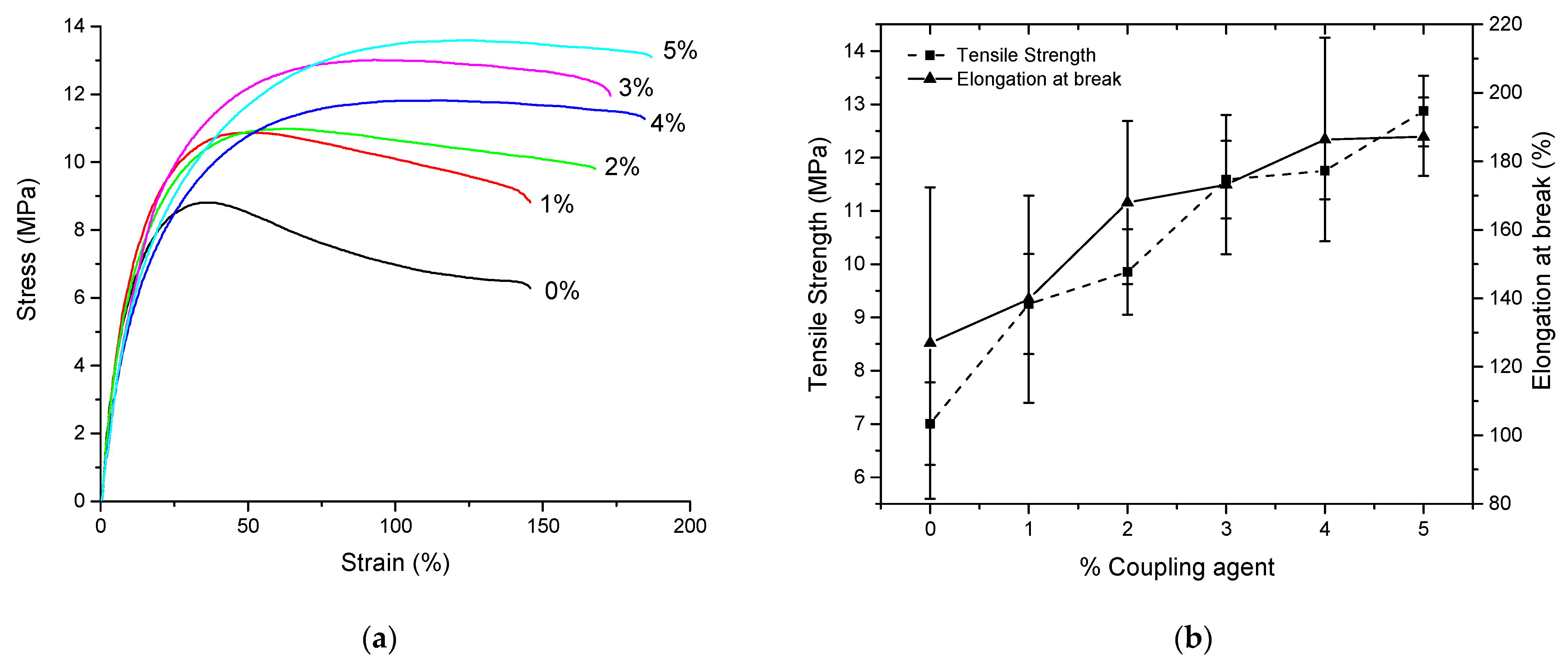
3.1. Study of the Amount of Coupling Agent
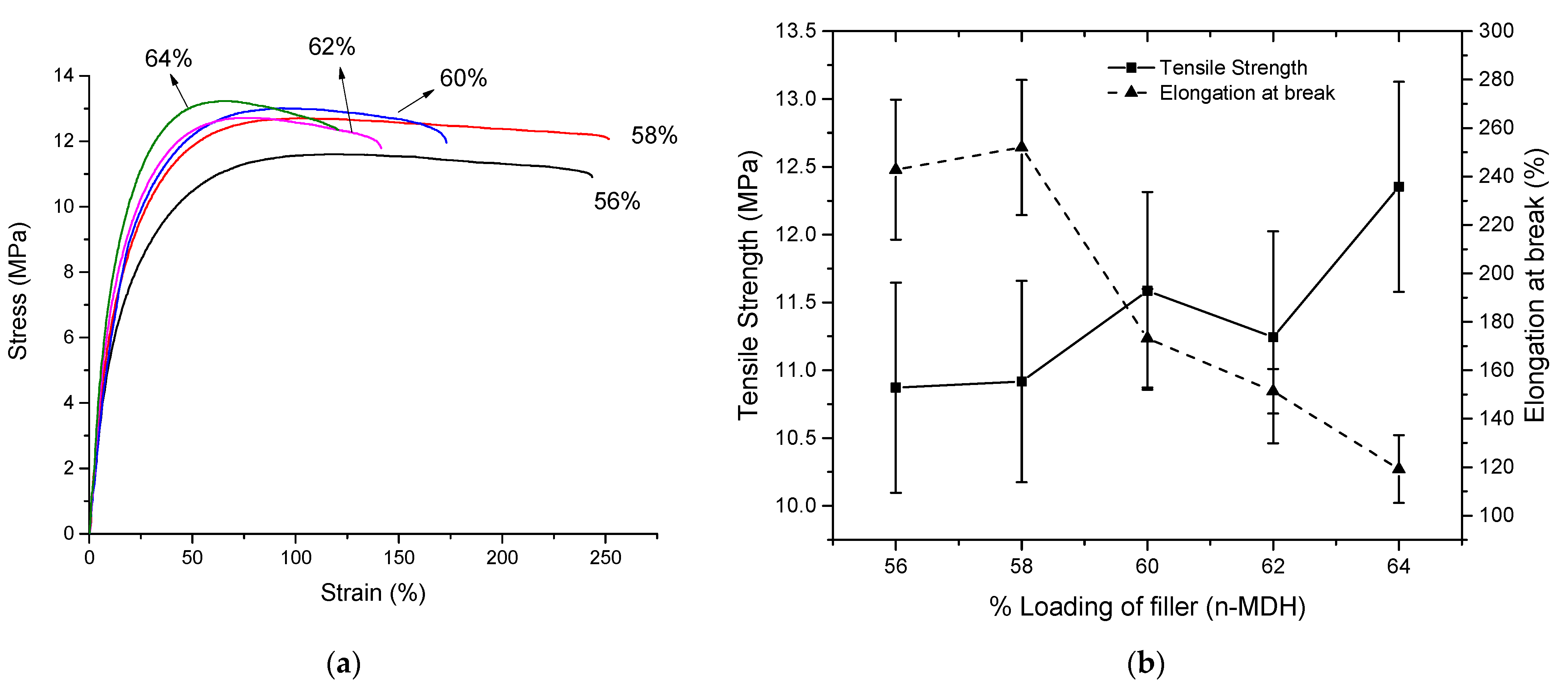
3.2. Variation of Content of n-MDH
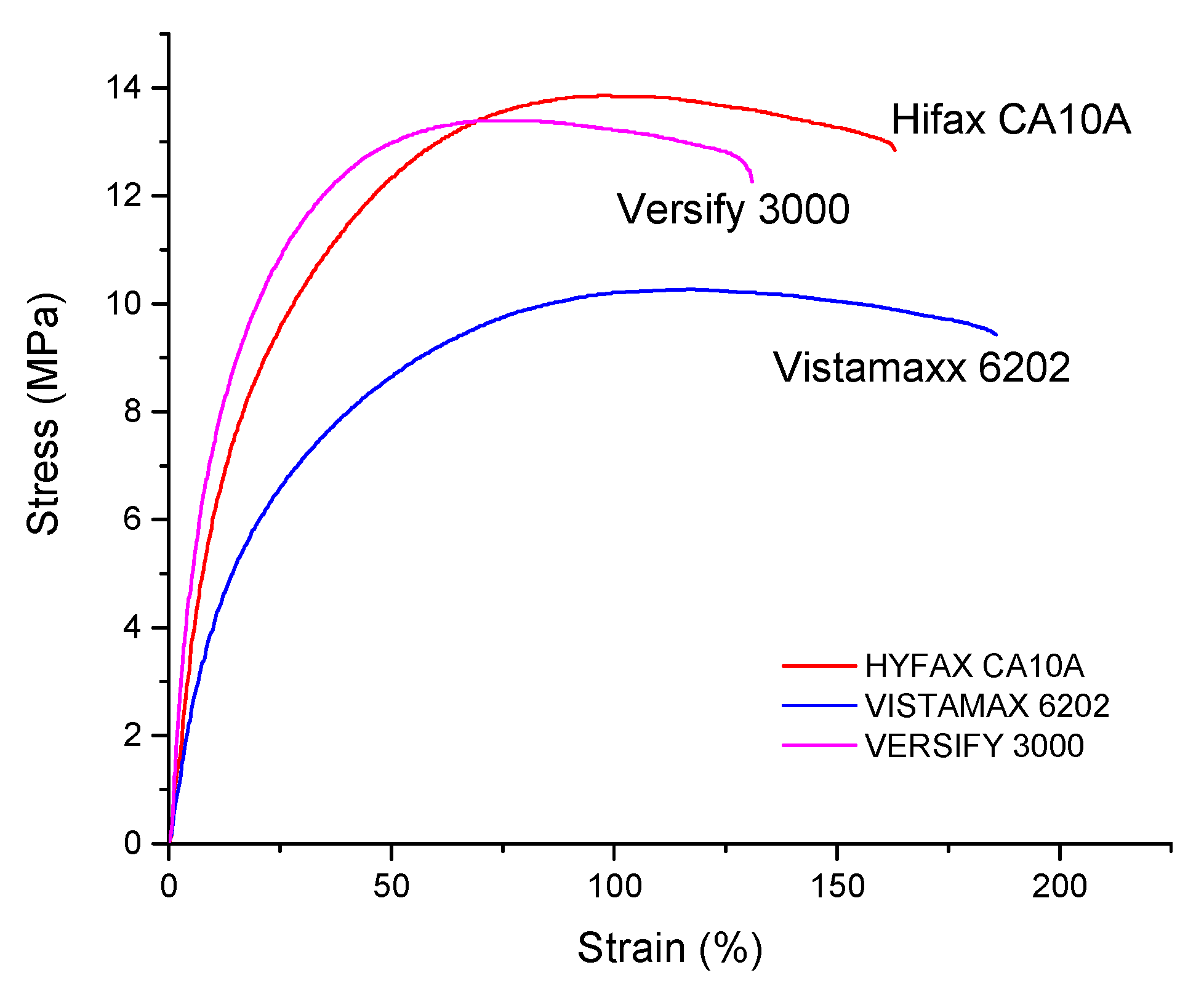
3.3. Variation of Type of Polyolefin Used in Combination with EVA28
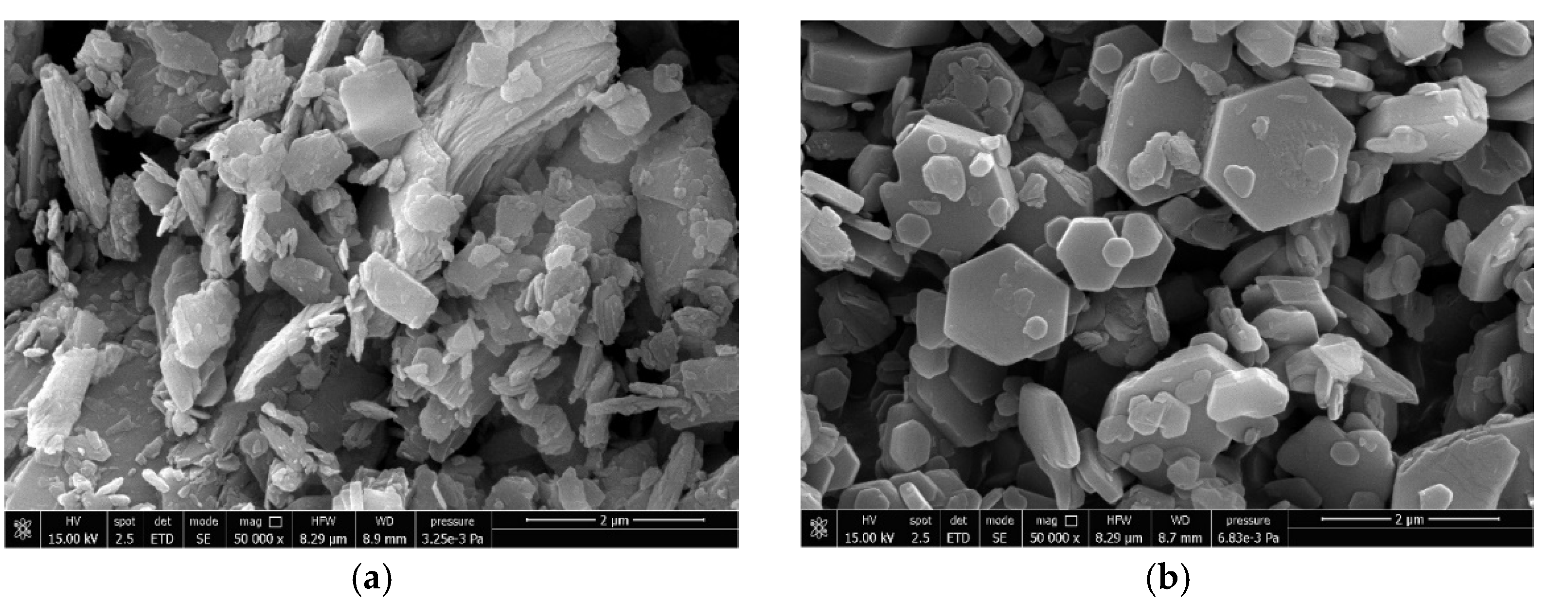
3.4. Variation of Type of Mineral Filler Used in Combination with Natural n-MDH
3.5. Flame Retardant Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jancar, J.; Fekete, E.; Hornsby, P.R.; Jancar, J.; Pukánszky, B.; Rothon, R.N. Mineral Fillers in Thermoplastics I. Adv. Polym. Sci. 1999. [Google Scholar] [CrossRef]
