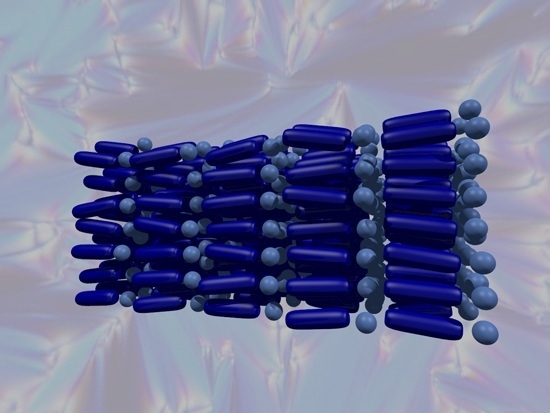
Key Developments in Ionic Liquid Crystals
Abstract
:1. Introduction
2. Liquid Crystals: The Fourth State of Matter
3. Ionic Liquid Crystals: Merging Liquid Crystals and Ionic Liquids
4. Materials Development in Ionic Liquid Crystals
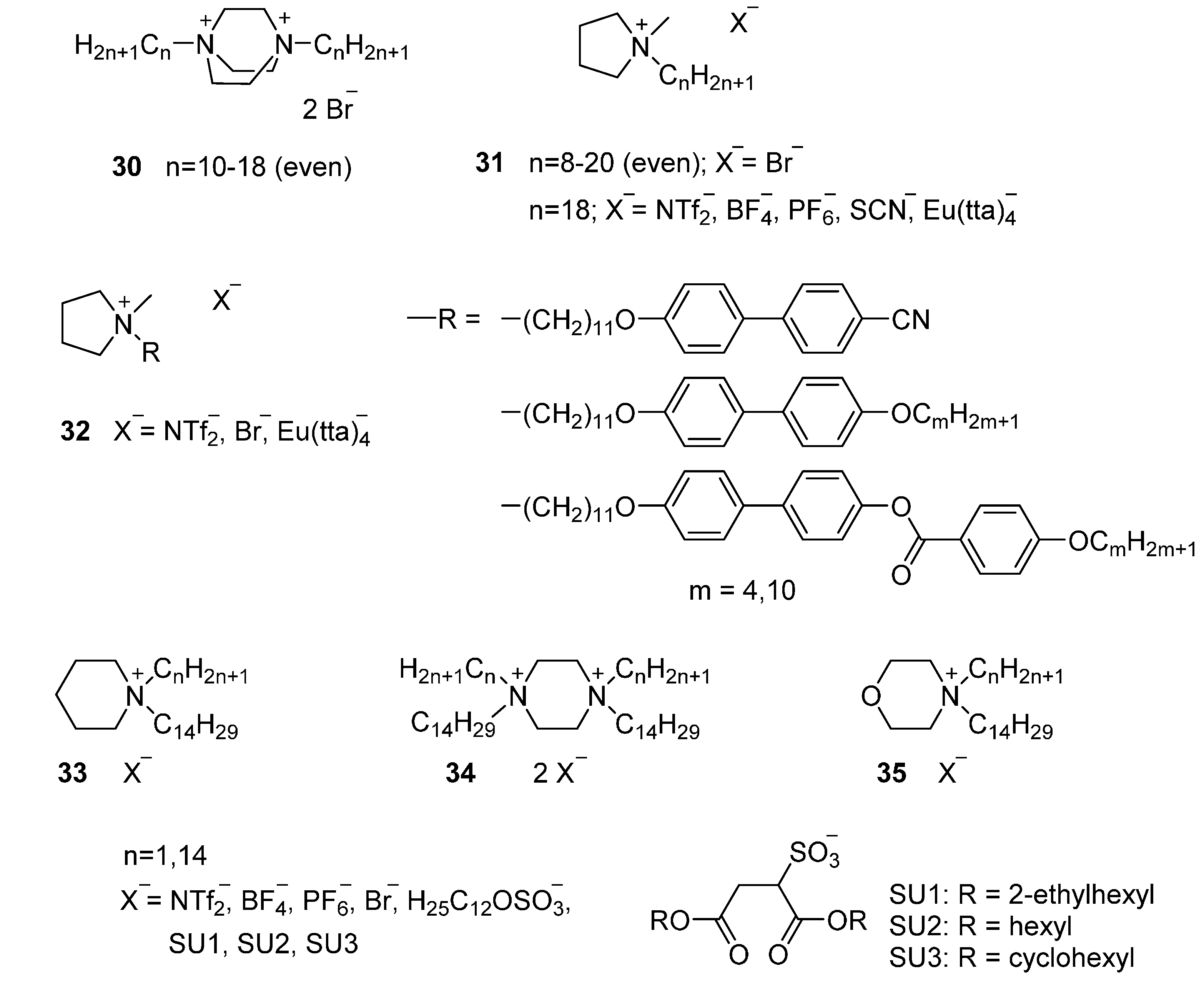
4.1. Aliphatic Cations
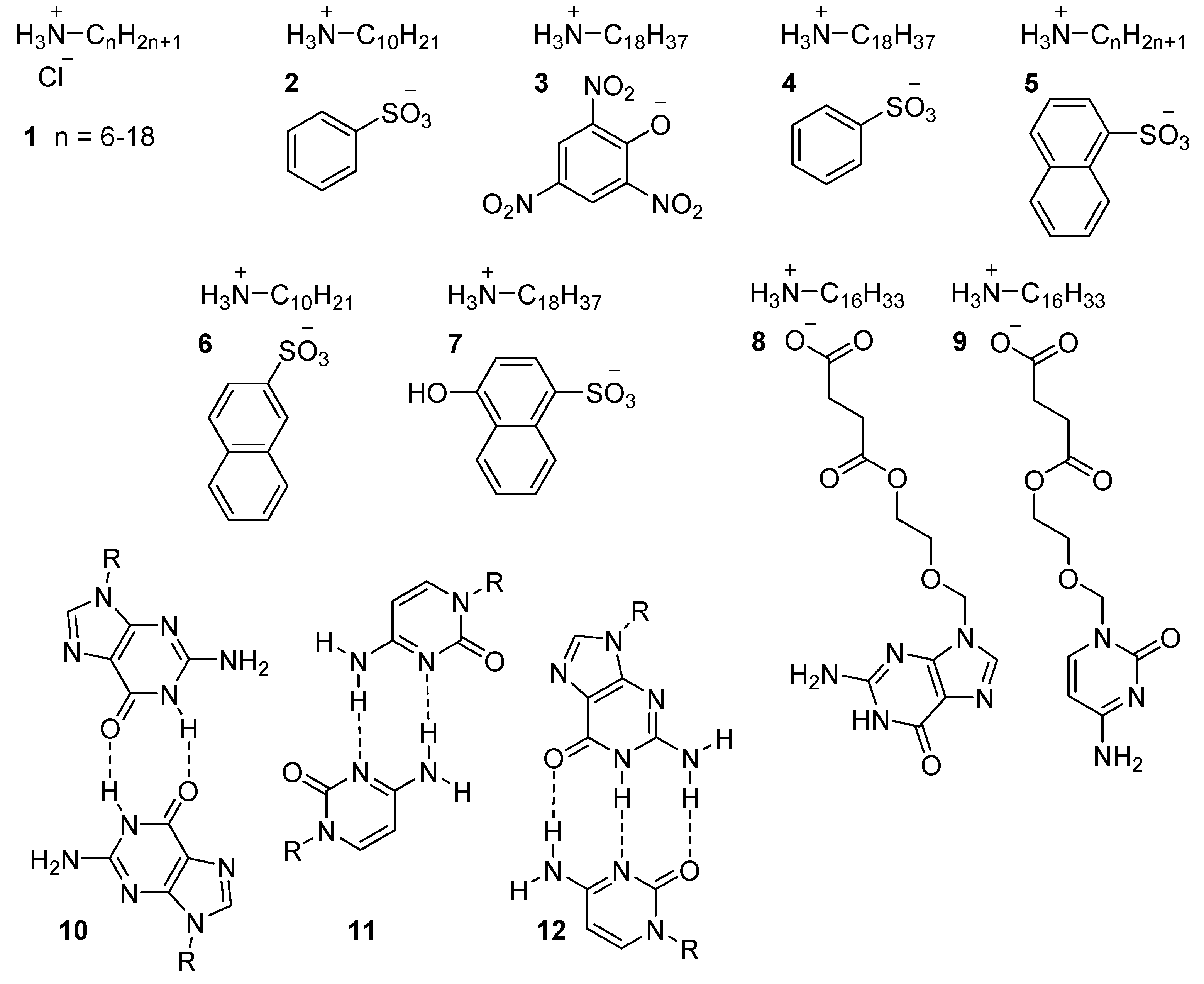
4.1.1. Protonated Amines
4.1.2. Quaternary Ammonium and Phosphonium Salts
One Long Alkyl Chain
Two Long Alkyl Chains
Three or Four Long Alkyl Chains
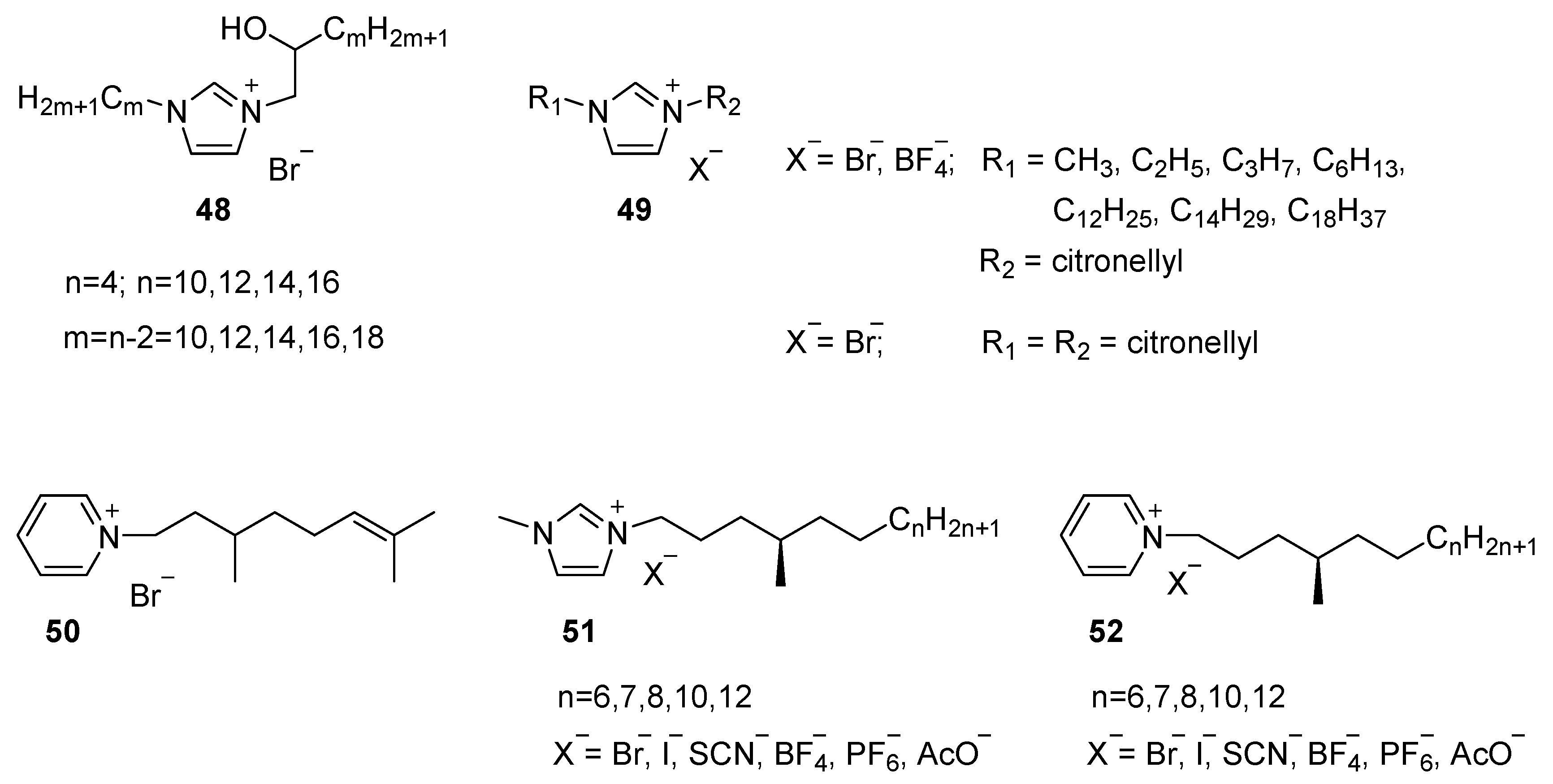
Aliphatic Heterocyclic Cations
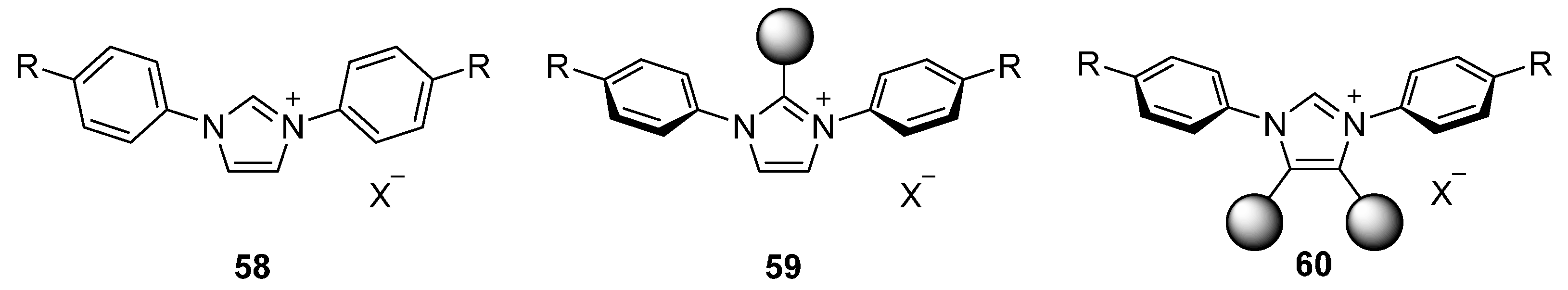
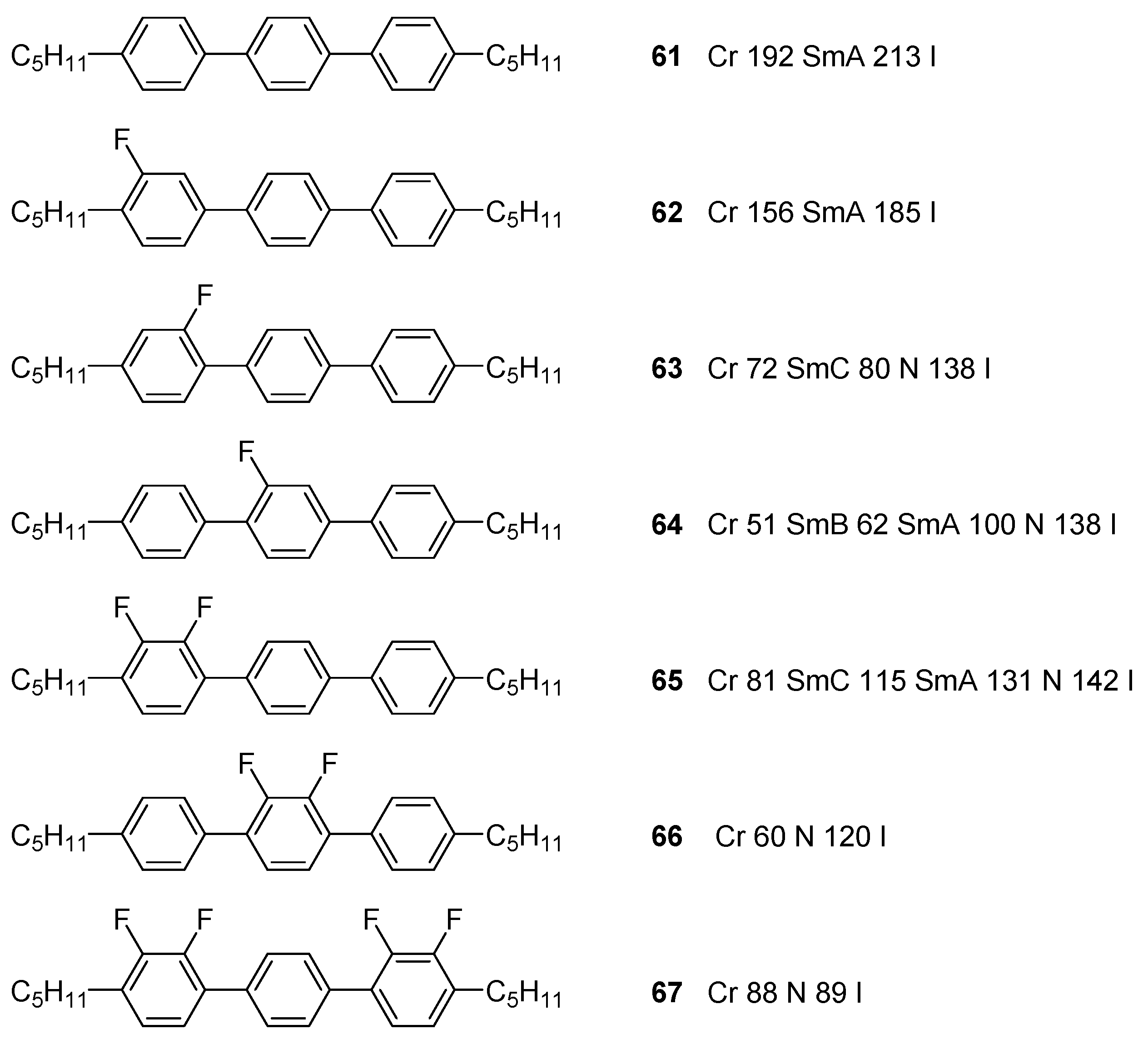
4.2. Aromatic Cations
4.2.1. Simple Aliphatic Tails
4.2.2. Mesogenic Moieties
4.3. Counter Ions
5. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ArIm | arylimidazole or arylimidazolium |
| AmImAr | arylimidazolearyl or arylimidazoliumaryl |
| Colh, Colr | hexagonally, rectangular packed columnar (mesophase) |
| Cubbis | biscontinuous cubic liquid crystal |
| IL | ionic liquid |
| ILC | ionic liquid crystal |
| LC | liquid crystal |
| N | nematic (mesophase) |
| SmA, SmC, SmT | smectic A, smectic C or smectic T (mesophases) |
| SmA* | chiral smectic A (mesophase) |
| X− | anion |
References and Notes
- Sluckin, T.J.; Dunmur, D.A.; Stegemeyer, H. Crystals that Flow: Classic Papers from the History of Liquid Crystals; Taylor & Francis: London, UK, 2004. [Google Scholar]
- De Vries, A.; Saeva, F.D. Liquid Crystals—The Fourth State of Matter; Marcel Dekker: New York, NY, USA, 1979. [Google Scholar]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Axenov, K.V.; Laschat, S. Thermotropic ionic liquid crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef]
- Lydon, J. Chromonics. In Handbook of Liquid Crystals Set; Demus, D., Goodby, J., Gray, G.W., Spiess, H.W., Vill, V., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 981–1007. [Google Scholar]
- Lydon, J. Chromonic review. J. Mater. Chem. 2010, 20, 10071–10099. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering-ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, C.E.; Fuller, S.; Gray, J.; Holmes, M.C.; Tiddy, G.J.T. Lyotropic surfactant liquid crystals. In Handbook of Liquid Crystals Set; Demus, D., Goodby, J., Gray, G.W., Spiess, H.W., Vill, V., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 341–392. [Google Scholar]
- Tam-Chang, S.-W.; Huang, L. Chromonic liquid crystals: Properties and applications as functional materials. Chem. Commun. 2008, 17, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Pucci, D.; Donnio, B. Metal-containing liquid cystals. In Handbook of Liquid Crystals Set; Goodby, J., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Ranynes, P., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2014; pp. 175–242. [Google Scholar]
- Reinitzer, F. Beiträge zur kenntniss des cholesterins. Monatsh. Chem. 1888, 9, 421–441. [Google Scholar] [CrossRef]
- Lehmann, O. On flowing liquid crystals. Z. Phys. Chem. 1889, 4, 462–472. [Google Scholar]
- Goodby, J.W. Phase structures of calamitic liquid crystals. In Handbook of Liquid Crystals; Goodby, J.W., Demus, D., Goodby, J., Gray, G.W., Spiess, H.W., Vill, V., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 3–21. [Google Scholar]
- Goossens, K.; Nockemann, P.; Driesen, K.; Goderis, B.; Görller-Walrand, C.; van Hecke, K.; van Meervelt, L.; Pouzet, E.; Binnemans, K.; Cardinaels, T. Imidazolium ionic liquid crystals with pendant mesogenic groups. Chem. Mater. 2007, 20, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, J.; Li, B.; Zhang, M.; Wu, L. Branched quaternary ammoniumamphiphiles: Nematic ionic liquid crystals near room temperature. Chem. Commun. 2009, 5269–5271. [Google Scholar] [CrossRef] [PubMed]
- Ringstrand, B.; Jankowiak, A.; Johnson, L.E.; Kaszynski, P.; Pociecha, D.; Górecka, E. Anion-driven mesogenicity: A comparative study of ionic liquid crystals based on the [closo-1-CB9H10]− and [closo-1-CB11H12]− clusters. J. Mater. Chem. 2012, 22, 4874–4880. [Google Scholar] [CrossRef]
- Alami, E.; Levy, H.; Zana, R.; Weber, P.; Skoulios, A. A new smectic mesophase with two dimensional tetragonal symmetry from dialkyldimethylammonium bromides: St. Liq. Cryst. 1993, 13, 201–212. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Armitage, D. Liquid crystal display device fundamentals. In Electro-Optical Displays; Karim, M.A., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1992; Volume 33, pp. 19–67. [Google Scholar]
