The Effect of the Environment on the Case Hardening Characteristics of AISI 1018 Steel during Cassava Leaf Pack Cyaniding
Abstract
:1. Introduction
Decomposition of Cassava
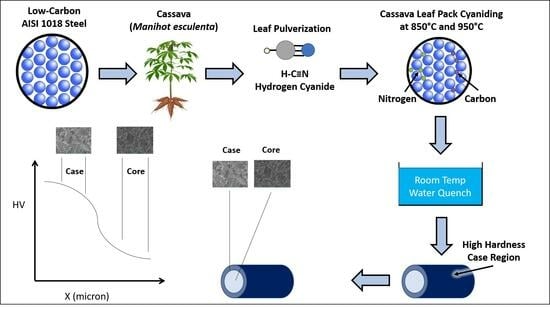
2. Experimental Section
3. Results and Discussion
3.1. Microhardness Profiles
3.2. Microstructure
3.3. Diffusion of Pulverized Cassava Leaf Media in Steel
4. Conclusions
- There was little or no change in the microhardness profile of AISI 1018 steel that was heat-treated in air (Process 1). However, a significant difference in hardness between the surface region and the interior was observed when the steel was processed in either pulverized cassava leaf (Process 2) or the mixture of pulverized cassava leaf and BaCO3 (Process 3). This confirms that the environment/medium containing cassava leaf (without energizer) resulted in the effective case hardening of AISI 1018 steel.
- At the completion of Process 2 and Process 3, in AISI 1018 steel, the case depth was found to be lower when the holding temperature was below the A3 transformation temperature.
- The addition of the barium carbonate (BaCO3) energizer (Process 3) produced the highest peak microhardness values (hmax), and resulted in the most rapid attainment of maximum case hardness, compared to Process 2. The average difference in peak microhardness (Δhmax) between Process 3 and Process 2 was above 100 HV for all samples.
- Process 2 resulted in a higher rate of diffusion compared to Process 3. Process 2 produced the deepest case depth of 1566 μm at 950 °C for 5 h.
- The microstructure of AISI 1018 after Process 2 and Process 3 was characterized by martensite platelets in the case region, while the core region retained its combined ferrite and pearlite microstructure.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adetunji, A.R. Metallographic Studies of Pack Cyanided Mild Steel Using Cassava Leaves. Mater. Manuf. Process. 2008, 23, 385–390. [Google Scholar] [CrossRef]
- Schmitt, M.; Gerstl, F.; Boesele, M.; Horn, M.; Schlick, G.; Schilp, J.; Reinhart, G. nfluence of Part Geometry and Feature Size on the Resulting Microstructure and Mechanical Properties of the Case Hardening Steel 16MnCr5 processed by Laser Powder Bed Fusion. Procedia CIRP 2021, 104, 726–731. [Google Scholar] [CrossRef]
- Bartels, D.; Fallqvist, M.; Heise, M.; Vetter, J.; Schmidt, M.; Krakhmalev, P. Development of a novel wear-resistant WC-reinforced coating based on the case-hardening steel Bainidur AM for the substitution of carburizing heat treatments. J. Mater. Res. Technol. 2023, 26, 186–198. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; You, Q.; Zhang, L. A novel martensitic steel powder for plasma arc direct energy deposition to remanufacture broken gear teeth. Mater. Lett. 2021, 301, 130111. [Google Scholar] [CrossRef]
- Edun, B.M.; Ajayi, O.O.; Babalola, P.O.; Salawu, E.Y. Effect of case hardening on the wear and hardness properties of medium carbon steel for bone crushing application. Heliyon 2023, 9, e17923. [Google Scholar] [CrossRef] [PubMed]
- Kayiwa, R.; Kasedde, M.L.H.; Kirabira, J. The potential for commercial scale production and application of activated carbon from cassava peels in Africa: A review. Bioresour. Technol. Rep. 2021, 15, 100772. [Google Scholar] [CrossRef]
- Oliver, G.D.; Cooke, K. An example of the infusion of endogenous technology in engineering curricula: The case hardening of mild steel using local Jamaica blue mountain coffee shells. In World Transactions on Engineering and Technology Education; Engineering and Technology Education: Kingston, Jamaica, 2005. [Google Scholar]