- Shrivastava, A. Additives for Plastics. In Introduction to Plastics Engineering; Elsevier: Oxford, UK, 2018; pp. 111–141. [Google Scholar] [CrossRef]
- Kiran, M.; Govindaraju, H.; Jayaraju, T.; Kumar, N. Review-Effect of Fillers on Mechanical Properties of Polymer Matrix Composites. Mater. Today Proc. 2018, 5, 22421–22424. [Google Scholar] [CrossRef]
- Rothon, R.N. Particulate-Filled Polymer Composites, 2nd ed.; Rapra Techonology Limited: Shrewsbury, UK, 2003; Chapter 6. [Google Scholar]
- Hornsby, P.; Rothon, R. Fire Retardant Fillers for Polymers: New Applications of Mineral Fillers; Bras, M.L., Wilkie, C., Bourbigot, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2005; Chapter 2. [Google Scholar]
- Hornsby, P. Fire retardant fillers for polymers. Int. Mater. Rev. 2001, 46, 199–210. [Google Scholar] [CrossRef]
- Polymer Data Handbook; Oxford University Press: Oxford, UK, 1999.
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Hull, R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Flame Retardant Composites. In Fire Properties of Polymer Composite Materials; Solid Mechanics and Its Applications; Springer: Berlin/Heidelberg, Germany, 2006; Volume 143.