- Yu, Y.; Ikeda, T. Soft actuators based on liquid-crystalline elastomers. Angew. Chem. Int. Ed. 2006, 45, 5416–5418. [Google Scholar] [CrossRef] [PubMed]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Lin, R.M.; Ren, Y. Design and fabrication of silicon condenser microphone using single deeply corrugated diaphragm technique. Microelectron. Int. 2003, 20, 36–40. [Google Scholar] [CrossRef]
- Broer, D.J.; Mol, G.N.; Haaren, J.A.M.M.V.; Lub, J. Photo-induced diffusion in polymerizing chiral-nematic media. Adv. Mater. 1999, 11, 573–578. [Google Scholar] [CrossRef]
- Van Oosten, C.L.; Bastiaansen, C.W.M.; Broer, D.J. Printed artificial cilia from liquid-crystal network actuators modularly driven by light. Nat. Mater. 2009, 8, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Zayas-Gonzalez, Y.M.; Carlton, R.J.; Caruso, F.; Abbott, N.L.; Lynn, D.M. Liquid crystal chemical sensors that cells can wear. Angew. Chem. Int. Ed. 2013, 52, 14011–14015. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Ravnik, M.; Pajk, S.; Musevic, I. Electrically tunable liquid crystal optical microresonators. Nat. Photonics 2009, 3, 595–600. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Paleos, C.M. Thermotropic liquid crystals derived from amphiphilic mesogens. Mol. Cryst. Liq. Cryst. 1994, 243, 159–183. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, H.; Hallett, J.P.; Villar-Garcia, I.J.; Hunt, P.A.; Welton, T. Mixtures of ionic liquids. Chem. Soc. Rev. 2012, 41, 7780–7802. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, T.; Tsuda, T.; Okazaki, K.-I.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Ionic liquid crystal as a hole transport layer of dye-sensitized solar cells. Chem. Commun. 2005, 6, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Mukai, T.; Ohno, H.; Kato, T. One-dimensional ion transport in self-organized columnar ionic liquids. J. Am. Chem. Soc. 2004, 126, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Self-assembly of phase-segregated liquid crystal structures. Science 2002, 295, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Nemade, P.R.; Lu, X.; Zeng, X.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. New type of membrane material for water desalination based on a cross-linked bicontinuous cubic lyotropic liquid crystal assembly. J. Am. Chem. Soc. 2007, 129, 9574–9575. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.S.; Jeon, H.; Kim, S.J.; Kim, D.J.; Kim, J.H. Ionic liquid crystals: Synthesis, structure and applications to I2-free solid-state dye-sensitized solar cells. Macromol. Res. 2013, 21, 315–320. [Google Scholar] [CrossRef]
- Sasi, R.; Rao, T.P.; Devaki, S.J. Bio-based ionic liquid crystalline quaternary ammonium salts: Properties and applications. ACS Appl. Mater. Interfaces 2014, 6, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, S.; Iimura, K. Ammonium halide type thermotropic liquid-crystalline polyethylenimines and those low-mass model compounds. Chem. Lett. 1990, 19, 995–998. [Google Scholar] [CrossRef]
- Kouwer, P.H.J.; Swager, T.M. Synthesis and mesomorphic properties of rigid-core ionic liquid crystals. J. Am. Chem. Soc. 2007, 129, 14042–14052. [Google Scholar] [CrossRef] [PubMed]
- Butschies, M.; Frey, W.; Laschat, S. Designer ionic liquid crystals based on congruently shaped guanidinium sulfonates. Chem. Eur. J. 2012, 18, 3014–3022. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, S.; Bier, M.; Harnau, L. Phase behavior of ionic liquid crystals. J. Chem. Phys. 2010, 132, 184901. [Google Scholar] [CrossRef]
- Wang, Y.; Voth, G.A. Unique spatial heterogeneity in ionic liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef] [PubMed]
- Canongia Lopes, J.N.; Costa Gomes, M.F.; Pádua, A.A.H. Nonpolar, polar, and associating solutes in ionic liquids. J. Mater. Chem. B 2006, 110, 16816–16818. [Google Scholar] [CrossRef] [PubMed]
- Blesic, M.; Swadźba-Kwaśny, M.; Holbrey, J.D.; Lopes, J.N.C.; Seddon, K.R.; Rebelo, L.P.N. New catanionic surfactants based on 1-alkyl-3-methylimidazolium alkylsulfonates, [CnH2n+1MIM][CmH2m+1SO3]: Mesomorphism and aggregation. Phys. Chem. Chem. Phys. 2009, 11, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Saielli, G. MD simulation of the mesomorphic behaviour of 1-hexadecyl-3-methylimidazolium nitrate: Assessment of the performance of a coarse-grained force field. Soft Matter 2012, 8, 10279–10287. [Google Scholar] [CrossRef]
