Poly(acrylic acid)-regulated Synthesis of Rod-Like Calcium Carbonate Nanoparticles for Inducing the Osteogenic Differentiation of MC3T3-E1 Cells
Abstract
:1. Introduction
2. Results and Discussion
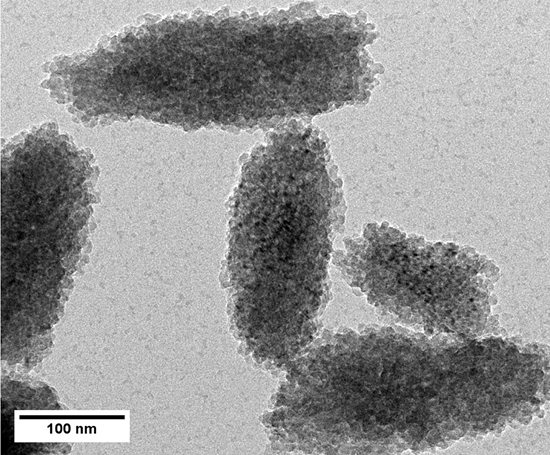
2.1. Preparation and Characterization of Rod-Like Calcium Carbonate Nanoparticles (Rod-CC NPs)
2.2. Cytocompatibility
2.3. Cell Proliferation Assay
2.4. Differentiation of Osteoblast Cells
3. Materials and Methods
3.1. Preparation of Rod-CC NPs
3.2. Characterization of the Rod-CC NPs
3.2.1. Physical–Chemical Characterization
3.2.2. Aqueous Stability of the Rod-CC NPs
3.2.3. In Vitro Rod-CC NPs Degradation
3.3. Cell Culture
3.4. Cytocompatibility Study
3.5. Cell Proliferation Study
3.6. Osteogenic Differentiation Study
3.6.1. Alkaline Phosphatase (ALP) Activity
3.6.2. Osteocalcin and Bone Sialoprotein Production
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Gusić, N.; Ivković, A.; VaFaye, J.; Vukasović, A.; Ivković, J.; Hudetz, D.; Janković, S. Nanobiotechnology and bone regeneration: A mini-review. Int. Orthop. 2014, 38, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.Y.; Chen, C.; Wang, X.M.; Lee, I.S. Advances in the surface modification techniques of bone-related implants for last 10 years. Regen. Biomater. 2014, 1, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Koupaei, N.; Karkhaneh, A.; Daliri Joupari, M. Preparation and characterization of (PCL-crosslinked-PEG)/hydroxyapatite as bone tissue engineering scaffolds. J. Biomed. Mater. Res. A 2015, 103, 3919–3926. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ren, C.; Zhang, B.; Yang, H.; Lang, Y.; Min, L.; Zhang, W.; Pei, F.; Yan, Y.; Li, H. Analyzing the behavior of a porous nano-hydroxyapatite/polyamide 66 (n-HA/PA66) composite for healing of bone defects. Int. J. Nanomed. 2014, 9, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Mao, K.; Liu, X.; Wang, X.; Cui, F. In vivo osteogenesis of vancomycin loaded nanohydroxyapatite/collagen/calcium sulfate composite for treating infectious bone defect induced by chronic osteomyelitis. J. Nanomater. 2015, 2015, 13. [Google Scholar] [CrossRef]
- Xu, S.J.; Qiu, Z.Y.; Wu, J.J.; Kong, X.D.; Weng, X.S.; Cui, F.Z.; Wang, X.M. Osteogenic differentiation gene expression profiling of hMSCs on hydroxyapatite and mineralized collagen. Tissue Eng. Part A 2015, 22, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Vuola, J.; Göransson, H.; Böhling, T.; Asko-Seljavaara, S. Bone marrow induced osteogenesis in hydroxyapatite and calcium carbonate implants. Biomaterials 1996, 17, 1761–1766. [Google Scholar] [CrossRef]
- Combes, C.; Bareille, R.; Rey, C. Calcium carbonate–calcium phosphate mixed cement compositions for bone reconstruction. J. Biomed. Mater. Res. A 2006, 79, 318–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, Z. Fabrication and characterization of electrospun PLGA/MWNTs/hydroxyapatite biocomposite scaffolds for bone tissue engineering. J. Bioact. Compat. Polym. 2010, 25, 241–259. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles; potential in bone and tooth disorders. Pharm. Sci. 2015, 20, 175–182. [Google Scholar]
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium carbonate nanoparticles: Synthesis, characterization and biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, J.; Yang, F.; Zhu, J.; Tian, X.; Chen, X. In vitro degradation and cell response of calcium carbonate composite ceramic in comparison with other synthetic bone substitute materials. Mater. Sci. Eng. C 2015, 50, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Koga, N.; Tsuru, K.; Takahashi, I. Fabrication of interconnected porous calcite by bridging calcite granules with dicalcium phosphate dihydrate and their histological evaluation. J. Biomed. Mater. Res. A 2015, 104, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, J.C.; Hsu, C.W.; Chang, W.H. Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnology 2009, 20, 375102. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Monchau, F.; Hivart, P.; Genestie, B.; Chai, F.; Descamps, M.; Hildebrand, H.F. Calcite as a bone substitute. Comparison with hydroxyapatite and tricalcium phosphate with regard to the osteoblastic activity. Mater. Sci. Eng. C 2013, 33, 490–498. [Google Scholar] [CrossRef] [PubMed]