- Tian, N.; Wen, X.; Jiang, Z.; Gong, J.; Wang, Y.; Xue, J.; Tang, T. Synergistic Effect between a Novel Char Forming Agent and Ammonium Polyphosphate on Flame Retardancy and Thermal Properties of Polypropylene. Ind. Eng. Chem. Res. 2013, 52, 10905–10915. [Google Scholar] [CrossRef]
- Witkowski, A.; Hollingbery, L.; Hull, T.R. Fire Retardancy of Mineral Fillers in EVA Copolymers. In Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; ACS Publication: Washington, DC, USA, 2012; pp. 97–111. [Google Scholar] [CrossRef]
- Musselman, L. Alumina Chemicals as Additives for Paper, Dentifrices, Paints, Coatings, Rubbers and Plastics with Emphasis on Fire-Retardant Products. In Handbook of Science and Technology of Alumina Chemicals; Wiley: Hoboken, NJ, USA, 1990; p. 195. [Google Scholar]
- Innes, J.; Innes, A. Plastic Flame Retardants: Technology and Current Developments; Rapra Technology Limited: Shrewsbury, UK, 2003. [Google Scholar]
- Weil, E.D.; Levchik, S.V. Flame Retardants for Plastics and Textiles; Hanser eLibrary: Munich, Germany, 2009. [Google Scholar] [CrossRef]
- Amin, S.; Amin, M. Thermoplastic elastomeric (TPE) materials and their use in outdoor electrical insulation. Rev. Adv. Mater. Sci. 2011, 29, 15–30. [Google Scholar]
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies—History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- B. V Europiren. Cables. 2019. Available online: https://www.ami.international/events/event?Code=C0948 (accessed on 1 May 2021).
- Costache, M.C.; Jiang, D.D.; Wilkie, C.A. Thermal degradation of ethylene–vinyl acetate coplymer nanocomposites. Polymer 2005, 46, 6947–6958. [Google Scholar] [CrossRef]
- Polanský, R.; Pinkerová, M.; Bartůňková, M.; Prosr, P. Mechanical Behavior and Thermal Stability of EVA Encapsulant Material Used in Photovoltaic Modules. J. Electr. Eng. 2013, 64, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Hull, T.; Stec, A. Polymers and Fire. In 11th Meeting, Proceedings of the Fire Retardancy of Polymers: New Strategies and Mechanisms, Bolton, UK, 7 July 2007; Hull, T.R., Kandola, B.K., Eds.; Royal Society of Chemistry: London, UK, 2009; p. 433. [Google Scholar] [CrossRef]
- Dando, N.R.; Clever, T.R.; Kolek, P.L.; Pearson, A.; Martin, E.S. Aluminum trihydroxide (ATH) as a filler for polymer composites: Comparative evaluation of precipitation and grinding on thermal stability and dehydration kinetics. In 51st Annual Conference of the Society of the Plastics Industry, Inc (SPI), Session 19-A, 5–7 February 1996; American Composites Manufacturers Association (ACMA): Arlington, VA, USA, 1996; pp. 1–9. [Google Scholar]
- Zhao, Z.-Y.; Dong, L.-P.; Chen, L.; Wang, Y.-Z. Morphology development of PP/POE blends with high loading of magnesium hydroxide. RSC Adv. 2015, 5, 17967–17975. [Google Scholar] [CrossRef]
- El Hage, R.; Viretto, A.; Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.-M. Flame retardancy of ethylene vinyl acetate (EVA) using new aluminum-based fillers. Polym. Degrad. Stab. 2014, 108, 56–67. [Google Scholar] [CrossRef]
- Tolinski, M. Coupling and Compatibilizing. Polyolefins 2015, 153–158. [Google Scholar] [CrossRef]
- Lin, T.S.; Bunker, S.P.; Whaley, P.D.; Cogen, J.M.; Bolz, K.A.; Alsina, M.F. Evaluation of Metal Hydroxides and Coupling Agents for Flame Resistant Industrial Cable Applications. In Proceedings of the 54th IWCS/FOCUS Conference: International Wire & Cable Symposium (IWCS), Providence, RI, USA, 13–16 November 2005; p. 229. [Google Scholar]
- Tolinski, M. Processing Aids for Molding. Polyolefins 2015, 129–134. [Google Scholar] [CrossRef]
- Tolinski, M. Processing Aids for Extrusion. Polyolefins 2015, 135–144. [Google Scholar] [CrossRef]
- Graft Polymer-Material Comparison Report. 2013, pp. 253–254. Available online: https://graftpolymer.com/wp-content/uploads/2019/08/Full-Materials-Report-DuPont-GPO.pdf (accessed on 30 April 2021).
- Fu, M.; Qu, B. Synergistic flame retardant mechanism of fumed silica in ethylene-vinyl acetate/magnesium hydroxide blends. Polym. Degrad. Stab. 2004, 85, 633–639. [Google Scholar] [CrossRef]
- Wypych, G. Flammability of Filled Materials. Handb. Fill. 2016, 589–604. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Liu, L.; Liang, W.; Zhang, L. Effect of particle size on flame retardancy of Mg(OH)2-filled ethylene vinyl acetate copolymer composites. J. Appl. Polym. Sci. 2006, 100, 4461–4469. [Google Scholar] [CrossRef]
- I Cavi di vErsalis-Versalis Wire & Cable. 2015, p. 24. Available online: https://www.versalis.eni.com/irj/go/km/docs/versalis/ContenutiVersalis/EN/Documenti/Documentazione/Brochure/Polietilene/Brochure/248x200Cavi_WEB.pdf (accessed on 12 May 2021).
- The Chemistry of Polyethylene Insulation. Available online: https://www.lyondellbasell.com/globalassets/documents/polymers-technical-literature/the-chemistry-of-polyethylene-insulation.pdf (accessed on 15 May 2021).