- Saielli, G. Fully atomistic simulations of the ionic liquid crystal [C16MIM][NO3]: Orientational order parameters and voids distribution. J. Phys. Chem. B 2016, 120, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shi, R.; Wang, Y.; Saielli, G. Effect of the chain length on the structure of ionic liquids: From spatial heterogeneity to ionic liquid crystals. J. Phys. Chem. B 2013, 117, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Saielli, G.; Bagno, A.; Wang, Y. Insights on the isotropic-to-smectic a transition in ionic liquid crystals from coarse-grained molecular dynamics simulations: The role of microphase segregation. J. Phys. Chem. B 2015, 119, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Viologen-based ionic liquid crystals: Induction of a smectic a phase by dimerisation. Phys. Chem. Chem. Phys. 2014, 16, 5048–5051. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Fernandez, A.; de Haan, L.T.; Kouwer, P.H.J. Towards room-temperature ionic liquid crystals. J. Mater. Chem. A 2013, 1, 354–357. [Google Scholar] [CrossRef]
- Butschies, M.; Mansueto, M.; Haenle, J.C.; Schneck, C.; Tussetschläger, S.; Giesselmann, F.; Laschat, S. Headgroups vs. symmetry in congruent ion pairs: Which one does the job in mesomorphic aryl guanidinium and aryl imidazolium sulphonates? Liq. Cryst. 2014, 41, 821–838. [Google Scholar] [CrossRef]
- Busico, V.; Corradini, P.; Vacatello, M. Thermal behavior and observation of a smectic phase in n-pentadecylammonium chloride. J. Phys. Chem. 1982, 86, 1033–1034. [Google Scholar] [CrossRef]
- Busico, V.; Cernicchiaro, P.; Corradini, P.; Vacatello, M. Polymorphism in anhydrous amphiphilic systems. Long-chain primary n-alkylammonium chlorides. J. Phys. Chem. 1983, 87, 1631–1635. [Google Scholar] [CrossRef]
- Vorländer, D. Verhalten der salze organischer säuren beim schmelzen. Ber. Dtsch. Chem. Ges. 1910, 43, 3120–3135. [Google Scholar] [CrossRef]
- Lawrence, A.S.C. The metal soaps and the gelation of their paraffin solutions. Trans. Faraday Soc. 1938, 34, 660–677. [Google Scholar] [CrossRef]
- Ito, M.; Matsunaga, Y.; Matsuzaki, H.; Shimojima, S. The thermotropic liquid-crystalline behavior of alkylammonium benzenesulfonates and related salts. Bull. Chem. Soc. Jpn. 1989, 62, 3919–3922. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Tsujimura, T. The thermotropic liquid-crystalline behavior of alkylammonium naphthalenesulfonates. Mol. Cryst. Liq. Cryst. 1991, 200, 103–108. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Mihou, A.P.; Couladouros, E.A.; Paleos, C.M. Liquid crystals resulting from combined ionic and hydrogen bonding interactions of nucleobase derivatives. Mol. Cryst. Liq. Cryst. 2001, 362, 177–184. [Google Scholar] [CrossRef]
- Ujiie, S.; Yano, Y.; Mori, A. Liquid-crystalline branched polymers having ionic moieties. Mol. Cryst. Liq. Cryst. 2004, 411, 483–489. [Google Scholar] [CrossRef]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Barberá, J.; Serrano, J.L. Ionic liquid crystal dendrimers with mono-, di- and trisubstituted benzoic acids. Chem. Mater. 2006, 18, 1206–1212. [Google Scholar] [CrossRef]
- Vergara, J.; Gimeno, N.; Cano, M.; Barberá, J.; Romero, P.; Serrano, J.L.; Ros, M.B. Mesomorphism from bent-core based ionic dendritic macromolecules. Chem. Mater. 2011, 23, 4931–4940. [Google Scholar] [CrossRef]
- Türkçü, H.N.; Ocak, H.; Gürbüz, M.U.; Çakar, F.; Bilgin-Eran, B.; Tülü, M. A study of dendritic ionic liquid crystals: Using (S)-4-citronellyloxybenzoic acid and polypropylene imine dendrimers. J. Mol. Liq. 2016, 216, 209–215. [Google Scholar] [CrossRef]
- Seki, S.; Hayamizu, K.; Tsuzuki, S.; Fujii, K.; Umebayashi, Y.; Mitsugi, T.; Kobayashi, T.; Ohno, Y.; Kobayashi, Y.; Mita, Y. Relationships between center atom species (N, P) and ionic conductivity, viscosity, density, self-diffusion coefficient of quaternary cation room-temperature ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.A.; Zhou, J.; Kohl, P.A. Electrochemical comparison and deposition of lithium and potassium from phosphonium-and ammonium-TFSI ionic liquids. J. Electrochem. Soc. 2009, 156, A253–A259. [Google Scholar] [CrossRef]
- Bradaric, C.J.; Downard, A.; Kennedy, C.; Robertson, A.J.; Zhou, Y. Industrial preparation of phosphonium ionic liquids. Green Chem. 2003, 5, 143–152. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Robertson, A.; Hsu, H.-F.; Weiss, R.G. Smectic liquid-crystalline phases of quaternary group VA (especially phosphonium) salts with three equivalent long n-alkyl chains. How do layered assemblies form in liquid-crystalline and crystalline phases? J. Am. Chem. Soc. 2000, 122, 3053–3062. [Google Scholar] [CrossRef]
- Henderson, W.A., Jr.; Buckler, S.A. The nucleophilicity of phosphines. J. Am. Chem. Soc. 1960, 82, 5794–5800. [Google Scholar] [CrossRef]
- Allman, T.; Goel, R.G. The basicity of phosphines. Can. J. Chem. 1982, 60, 716–722. [Google Scholar] [CrossRef]