- Ihom, P.A. Case hardening of mild steel using cowbone as energiser. Afr. J. Eng. Res. 2013, 1, 97–101. [Google Scholar]
- Akanji, O.L.; Fatoba, O.S.; Aasa, A.S. The Influence of Particle Size and Soaking Time on Surface Hardness of Carburized AISI 1018 Steel. Br. J. Appl. Sci. Technol. 2015, 7, 37–44. [Google Scholar] [CrossRef]
- Ibironke, O.J.; Falaiye, A.; Ojumu, T.V.; Odo, E.A.; Adewoye, O.O. Case-Depth Studies of Pack Cyaniding of Mild Steel Using Cassava Leaves. Mater. Manuf. Process. 2004, 19, 899–905. [Google Scholar] [CrossRef]
- Sun, Y. Kinetics of low temperature plasma carburizing of austenitic stainless steels. J. Mater. Process. Technol. 2005, 168, 189–194. [Google Scholar] [CrossRef]
- Wang, C.; Mandelis, A. Case depth determination in heat-treated industrial steel products using photothermal radiometric interferometric phase minima. NDTE Int. 2007, 40, 158–167. [Google Scholar] [CrossRef]
- Katsamas, A.; Haidemenopoulos, G. Surface hardening of low-alloy 15CrNi6 steel by CO2 laser beam. Surf. Coat. Technol. 1999, 115, 249–255. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Sowmiya, K.; Pragasan, L.A.; Rajagopal, R.; Sathya, R.; Ragupathy, S.; Krishnakumar, M.; Reddy, V.R.M. Enhanced photocatalytic degradation of organic pollutants by Ag–TiO2 loaded cassava stem activated carbon under sunlight irradiation. Chemosphere 2022, 302, 134844. [Google Scholar] [CrossRef] [PubMed]
- Cock, J. Cassava: New Potential for a Neglected Crop; Westview Press: Boulder, CO, USA, 1985. [Google Scholar]
- Arthur, E.K.; Azeko, S.T. Surface hardening of ferrous materials with cassava (Manihot spp.) waste: A review. Sci. Afr. 2020, 9, e00483. [Google Scholar] [CrossRef]
- Watcharamongkol, T.; Khaopueak, P.; Seesau, C.; Wechakorn, K. Green Hydrothermal Synthesis of Multifunctional Carbon Dots from Cassava Pulps for Metal Sensing, Antioxidant, and Mercury Detoxification in Plants. Carbon Resour. Convers. 2023, 2023, 100206. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, F.; Ma, X.; Yin, F.; Li, D.; Li, J. Carbon quantum dots derived from cassava stems via acid/alkali-assisted hydrothermal carbonization: Formation, mechanism and application in drug release. Ind. Crops Prod. 2023, 204, 117243. [Google Scholar] [CrossRef]
- Amakoromo, T.; Abumere, O.; Amusan, J.; Anye, V.; Bello, A. Porous carbon from Manihot Esculenta (cassava) peels waste for charge storage applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100098. [Google Scholar] [CrossRef]
- Kayiwa, H.K.R.; Lubwama, M.; Kirabira, J. Characterization and pre-leaching effect on the peels of predominant cassava varieties in Uganda for production of activated carbon. Curr. Res. Green Sustain. Chem. 2021, 4, 100083. [Google Scholar] [CrossRef]
- Jørgensen, K.; Morant, A.V.; Morant, M.; Jensen, N.B.; Olsen, C.E.; Kannangara, R.; Motawia, M.S.; Møller, B.L.; Bak, S. Biosynthesis of the Cyanogenic Glucosides Linamarin and Lotaustralin in Cassava: Isolation, Biochemical Characterization, and Expression Pattern of CYP71E7, the Oxime-Metabolizing Cytochrome P450 Enzyme. Plant Physiol. 2011, 155, 282–292. [Google Scholar] [CrossRef]
- Zagrobelnya, M.; Bak, S.; Rasmussena, A.V.; Jørgensen, B.; Naumann, C.M.; Møller, B.L. Cyanogenic glucosides and plant–insect interactions. Phytochemistry 2004, 65, 293–306. [Google Scholar] [CrossRef]