- Tan, Y.; Wachtendorf, V.; Kukofka, T.; Klack, P.; Ruder, J.; Lin, X.; Schartel, B. Degradation of flame retardance: A comparison of ethylene-vinyl acetate and low-density polyethylene cables with two different metal hydroxides. J. Appl. Polym. Sci. 2020, 138, app50149. [Google Scholar] [CrossRef]
- Batistini, A. New polyolefin plastomers and elastomers made with insite™ technology: Structure—Property relationship and benefits in flexible thermoplastic applications. Macromol. Symp. 1995, 100, 137–142. [Google Scholar] [CrossRef]
- DeArmitt, C.; Rothon, R. Particulate Fillers, Selection, and Use in Polymer Composites. In Polymers and Polymeric Composites: A Reference Series; Springer: Heidelberg/Berlin, Germany, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Yang, J.; Li, H.; Liang, W.; Zhang, L.; Li, X. Stearic acid surface modifying Mg(OH)2: Mechanism and its effect on properties of ethylene vinyl acetate/Mg(OH)2 composites. J. Appl. Polym. Sci. 2008, 107, 3325–3331. [Google Scholar] [CrossRef]
- Osman, M.A.; Atallah, A.; Suter, U.W. Influence of excessive filler coating on the tensile properties of LDPE–calcium carbonate composites. Polymer 2004, 45, 1177–1183. [Google Scholar] [CrossRef]
- Cardelli, A. Rheological, Mechanical, Thermal and Flame Retardant Properties of EVA Composites Highly Filled with Natural Inorganic Fillers. 2012. Available online: https://etd.adm.unipi.it/theses/available/etd-01182012-124202/unrestricted/PhD_thesis_Angela_Cardelli.pdf (accessed on 1 July 2021).
- Cardelli, C. Combination of natural and synthetic FR mineral fillers: Opportunities for flame retardant not toxic cables. In Proceedings of the FRPM 2019 European Meeting on fire Retardant Polymeric Materials, Turku, Finland, 26–28 June 2019; Available online: https://frpm19.com/programme (accessed on 1 July 2021).
- Zhang, L.; Li, C.; Zhou, Q.; Shao, W. Aluminum hydroxide filled ethylene vinyl acetate (EVA) composites: Effect of the interfacial compatibilizer and the particle size. J. Mater. Sci. 2007, 42, 4227–4232. [Google Scholar] [CrossRef]



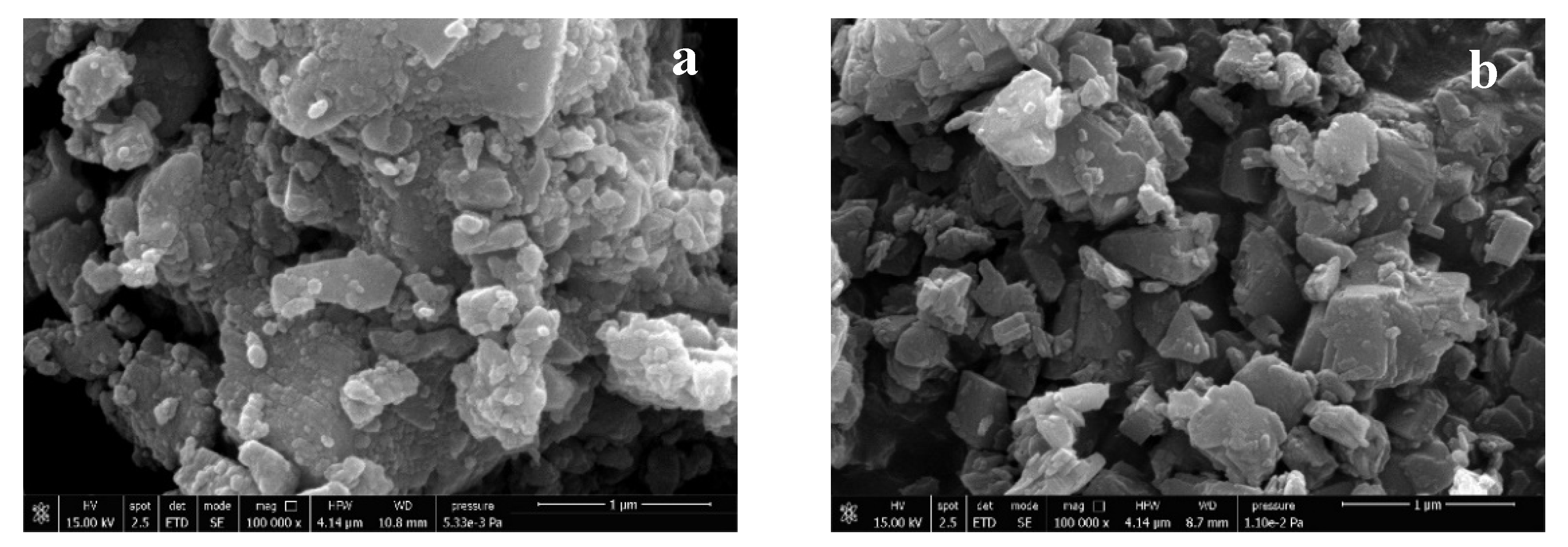


| Ingredient | Chemical Formula | Origin | Trade Name | Supplier | D50 *1 (μm) | BET *2 (m2/g) |
|---|---|---|---|---|---|---|
| n-MDH | Mg(OH)2 | Natural | Ecopiren 3.5 | Europiren | 3.43 | 11–13 |
| CaCO3 stearic coated | CaCO3 | Natural | Polyplex 0 | Calcit | 2.10 | 9.5 |
| Boehmite | AlO(OH) | Synthetic | Aluprem TB 1/T | Tor Minerals | 1.21 | 12 |