- Iwamoto, K.; Ohnuki, Y.; Sawada, K.; Senō, M. Solid-solid phase transitions of long-chain n-alkyltrimethylammonium halides. Mol. Cryst. Liq. Cryst. 1981, 73, 95–103. [Google Scholar] [CrossRef]
- Margomenou-Leonidopoulou, G.; Malliaris, A.; Paleos, C.M. Thermal behavior of some long-chain quaternary ammonium salts. Thermochim. Acta 1985, 85, 147–150. [Google Scholar] [CrossRef]
- Arkas, M.; Yannakopoulou, K.; Paleos, C.M.; Weber, P.; Skoulios, A. The mesomorphic behavior of cyanopropylalkyldimethylammonium bromides. Liq. Cryst. 1995, 18, 563–569. [Google Scholar] [CrossRef]
- Paleos, C.M.; Arkas, M.; Skoulios, A. Mesomorphic character of quaternary ammonium salts affected by secondary hydrogen bonding interactions. Mol. Cryst. Liq. Cryst. 1998, 309, 237–250. [Google Scholar] [CrossRef]
- Arkas, M.; Paleos, C.M.; Skoulios, A. Crystal and liquid crystal behavior of N-cyanoalkyl-N-alkyl-N,N-dimethylammonium bromides: Role of the dipole interactions of the cyano groups. Liq. Cryst. 1997, 22, 735–742. [Google Scholar] [CrossRef]
- Paleos, C.; Arkas, M.; Seghrouchni, R.; Skoulios, A. Smectic mesophases from quaternary amphiphilic ammonium salts functionalized with interacting endgroups. Mol. Cryst. Liq. Cryst. 1995, 268, 179–182. [Google Scholar] [CrossRef]
- Paleos, C.M.; Margomenou-Leonidopoulou, G.; Malliaris, A. Organizational and aggregational characteristics of some monomeric and polymerized quaternary ammonium salts. Mol. Cryst. Liq. Cryst. 1988, 161, 385–394. [Google Scholar] [CrossRef]
- Chen, H.; Kwait, D.C.; Gönen, Z.S.; Weslowski, B.T.; Abdallah, D.J.; Weiss, R.G. Phase characterization and properties of completely saturated quaternary phosphonium salts. Ordered, room-temperature ionic liquids. Chem. Mater. 2002, 14, 4063–4072. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Chen, H.; Khetrapal, C.L.; Weiss, R.G. Amphotropic ionic liquid crystals with low order parameters. Chem. Mater. 2004, 16, 2101–2106. [Google Scholar] [CrossRef]
- Ohta, K.; Sugiyama, T.; Nogami, T. A smectic T phase of 1,4-dialkyl-1,4-diazoniabicyclo[2.2.2]octane dibromides. J. Mater. Chem. 2000, 10, 613–616. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Tsiourvas, D.; Arkas, M.; Sideratou, Z.; Paleos, C.M. Thermotropic liquid crystalline behaviour of piperazinium and homopiperazinium alkylsulphates. Liq. Cryst. 2002, 29, 1547–1553. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M.; Skoulios, A. Smectic mesophases from dihydroxy derivatives of quaternary alkylammonium salts. Chem. Eur. J. 1999, 5, 3202–3207. [Google Scholar] [CrossRef]
- Kumano, A.; Kajiyama, T.; Takayanagi, M.; Kunitake, T.; Okahata, Y. Phase transition behavior and permeation properties of cationic and anionic artificial lipids with two alkyl chains. Ber. Bunsenges. Phys. Chem. 1984, 88, 1216–1222. [Google Scholar] [CrossRef]
- Kanazawa, A.; Tsutsumi, O.; Ikeda, T.; Nagase, Y. Novel thermotropic liquid crystals without a rigid core formed by amphiphiles having phosphonium ions. J. Am. Chem. Soc. 1997, 119, 7670–7675. [Google Scholar] [CrossRef]
- Fraser, K.J.; Izgorodina, E.I.; Forsyth, M.; Scott, J.L.; MacFarlane, D.R. Liquids intermediate between “molecular” and “ionic” liquids: Liquid ion pairs? Chem. Commun. 2007, 3817–3819. [Google Scholar] [CrossRef]
- Ma, K.; Somashekhar, B.S.; Nagana Gowda, G.A.; Khetrapal, C.L.; Weiss, R.G. Induced amphotropic and thermotropic ionic liquid crystallinity in phosphonium halides: “Lubrication” by hydroxyl groups. Langmuir 2008, 24, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Scibona, G.; Basol, S.; Danesi, P.R.; Orlandini, F. Aggregation studies on long chain alkyl-ammonium salts in organic diluents—I The chloride, nitrate salts in benzene. J. Inorg. Nuclear Chem. 1966, 28, 1441–1450. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Lu, L.; Cocker, T.M.; Bachman, R.E.; Weiss, R.G. The crystalline and liquid crystalline structures of benzyl-tri-octadecylammonium bromide complete the puzzle: How do group VA halide salts with one-four long n-alkyl chains pack? Liq. Cryst. 2000, 27, 831–837. [Google Scholar] [CrossRef]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Kagimoto, J.; Ohno, H.; Kato, T. 3D interconnected ionic nano-channels formed in polymer films: Self-organization and polymerization of thermotropic bicontinuous cubic liquid crystals. J. Am. Chem. Soc. 2011, 133, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Frise, A.E.; Ichikawa, T.; Yoshio, M.; Ohno, H.; Dvinskikh, S.V.; Kato, T.; Furó, I. Ion conductive behaviour in a confined nanostructure: NMR observation of self-diffusion in a liquid-crystalline bicontinuous cubic phase. Chem. Commun. 