- Narayanan, N.; Ihemere, U.; Ellery, C.; Sayre, R.T. Overexpression of Hydroxynitrile Lyase in Cassava Roots Elevates Protein and Free Amino Acids while Reducing Residual Cyanogen Levels. PLoS ONE 2011, 6, e21996. [Google Scholar] [CrossRef] [PubMed]
- Siritunga, D.; Arias-Garzon, D.; White, W.; Sayre, R.T. Over-expression of hydroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification. Plant Biotechnol. J. 2004, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Matlock, D.K.; Van Tyne, C.J. Carbon diffusivity in multi-component austenite. Scr. Mater. 2011, 64, 805–808. [Google Scholar] [CrossRef]
- Pous-Romero, H.; Bhadeshia, H.K.D.H. Coalesced Martensite in Pressure Vessel Steels. ASME J. Press. Vessel Technol. 2014, 136, 031402-1-6. [Google Scholar] [CrossRef]
- Arvanitidis, S.S. A Study of the Thermal Decomposition of BaCO3. Metall. Mater. Trans. B 1996, 27B, 409–416. [Google Scholar] [CrossRef]
- Sugianto, A.; Narazaki, M.; Kogawara, M.; Shirayori, A.; Kim, S.-Y.; Kubota, S. Numerical simulation and experimental verification of carburizing-quenching process of SCr420H steel helical gear. J. Mater. Process. Technol. 2009, 209, 3597–3609. [Google Scholar] [CrossRef]
- Sarıkaya, Y.; Onal, M. High temperature carburizing of a stainless steel with uranium carbide. J. Alloys Compd. J. 2012, 542, 253–256. [Google Scholar] [CrossRef]
- Grube, W.L.; Gay, J.G. High-rate carburizing in a glow- discharge methane plasma. Metall. Trans. A 1978, 9, 1421–1429. [Google Scholar] [CrossRef]









| C | Mn | Si | P | S |
|---|---|---|---|---|
| 0.15–0.20 | 0.60–0.90 | 0.15–0.30 | 0–0.04 | 0–0.05 |
| Process Identification | Medium/Environment | Details |
|---|---|---|
| Unprocessed | N/A | As-received AISI 1018 steel. |
| Process 1 | Air | Heat treatment of AISI 1018 steel in air (with no cassava leaf present). |
| Process 2 | Pulverized Cassava Leaf | Heat treatment of AISI 1018 steel in pulverized cassava leaf. |
| Process 3 | Pulverized Cassava Leaf + Barium Carbonate (BaCO3) * also called the “CBC Mixture” | Heat treatment of AISI 1018 steel in pulverized cassava leaf with BaCO3 as energizer (CBC mixture). The ratio of pulverized cassava leaf to energizer was 4:1 by weight. |
| Temperature (°C) | Holding Time | |
| 850 | 1 h | |
| 5 h | ||
| 950 | 1 h | |
| 5 h | ||
| Medium/Environment | Temperature (°C) | D (10−9 m2/s) |
|---|---|---|
| Pulverized Cassava Leaf (Process 2) | 850 | 1.568 |
| 950 | 1.893 | |
| Cassava + BaCO3 (Process 3) | 850 | 0.177 |
| 950 | 0.844 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, R.E.; Kalu, E.E.; Adetunji, A.R.; Campbell, D.; Kalu, P.N. The Effect of the Environment on the Case Hardening Characteristics of AISI 1018 Steel during Cassava Leaf Pack Cyaniding. Alloys 2024, 3, 1-14. https://0-doi-org.brum.beds.ac.uk/10.3390/alloys3010001
Gordon RE, Kalu EE, Adetunji AR, Campbell D, Kalu PN. The Effect of the Environment on the Case Hardening Characteristics of AISI 1018 Steel during Cassava Leaf Pack Cyaniding. Alloys. 2024; 3(1):1-14. https://0-doi-org.brum.beds.ac.uk/10.3390/alloys3010001
Chicago/Turabian StyleGordon, Renee Erica, Egwu Eric Kalu, Adelana Rasak Adetunji, Dorr Campbell, and Peter N. Kalu. 2024. "The Effect of the Environment on the Case Hardening Characteristics of AISI 1018 Steel during Cassava Leaf Pack Cyaniding" Alloys 3, no. 1: 1-14. https://0-doi-org.brum.beds.ac.uk/10.3390/alloys3010001