| S–MDH | Mg(OH)2 | Synthetic | Magnifin H5 | Huber | 1.50 | 4–6 |
| Mg (OH)2 coated with silane | Mg(OH)2 | Synthetic | Magnifin H5GV | Huber | 1.50 | 2–5 |
| coated with stearic acid | Mg(OH)2 | Synthetic | Kisuma 5A | Kisuma | 1.50 | 4–7 |
| CaCO3 | CaCO3 | Synthetic | Winnofil S | Solvay | 0.3 | 14–16 |
| Huntite | CaMg3(CO3)4 | Natural | Portafill H5 | Sibelco | 3.27 | 18 |
| Ingredient *3 | Trade Name | Supplier | Density *1 | MFI *2 | Catalysis |
|---|---|---|---|---|---|
| C4–LLDPE | Flexirene CL10U | Versalis | 0.918 | 2.5 | Z-N |
| C6–mLLDPE | Exceed 3518 | ExxonMobil | 0.918 | 3.5 | metallocene |
| C6–mLLDPE | Exceed 3812 | ExxonMobil | 0.912 | 3.8 | metallocene |
| C6–mLLDPE | Evolue SP1071C | Prime Polymer | 0.910 | 10 | metallocene |
| C6–mLLDPE | Exceed 0015XC | ExxonMobil | 0.918 | 15 | metallocene |
| C8-ULDPE | Engage 8450 | Dow | 0.902 | 3 | metallocene |
| C4-VLDPE | Clearflex MBQ0 | Versalis | 0.911 | 13 | Z-N |
| Ingredient | Trade Name | Supplier | Density *1 | MFI *2 | Catalysis |
|---|---|---|---|---|---|
| Heterophasic PP-EPR | Hifax CA10A | Lyndell-Basell | 0.880 | 0.6 | Z-N |
| C3–C2 copolymer | Vistamaxx 6202 | Exxon-Mobil | 0.862 | 9.1 | metallocene |
| C3–C2 copolymer | Versify 3000 | Dow | 0.891 | 8 | metallocene |
| Ingredients | wt.% | Role |
|---|---|---|
| EVA 28–3 | 27 | Vinyl acetate comonomer contributes to flexibility, polarity, good behavior in fire tests (char forming) |
| Ethylene α-olefin copolymer | 7–12 | Resistance to deformation at high temperature |
| Mineral filler | 56–64 | Flame retardant |
| ULDPE–g–MAH | 0–5 | Coupling agent |
| Silicon MB | 1 | Processing aid |
| Ingredient | Trade Name | A (%) | B (%) | C (%) | D (%) | E (%) | F (%) |
|---|---|---|---|---|---|---|---|
| EVA 28–3 | Elvax 265 | 27 | 27 | 27 | 27 | 27 | 27 |
| C8–ULDPE | Engage 8450 | 12 | 11 | 10 | 9 | 8 | 7 |
| ULDPE–g–MAH | Fusabond N 525 | 0 | 1 | 2 | 3 | 4 | 5 |
| N–MDH | Ecopiren 3.5 | 60 | 60 | 60 | 60 | 60 | 60 |
| Silicon MB | Silmaprocess AL1142A | 1 | 1 | 1 | 1 | 1 | 1 |
| Properties | Unit | A 0% | B 1% | C 2% | D 3% | E 4% | F 5% |
|---|---|---|---|---|---|---|---|
| Density *1 (±0.1) | g/cm3 | 1.475 | 1.471 | 1.474 | 1.469 | 1.471 | 1.471 |
| Calculated Density | g/cm3 | 1.472 | 1.471 | 1.471 | 1.470 | 1.469 | 1.469 |
| MFI *2 | g/10 min | 14.7 ± 0.7 | 11.6 ± 0.6 | 10.3 ± 0.5 | 9.2 ± 0.5 | 7 ± 0.3 | 6.6 ± 0.4 |
| Young’s Modulus | MPa | 99 ± 6 | 94 ± 12 | 89 ± 8 | 81 ± 7 | 74 ± 12 | 70 ± 2 |