2010, 46, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Self-organization of room-temperature ionic liquids exhibiting liquid-crystalline bicontinuous cubic phases: Formation of nano-ion channel networks. J. Am. Chem. Soc. 2007, 129, 10662–10663. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Taguchi, S.; Liu, F.; Zeng, X.-B.; Ungar, G.; Ohno, H.; Kato, T. Induction of thermotropic bicontinuous cubic phases in liquid-crystalline ammonium and phosphonium salts. J. Am. Chem. Soc. 2012, 134, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Lava, K.; Binnemans, K.; Cardinaels, T. Piperidinium, piperazinium and morpholinium ionic liquid crystals. J. Mater. Chem. B 2009, 113, 9506–9511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, K.; Lava, K.; Nockemann, P.; van Hecke, K.; van Meervelt, L.; Driesen, K.; Görller-Walrand, C.; Binnemans, K.; Cardinaels, T. Pyrrolidinium ionic liquid crystals. Chem. Eur. J. 2009, 15, 656–674. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Lava, K.; Nockemann, P.; van Hecke, K.; van Meervelt, L.; Pattison, P.; Binnemans, K.; Cardinaels, T. Pyrrolidinium ionic liquid crystals with pendant mesogenic groups. Langmuir 2009, 25, 5881–5897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnemans, K.; Galyametdinov, Y.G.; Collinson, S.R.; Bruce, D.W. Reduction of the transition temperatures in mesomorphic lanthanide complexes by the exchange of counter-ions. J. Mater. Chem. 1998, 8, 1551–1553. [Google Scholar] [CrossRef]
- Mathevet, F.; Masson, P.; Nicoud, J.F.; Skoulios, A. Smectic liquid crystals from supramolecular guanidinium alkylbenzenesulfonates. Chem. Eur. J. 2002, 8, 2248–2254. [Google Scholar] [CrossRef]
- Kato, T.; Yasuda, T.; Kamikawa, Y.; Yoshio, M. Self-assembly of functional columnar liquid crystals. Chem. Commun. 2009, 7, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Suzui, Y.; Hasegawa, M.; Kato, T. Full-color tunable photoluminescent ionic liquid crystals based on tripodal pyridinium, pyrimidinium, and quinolinium salts. J. Am. Chem. Soc. 2012, 134, 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Bonchio, M.; Carraro, M.; Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Thermal behaviour and electrochemical properties of bis(trifluoromethanesulfonyl) amide and dodecatungstosilicate viologen dimers. Phys. Chem. Chem. Phys. 2012, 14, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pal, S.K. Synthesis and characterization of novel imidazolium-based ionic discotic liquid crystals with a triphenylene moiety. Tetrahedron Lett. 2005, 46, 2607–2610. [Google Scholar] [CrossRef]
- Bowlas, C.J.; Bruce, D.W.; Seddon, K.R. Liquid-crystalline ionic liquids. Chem. Commun. 1996, 1625–1626. [Google Scholar] [CrossRef]
- Bradley, A.E.; Hardacre, C.; Holbrey, J.D.; Johnston, S.; McMath, S.E.J.; Nieuwenhuyzen, M. Small-angle X-ray scattering studies of liquid crystalline 1-alkyl-3-methylimidazolium salts. Chem. Mater. 2002, 14, 629–635. [Google Scholar] [CrossRef]
- Wang, X.; Heinemann, F.W.; Yang, M.; Melcher, B.U.; Fekete, M.; Mudring, A.-V.; Wasserscheid, P.; Meyer, K. A new class of double alkyl-substituted, liquid crystalline imidazolium ionic liquids—A unique combination of structural features, viscosity effects, and thermal properties. Chem. Commun. 2009, 7405–7407. [Google Scholar] [CrossRef] [PubMed]
- Downard, A.; Earle, M.J.; Hardacre, C.; McMath, S.E.J.; Nieuwenhuyzen, M.; Teat, S.J. Structural studies of crystalline 1-alkyl-3-methylimidazolium chloride salts. Chem. Mater. 2004, 16, 43–48. [Google Scholar] [CrossRef]
- Hardacre, C.; Holbrey, J.D.; Mullan, C.L.; Youngs, T.G.A.; Bowron, D.T. Small angle neutron scattering from 1-alkyl-3-methylimidazolium hexafluorophosphate ionic liquids ([CnMIm][PF6], n = 4, 6, and 8). J. Chem. Phys. 2010, 133. [Google Scholar] [CrossRef] [PubMed]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. Dalton Trans. J. Chem. Soc. 1999, 2133–2140. [Google Scholar] [CrossRef]
- Knight, G.A.; Shaw, B.D. 121. Long-chain alkylpyridines and their derivatives. New examples of liquid crystals. J. Chem. Soc. 1938, 682–683. [Google Scholar] [CrossRef]
- Somashekar, R. Mesomorphic behaviour of n-4-hexadecylpyridinium chloride. Mol. Cryst. Liq. Cryst. 1987, 146, 225–233. [Google Scholar] [CrossRef]
- Gordon, C.; Holbrey, J.; Kennedy, A.; Seddon, K. Ionic liquid crystals: Hexafluorophosphate salts. J. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Yang, M.; Mallick, B.; Mudring, A.-V. On the mesophase formation of 1,3-dialkylimidazolium ionic liquids. Cryst. Growth Des. 2013, 13, 3068–3077. [Google Scholar] [CrossRef]
- Yang, M.; Mallick, B.; Mudring, A.-V. A systematic study on the mesomorphic behavior of asymmetrical 1-alkyl-3-dodecylimidazolium bromides. Cryst. Growth Des. 2014, 14, 1561–1571. [Google Scholar] [CrossRef]