- YináWin, K.; HináTeoh, S. Bioinspired fabrication of 3D hierarchical porous nanomicrostructures of calcium carbonate for bone regeneration. Chem. Commun. 2010, 46, 6578–6580. [Google Scholar]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule loading into spherical, elliptical, star-like and cubic calcium carbonate carriers. ChemPhysChem 2014, 15, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnol. 2015, 13, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, B.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shakibaie, M.; Rezaie, S.; Shahverdi, A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012, 47, 3719–3725. [Google Scholar] [CrossRef]
- Zhao, X.; Heng, B.C.; Xiong, S.; Guo, J.; Tan, T.T.Y.; Boey, F.Y.C.; Ng, K.W.; Loo, J.S.C. In vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areas. Nanotoxicology 2011, 5, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Synergistic targeting of cell membrane, cytoplasm, and nucleus of cancer cells using rod-shaped nanoparticles. ACS Nano 2013, 7, 9558–9570. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Liu, S.; Zhang, A.; Lu, Q.; Kaplan, D.L.; Zhu, H. Biomineralization regulation by nano-sized features in silk fibroin proteins: Synthesis of water-dispersible nano-hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Porsio, B.; Bondì, M.L.; Giammona, G.; Cavallaro, G. Evaluation of biodegradability on polyaspartamide-polylactic acid based nanoparticles by chemical hydrolysis studies. Polym. Degrad. Stab. 2015, 119, 56–67. [Google Scholar] [CrossRef]
- Ogomi, D.; Serizawa, T.; Akashi, M. Bioinspired organic-inorganic composite materials prepared by an alternate soaking process as a tissue reconstitution matrix. J. Biomed. Mater. Res. A 2003, 67, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Cotton, N.J.; Stephens, P.; Brunelle, J.E.; Langdown, A.; Auld, J.; Vizesi, F.; Bruce, W. Comparison of poly-l-lactide and polylactide carbonate interference screws in an ovine anterior cruciate ligament reconstruction model. Arthroscopy 2007, 23, 757–765.e2. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gemeinhart, R.A. Cisplatin delivery from poly(acrylic acid-co-methyl methacrylate) microparticles. J. Control. Release 2005, 106, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Wu, S.Y.; Wu, T.; Chang, Y.J.; Hua, M.Y.; Chen, J.P. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials 2009, 30, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N.; Quinn, S.J.; Kifor, O.; Ye, C.; Brown, E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.; Liu, H. Nanomaterials enhance osteogenic differentiation of human mesenchymal stem cells similar to a short peptide of BMP-7. Int. J. Nanomed. 2011, 6, 2769–2777. [Google Scholar]
- Aubin, J.; Liu, F.; Malaval, L.; Gupta, A. Osteoblast and chondroblast differentiation. Bone 1995, 17, S77–S83. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Yamaguchi, T.; Kaji, H.; Sugimoto, T.; Chihara, K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E608–E616. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Naka, K.; Chujo, Y. A carbonate controlled-addition method for amorphous calcium carbonate spheres stabilized by poly(acrylic acid)s. Langmuir 2007, 23, 12086–12095. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.R.; Kim, A.R.; Hwang, J.H.; Kim, K.M.; Hwang, E.S.; Hong, J.H. FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression. Bone 2014, 58, 72–80. [Google Scholar] [CrossRef] [PubMed]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Yao, C.; Cui, Z.; Luo, D.; Lee, I.-S.; Yao, J.; Chen, C.; Kong, X. Poly(acrylic acid)-regulated Synthesis of Rod-Like Calcium Carbonate Nanoparticles for Inducing the Osteogenic Differentiation of MC3T3-E1 Cells. Int. J. Mol. Sci. 2016, 17, 639. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050639
Yang W, Yao C, Cui Z, Luo D, Lee I-S, Yao J, Chen C, Kong X. Poly(acrylic acid)-regulated Synthesis of Rod-Like Calcium Carbonate Nanoparticles for Inducing the Osteogenic Differentiation of MC3T3-E1 Cells. International Journal of Molecular Sciences. 2016; 17(5):639. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050639
Chicago/Turabian StyleYang, Wei, Chenxue Yao, Zhengyang Cui, Dandan Luo, In-Seop Lee, Juming Yao, Cen Chen, and Xiangdong Kong. 2016. "Poly(acrylic acid)-regulated Synthesis of Rod-Like Calcium Carbonate Nanoparticles for Inducing the Osteogenic Differentiation of MC3T3-E1 Cells" International Journal of Molecular Sciences 17, no. 5: 639. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050639