| Tensile Strength *3 | MPa | 7.0 ± 0.8 | 9.3 ± 0.9 | 9.9 ± 0.8 | 11.6 ± 0.7 | 11.8 ± 0.5 | 12.9 ± 0.7 |
| Elongation at break *3 | % | 127 ± 45 | 140 ± 30 | 168 ± 24 | 173 ± 20 | 199 ± 18 | 187 ± 11 |
| Properties | Unit | 56% | 58% | 60% | 62% | 64% |
|---|---|---|---|---|---|---|
| Density (±0.1) | g/cm3 | 1.416 | 1.445 | 1.469 | 1.502 | 1.529 |
| Calculated Density | g/cm3 | 1.418 | 1.444 | 1.470 | 1.497 | 1.526 |
| MFI | g/10 min | 12.4 ± 0.6 | 11.4 ± 0.6 | 9.2 ± 0.5 | 5.6 ± 0.3 | 3.8 ± 0.2 |
| Young’s Modulus | MPa | 70 ± 5 | 80 ± 7 | 81 ± 7 | 83 ± 6 | 97 ± 7 |
| LOI (±0.5) * | % | 34 | 34 | 36 | 37 | 40 |
| Tensile Strength | MPa | 10.9 ± 0.8 | 10.9 ± 0.7 | 11.6 ± 0.7 | 11.2 ± 0.8 | 12.4 ± 0.8 |
| Elongation at break | % | 243 ± 29 | 252 ± 28 | 173 ± 20 | 151 ± 9 | 119 ± 14 |
| Properties | Unit | 4 C4-LLDPE | 5 C6-mLLDPE | 6 C6-mLLDPE | 7 C6-mLLDPE | 8 C6-mLLDPE | 9 C8-ULDPE | 10 C4-VLDPE |
|---|---|---|---|---|---|---|---|---|
| Second polymer | Trade Name | Flexirene CL10U | Exceed 3518 | Exceed 3812 | Evolue SP1071 | Exceed 0015XC | Engage 8450 | Clearflex MBQ0 |
| Density (±0.1) | g/cm3 | 1.473 | 1.478 | 1.477 | 1.475 | 1.473 | 1.469 | 1.478 |
| Calculated Density | g/cm3 | 1.474 | 1.474 | 1.472 | 1.472 | 1.474 | 1.470 | 1.472 |
| MFI | g/10 min | 10 ± 0.5 | 5.7 ± 0.3 | 7.1 ± 0.4 | 10.3 ± 0.5 | 14.9±0.7 | 9.2 ± 0.5 | 13 ± 0.7 |
| Young’s Modulus | MPa | 88 ± 5 | 105 ± 5 | 95 ± 9 | 96 ± 4 | 95 ± 6 | 81 ± 7 | 101 ± 10 |
| Tensile Strengh | MPa | 14.1 ± 1.3 | 12.7 ± 0.5 | 11.9 ± 0.8 | 12.5 ± 0.8 | 12.0 ± 0.7 | 11.6 ± 0.7 | 11.3 ± 0.7 |
| Elongation at Break | % | 114 ± 11 | 149 ± 16 | 138 ± 17 | 126 ± 20 | 111 ± 12 | 173 ± 20 | 194 ± 18 |
| Properties | Unit | 11 Heterophasic PP-EPR | 12 C3-C2 Copolymer | 13 C3-C2 Copolymer |
|---|---|---|---|---|
| Second polymer | Trade Name | Hifax CA10A | Vistamaxx 6202 | Versify 3000 |
| Density (±0.1) | g/cm3 | 1.472 | 1.472 | 1.472 |
| Calculated Density | g/cm3 | 1.470 | 1.470 | 1.470 |
| MFI | g/10 min | 5.6 ± 0.3 | 11 ± 0.6 | 13 ± 0.6 |
| Young’s Modulus | MPa | 76 ± 3 | 58 ± 8 | 109 ± 7 |
| Tensile Strength | MPa | 12.7 ± 0,5 | 10.9 ± 0.7 | 11.9 ± 0.9 |
| Elongation at Break | % | 163 ± 12 | 181 ± 20 | 128 ± 24 |
| Properties | Unit | 9 Ecopiren 3.5 (n-MDH) | 14 Magnifin H5 (s-MDH) |
|---|---|---|---|
| Density (±0.1) | g/cm3 | 1.469 | 1.462 |
| MFI | g/10 min | 9.2 ± 0.5 | 12.8 ± 0.6 |
| Young’s Modulus | MPa | 81 ± 7 | 72 ± 19 |
| Tensile Strength | MPa | 11.6 ± 0.7 | 11.8 ± 0.2 |