- Wang, X.; Sternberg, M.; Kohler, F.T.U.; Melcher, B.U.; Wasserscheid, P.; Meyer, K. Long-alkyl-chain-derivatized imidazolium salts and ionic liquid crystals with tailor-made properties. RSC Adv. 2014, 4, 12476–12481. [Google Scholar] [CrossRef]
- Costa, R.D.; Werner, F.; Wang, X.; Grönninger, P.; Feihl, S.; Kohler, F.T.U.; Wasserscheid, P.; Hibler, S.; Beranek, R.; Meyer, K.; et al. Beneficial effects of liquid crystalline phases in solid-state dye-sensitized solar cells. Adv. Energy Mater. 2013, 3, 657–665. [Google Scholar] [CrossRef]
- Sudholter, E.J.R.; Engberts, J.B.F.N.; de Jeu, W.H. Thermotropic liquid-crystalline behavior of some single- and double-chained pyridinium amphiphiles. J. Phys. Chem. 1982, 86, 1908–1913. [Google Scholar] [CrossRef]
- Nusselder, J.J.H.; Engberts, J.B.F.N.; van Doren, H.A. Liquid-crystalline and thermochromic behavior of 4-substituted 1-methylpyridinium iodide surfactants. Liq. Cryst. 1993, 13, 213–225. [Google Scholar] [CrossRef]
- Mukai, T.; Yoshio, M.; Kato, T.; Ohno, H. Effect of methyl groups onto imidazolium cation ring on liquid crystallinity and ionic conductivity of amphiphilic ionic liquids. Chem. Lett. 2004, 33, 1630–1631. [Google Scholar] [CrossRef]
- Chiou, J.Y.Z.; Chen, J.N.; Lei, J.S.; Lin, I.J.B. Ionic liquid crystals of imidazolium salts with a pendant hydroxyl group. J. Mater. Chem. 2006, 16, 2972–2977. [Google Scholar] [CrossRef]
- Tosoni, M.; Laschat, S.; Baro, A. Synthesis of novel chiral ionic liquids and their phase behavior in mixtures with smectic and nematic liquid crystals. Helv. Chim. Acta 2004, 87, 2742–2749. [Google Scholar] [CrossRef]
- Kohnen, G.; Tosoni, M.; Tussetschläger, S.; Baro, A.; Laschat, S. Counterion effects on the mesomorphic properties of chiral imidazolium and pyridinium ionic liquids. Eur. J. Org. Chem. 2009, 2009, 5601–5609. [Google Scholar] [CrossRef]
- Dobbs, W.; Douce, L.; Heinrich, B. 1-(4-alkyloxybenzyl)-3-methyl-1H-imidazol-3-ium organic backbone: A versatile smectogenic moiety. Beilstein J. Org. Chem. 2009, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Ichikawa, T.; Shimura, H.; Kagata, T.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Columnar liquid-crystalline imidazolium salts. Effects of anions and cations on mesomorphic properties and ionic conductivities. Bull. Chem. Soc. Jpn. 2007, 80, 1836–1841. [Google Scholar] [CrossRef]
- Yoshio, M.; Kagata, T.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. One-dimensional ion-conductive polymer films: Alignment and fixation of ionic channels formed by self-organization of polymerizable columnar liquid crystals. J. Am. Chem. Soc. 2006, 128, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Trilla, M.; Pleixats, R.; Parella, T.; Blanc, C.; Dieudonné, P.; Guari, Y.; Man, M.W.C. Ionic liquid crystals based on mesitylene-containing bis-and trisimidazolium salts. Langmuir 2008, 24, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P. Cyclizing a Diimine at or below Room Temperature by Reaction with Paraformaldehyde and a Protic Acid; Particularly Reacting a 1,3, Diaryldiazabutadiene, 1,3, Dialkyldiazabutadiene, or 1,3, Arylalkyldiazabutadiene, Paraformaldehyde and HCl, HBF4, or HPF6 to Form an Imidazolium Salt. U.S. Patent 7,109,348, 19 September 2006. [Google Scholar]
- Strassner, T.; Ahrens, S. Salts Comprising Aryl-alkyl-substituted Imidazolium and Triazolium Cations and the Use Thereof. U.S. Patent 20,110,105,761, 5 May 2011. [Google Scholar]
- Fouchet, J.; Douce, L.; Heinrich, B.; Welter, R.; Louati, A. A convenient method for preparing rigid-core ionic liquid crystals. Beilstein J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.A.; Buchwald, S.L. 4, 7-dimethoxy-1, 10-phenanthroline: An excellent ligand for the Cu-catalyzed n-arylation of imidazoles. Org. Lett. 2006, 8, 2779–2782. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Cai, Q.; Zhang, H. Mild method for ullmann coupling reaction of amines and aryl halides. Org. Lett. 2003, 5, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, Q.; Ma, D. Amino acid promoted cui-catalyzed C–N bond formation between aryl halides and amines or N-containing heterocycles. J. Org. Chem. 2005, 70, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Suisse, J.M.; Douce, L.; Bellemin-Laponnaz, S.; Maisse-François, A.; Welter, R.; Miyake, Y.; Shimizu, Y. Liquid crystal imidazolium salts: Towards materials for catalysis and molecular electronics. Eur. J. Inorg. Chem. 2007, 2007, 3899–3905. [Google Scholar] [CrossRef]
- Mallo, N.; Alvarez Fernandez, A.; Kouwer, P.H.J. Unpublished work. 2016.
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bruce, D.W.; Jean′ne, M.S. Dicationic imidazolium-based ionic liquids and ionic liquid crystals with variously positioned fluoro substituents. J. Mater. Chem. 2009, 19, 8232–8238. [Google Scholar] [CrossRef]
- Starkulla, G.; Kaller, M.; Frey, W.; Axenov, K.V.; Laschat, S. Liquid crystalline imidazolium salts bearing 5-phenylpyrimidine: Dependence of mesomorphic properties on spacer lengths, terminal n-alkyl group and counterions. Liq. Cryst. 2011, 38, 1515–1529. [Google Scholar] [CrossRef]
- Starkulla, G.F.; Klenk, S.; Butschies, M.; Tussetschlaeger, S.; Laschat, S. Towards room temperature ionic liquid crystals: Linear vs. bent imidazolium phenylpyrimidines. J. Mater. Chem. 2012, 22, 21987–21997. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiao, L.; Shan, C.; Hou, P.; Chen, B.; Xu, X.; Niu, L. Synthesis and characterisation of novel imidazolium-based ionic liquid crystals with a p-nitroazobenzene moiety. Liq. Cryst. 2008, 35, 765–772. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, C.; Wang, X.; Chen, L.; Niu, L.; Chen, B. New ionic liquid crystals based on azobenzene moiety with two symmetric imidazolium ion group substituents. Liq. Cryst. 2008, 35, 1299–1305. [Google Scholar] [CrossRef]
- Ahn, S.; Yamakawa, S.; Akagi, K. Liquid crystallinity-embodied imidazolium-based ionic liquids and their chiral mesophases induced by axially chiral tetra-substituted binaphthyl derivatives. J. Mater. Chem. C 2015, 3, 3960–3970. [Google Scholar] [CrossRef]
- Butschies, M.; Neidhardt, M.M.; Mansueto, M.; Laschat, S.; Tussetschläger, S. Synthesis of guanidinium–sulfonimide ion pairs: Towards novel ionic liquid crystals. Beilstein J. Org. Chem. 2013, 9, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Dule, M.; Samanta, P.N.; Ghosh, S.; Mandal, T.K. Imidazolium-based ionic liquids with different fatty acid anions: Phase behavior, electronic structure and ionic conductivity investigation. Phys. Chem. Chem. Phys. 2014, 16, 16255–16263. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Fernandez, A. Novel Anisotropic Materials for Biosensing Applications; Ipskamp, B.V.: Nijmegen, The Netherlands, 2015; pp. 117–133. [Google Scholar]
- Bridges, N.J.; Hines, C.C.; Smiglak, M.; Rogers, R.D. An intermediate for the clean synthesis of ionic liquids: Isolation and crystal structure of 1,3-dimethylimidazolium hydrogen carbonate monohydrate. Chem. Eur. J. 2007, 13, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Gurau, G.; Rodríguez, H.; Kelley, S.P.; Janiczek, P.; Kalb, R.S.; Rogers, R.D. Demonstration of chemisorption of carbon dioxide in 1,3-dialkylimidazolium acetate ionic liquids. Angew. Chem. Int. Ed. 2011, 50, 12024–12026. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Gurau, G.; Holbrey, J.D.; Rogers, R.D. Reaction of elemental chalcogens with imidazolium acetates to yield imidazole-2-chalcogenones: Direct evidence for ionic liquids as proto-carbenes. Chem. Commun. 2011, 47, 3222–3224. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Fernandez, A.; van Dongen, M.J.; Blanco-Ania, D.; Kouwer, P.H. A facile route to hydrophilic ionic liquids. RSC Adv. 2014, 4, 30267–30273. [Google Scholar] [CrossRef]