| Elongation at break | % | 173 ± 20 | 272 ± 71 |
| Properties | Unit | 9 n-MDH | 15 Coated CaCO3 | 16 Huntite/Hydromagnesite |
|---|---|---|---|---|
| Secondary filler | Trade Name | Ecopiren 3.5 | Polyplex 0 | Portafill H5 |
| Density (±0.1) | g/cm3 | 1.469 | 1.487 | 1.489 |
| MFI | g/10 min | 9.2 ± 0.5 | 10.4 ± 0.5 | 6.6 ± 0.3 |
| Young’s Modulus | MPa | 81 ± 7 | 65 ± 4 | 87 ± 4 |
| Tensile Strength | MPa | 11.6 ± 0.7 | 10.1 ± 0.5 | 11.8 ± 0.2 |
| Elongation at break | % | 173 ± 20 | 198 ± 16 | 177 ± 24 |
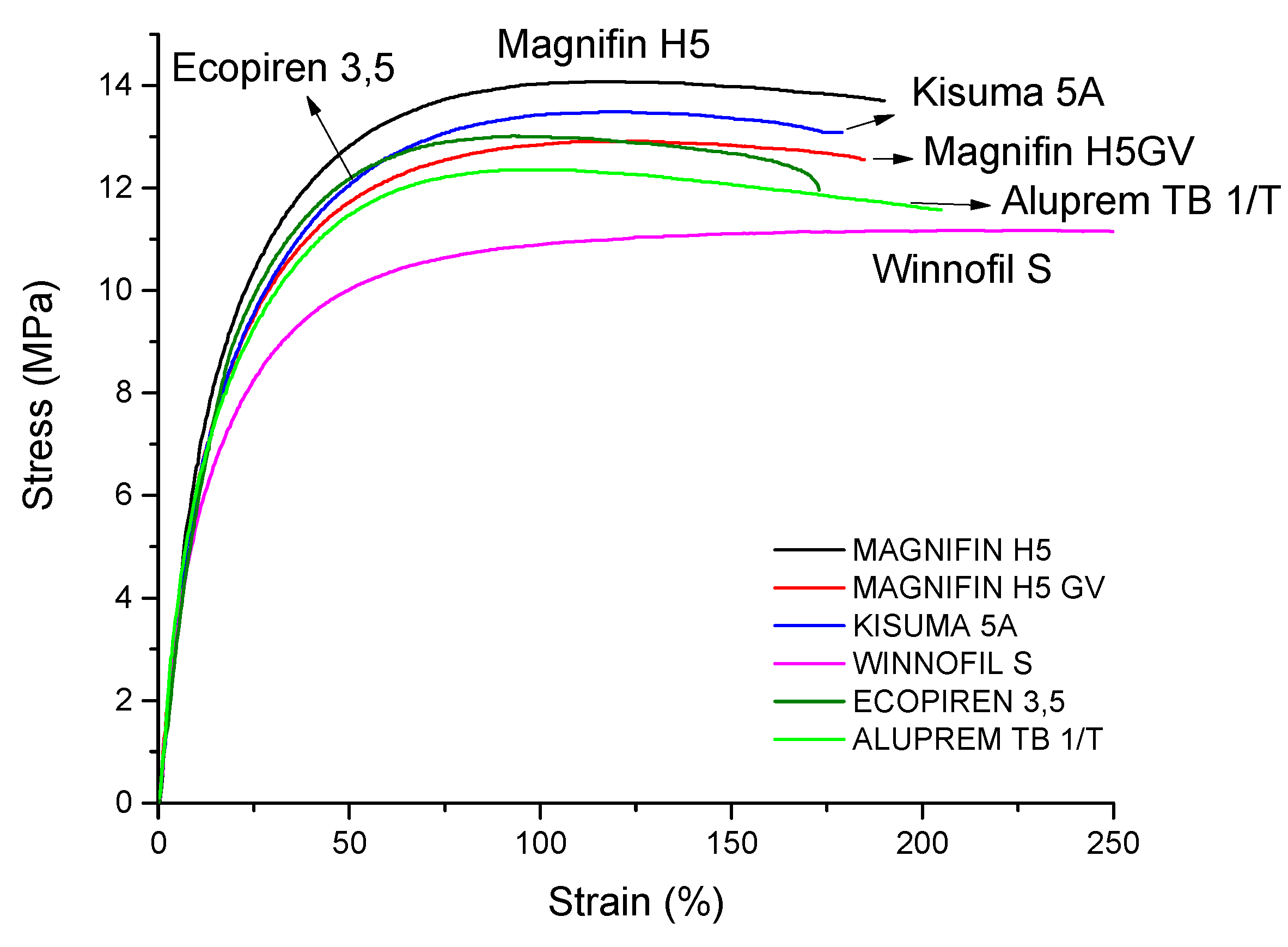
| Properties | Unit | 9 n-MDH | 17 AlOOH | 18 s-MDH | 19 Coated s-MDH | 20 Coated s-MDH | 21 Coated CaCO3 |
|---|---|---|---|---|---|---|---|
| Secondary filler | Trade Name | Ecopiren 3.5 | Aluprem TB 1/T | Magnifin H5 | Magnifin H5 GV | Kisuma 5A | Winnofil S |
| Density (±0.1) | g/cm3 | 1.469 | 1.501 | 1.479 | 1.466 | 1.471 | 1.482 |
| MFI | g/10 min | 9.2 ± 0.5 | 8.8 ± 0.4 | 8.8 ± 0.4 | 10.3 ± 0.5 | 9.6 ± 0.5 | 4.2 ± 0.2 |
| Young’s Modulus | MPa | 81 ± 7 | 72 ± 6 | 72 ± 4 | 76 ± 6 | 71 ± 3 | 74 ± 9 |
| Tensile Strength | MPa | 11.6 ± 0.7 | 11.3 ± 0.6 | 13.4 ± 0.4 | 12.6 ± 0.5 | 13.1 ± 0.3 | 11.8 ± 0.1 |
| Elongation at break | % | 173 ± 20 | 201 ± 17 | 188 ± 14 | 190 ± 15 | 179 ± 22 | 234 ± 30 |
| Properties | Unit | 9 n-MDH | 14 s-MDH | 17 AlOOH | 18 s-MDH | 21 Coated CaCO3 |
|---|---|---|---|---|---|---|
| Secondary filler | Trade Name | 100% Ecopiren 3.5 | 100% Magnifin H5 | 15% Aluprem TB 1/T | 15% Magnifin H5 | 15% Winnofil S |
| LOI | % | 36 | 38,5 | 37 | 36.5 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haveriku, S.; Meucci, M.; Badalassi, M.; Cardelli, C.; Ruggeri, G.; Pucci, A. Optimization of the Mechanical Properties of Polyolefin Composites Loaded with Mineral Fillers for Flame Retardant Cables. Micro 2021, 1, 102-119. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010008
Haveriku S, Meucci M, Badalassi M, Cardelli C, Ruggeri G, Pucci A. Optimization of the Mechanical Properties of Polyolefin Composites Loaded with Mineral Fillers for Flame Retardant Cables. Micro. 2021; 1(1):102-119. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010008
Chicago/Turabian StyleHaveriku, Sara, Michela Meucci, Marco Badalassi, Camillo Cardelli, Giacomo Ruggeri, and Andrea Pucci. 2021. "Optimization of the Mechanical Properties of Polyolefin Composites Loaded with Mineral Fillers for Flame Retardant Cables" Micro 1, no. 1: 102-119. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010008