- Amann, T.; Dold, C.; Kailer, A. Rheological characterization of ionic liquids and ionic liquid crystals with promising tribological performance. Soft Matter 2012, 8, 9840–9846. [Google Scholar] [CrossRef]
- Abate, A.; Petrozza, A.; Cavallo, G.; Lanzani, G.; Matteucci, F.; Bruce, D.W.; Houbenov, N.; Metrangolo, P.; Resnati, G. Anisotropic ionic conductivity in fluorinated ionic liquid crystals suitable for optoelectronic applications. J. Mater. Chem. A 2013, 1, 6572–6578. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xie, C.; Chen, Y. Optimization of the power conversion efficiency of room temperature-fabricated polymer solar cells utilizing solution processed tungsten oxide and conjugated polyelectrolyte as electrode interlayer. Adv. Funct. Mater. 2014, 24, 3986–3995. [Google Scholar] [CrossRef]
- Do, T.D.; Schmitzer, A.R. Intramolecular diels alder reactions in highly organized imidazolium salt-based ionic liquid crystals. RSC Adv. 2015, 5, 635–639. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, L.; Chen, Y. Direct anisotropic growth of CDS nanocrystals in thermotropic liquid crystal templates for heterojunction optoelectronics. Chem. Eur. J. 2014, 20, 11488–11495. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.; Coppel, Y.; Achard, M.F.; Mingotaud, C.; Marty, J.D.; Kahn, M.L. Thermotropic liquid crystals as templates for anisotropic growth of nanoparticles. Angew. Chem. Int. Ed. 2011, 123, 12238–12241. [Google Scholar] [CrossRef]
- Lee, J.J.; Yamaguchi, A.; Alam, M.A.; Yamamoto, Y.; Fukushima, T.; Kato, K.; Takata, M.; Fujita, N.; Aida, T. Discotic ionic liquid crystals of triphenylene as dispersants for orienting single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2012, 51, 8490–8494. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.; Suisse, J.-M.; Douce, L.; Welter, R. Electrodeposition of silver particles and gold nanoparticles from ionic liquid-crystal precursors. Angew. Chem. Int. Ed. 2006, 45, 4179–4182. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.F.J. Ionic self-assembly for functional hierarchical nanostructured materials. Acc. Chem. Res. 2014, 47, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Santella, M.; Amini, F.; Andreasen, K.B.; Aswad, D.S.; Ausar, H.; Austin, L.M.; Bora, I.; Boye, I.M.I.; Brinkenfeldt, N.K.; Bøe, M.F.; et al. Template-guided ionic self-assembled molecular materials and thin films with nanoscopic order. ChemNanoMat 2015, 1, 253–258. [Google Scholar] [CrossRef]
- Lozano, P.; de Diego, T.; Carrié, D.; Vaultier, M.; Iborra, J.L. Enzymatic ester synthesis in ionic liquids. J. Mol. Catal. B Enzym. 2003, 21, 9–13. [Google Scholar] [CrossRef]



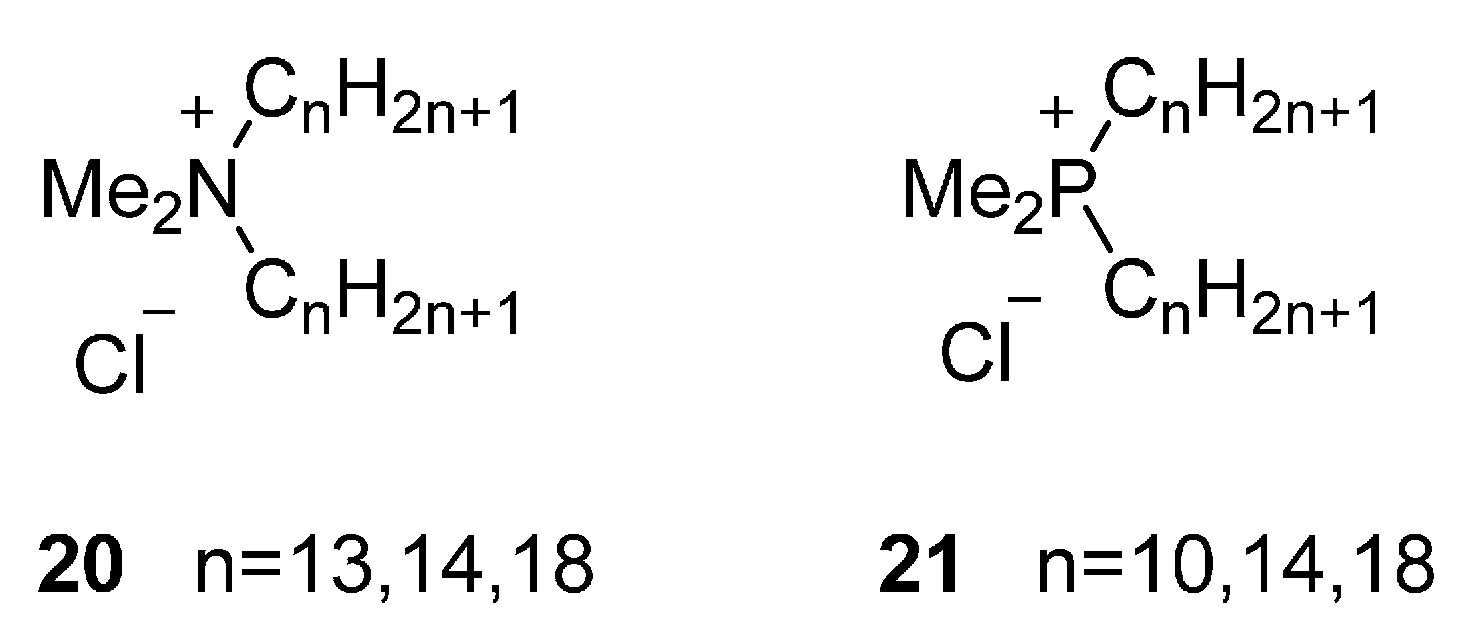
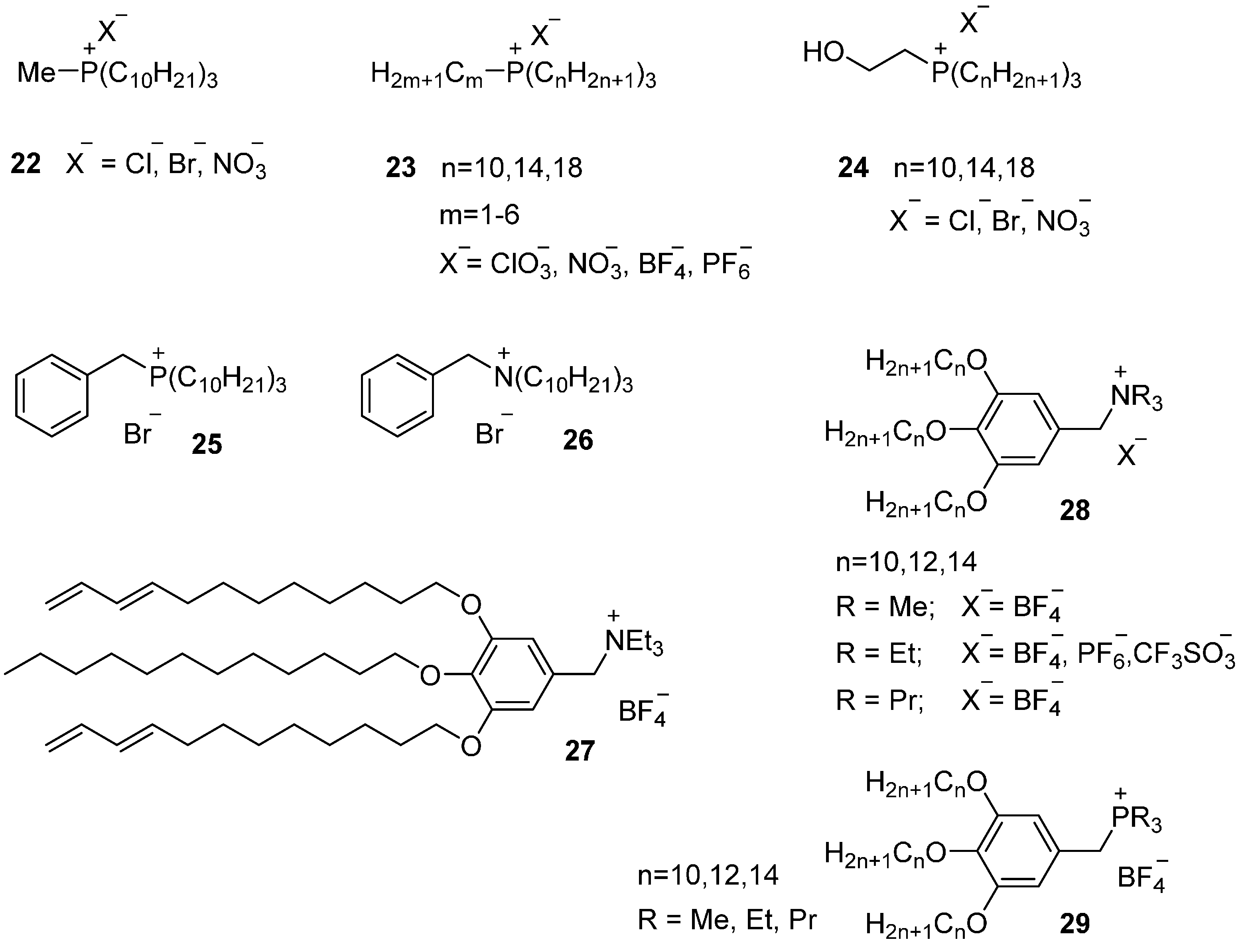









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez Fernandez, A.; Kouwer, P.H.J. Key Developments in Ionic Liquid Crystals. Int. J. Mol. Sci. 2016, 17, 731. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050731
Alvarez Fernandez A, Kouwer PHJ. Key Developments in Ionic Liquid Crystals. International Journal of Molecular Sciences. 2016; 17(5):731. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050731
Chicago/Turabian StyleAlvarez Fernandez, Alexandra, and Paul H. J. Kouwer. 2016. "Key Developments in Ionic Liquid Crystals" International Journal of Molecular Sciences 17, no. 5: 731. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050731