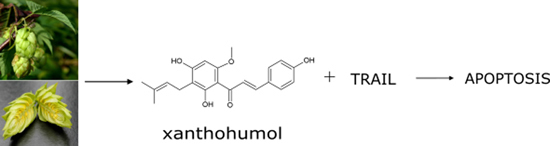
Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Prostate Cancer Cells after Treatment with Xanthohumol—A Natural Compound Present in Humulus lupulus L.
Abstract
:1. Introduction
2. Results
2.1. Cytotoxic and Apoptotic Activities of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) in Androgen-Sensitive Human Prostate Adenocarcinoma Cells (LNCaP)
2.2. Cytotoxic and Apoptotic Activities of Xanthohumol in LNCaP Cancer Cells
2.3. Apoptotic and Cytotoxic Activities of TRAIL in Combination with Xanthohumol on LNCaP
2.4. Necrotic Effect of TRAIL in Combination with Xanthohumol on LNCaP Cancer Cells
2.5. Effects of Xanthohumol on DR4/TRAIL-R1 and DR5/TRAIL-R2 Expression on Surface of LNCaP Cancer Cells
2.6. Effects of TRAIL and/or Xanthohumol on the Mitochondrial Membrane Potential in LNCaP Cancer Cells
2.7. Effect of TRAIL in Combination with Xanthohumol on the Protein Expression in LNCaP Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Cytotoxicity Assay
4.4. Lactate Dehydrogenase Release Assay
4.5. Detection of Apoptotic Cell Death by Flow Cytometry
4.6. Flow Cytometric Analysis of Death Receptor Expression on the Cancer Cell Surface
4.7. Mitochondrial Depolarization Assay
4.8. Western Blotting
4.9. Apoptosis Inhibition Using Caspase Inhibitors
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996, 271, 12687–12690. [Google Scholar] [PubMed]
- Secchiero, P.; Gonelli, A.; Corallini, F.; Ceconi, C.; Ferrari, R.; Zauli, G. Metalloproteinase 2 cleaves in vitro recombinant TRAIL: Potential implications for the decreased serum levels of TRAIL after acute myocardial infarction. Atherosclerosis 2010, 211, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Halaas, O.; Vik, R.; Ashkenazi, A.; Espevik, T. Lipopolysaccharide induces expression of Apo-2 ligand/TRAIL in human monocytes and macrophages. Scand. J. Immunol. 2000, 51, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, Y.; Zhang, M.; Wang, W.; Cao, X. The involvement of TNF-α-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-β-stimulated human dendritic cells to tumor cells. J. Immunol. 2001, 166, 5407–5415. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.C.; Haux, J.; Steinkjer, B.; Nonstad, U.; Egeberg, K.; Sundan, A.; Ashkenazi, A.; Espevik, T. Regulation of Apo-2 ligand/TRAIL expression in NK cells-involvement in NK cell-mediated cytotoxicity. Cytokine 1999, 11, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.D.; Hersey, P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J. Immunol. 1998, 161, 2195–2200. [Google Scholar] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Nguyen, T.; Thomas, W.D.; Sanders, J.E.; Hersey, P. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000, 482, 193–199. [Google Scholar] [CrossRef]
- Almasan, A.; Ashkenazi, A. Apo2L/TRAIL: Apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003, 14, 337–348. [Google Scholar] [CrossRef]
- Pan, G.; O′Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Ni, J.; Wei, Y.F.; Yu, G.; Gentz, R.; Dixit, V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Degli-Esposti, M.A.; Smolak, P.J.; Walczak, H.; Waugh, J.; Huang, C.P.; DuBose, R.F.; Goodwin, R.G.; Smith, C.A. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 1997, 186, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, M.; Ahmad, M.; Srinivasula, S.M.; Fernandes-Alnemri, T.; Cohen, G.M.; Alnemri, E.S. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 1997, 272, 25417–25420. [Google Scholar] [CrossRef] [PubMed]
- Pennarun, B.; Meijer, A.; de Vries, E.G.; Kleibeuker, J.H.; Kruyt, F.; de Jong, S. Playing the DISC: Turning on TRAIL death receptor-mediated apoptosis in cancer. Biochim. Biophys. Acta 2010, 1805, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Holoch, P.A.; Griffith, T.S. TNF-related apoptosis-inducing ligand (TRAIL): A new path to anti-cancer therapies. Eur. J. Pharmacol. 2009, 625, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.; Pils, D.; Haller, G.; Pribill, I.; Roessler, M.; Tomek, S.; Horvat, R.; Zeillinger, R.; Zielinski, C.; Krainer, M. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol. Cancer Res. 2005, 3, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Sanlioglu, A.D.; Dirice, E.; Aydin, C.; Erin, N.; Koksoy, S.; Sanlioglu, S. Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer 2005, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, T.S.; Lynch, D.H. TRAIL: A molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 1998, 10, 559–563. [Google Scholar] [CrossRef]
- Krajewska, M.; Krajewski, S.; Banares, S.; Huang, X.; Turner, B.; Bubendorf, L.; Kallioniemi, O.P.; Shabaik, A.; Vitiello, A.; Peehl, D.; et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin. Cancer Res. 2003, 9, 4914–4925. [Google Scholar] [PubMed]
- Sinicrope, F.A.; Penington, R.C.; Tang, X.M. Tumor necrosis factor-related apoptosis -inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14–1, in human colon cancer cells. Clin. Cancer Res. 2004, 10, 8284–8292. [Google Scholar] [CrossRef] [PubMed]
- Hinz, S.; Trauzold, A.; Boenicke, L.; Sandberg, C.; Beckmann, S.; Bayer, E.; Walczak, H.; Kalthoff, H.; Ungefroren, H. Bcl-xl protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene 2000, 19, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xiang, L.; Mackall, C.; Pastan, I. Killing of resistant cancer cells with low Bak by a combination of an antimesothelin immunotoxin and a TRAIL receptor 2 agonist antibody. Clin. Cancer Res. 2011, 17, 5926–5934. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, B.; Wendt, J.; Richter, A.; Müer, A.; Overkamp, T.; Gebhardt, N.; Preissner, R.; Belka, C.; Dörken, B.; Daniel, P.T. Endogenous Bak inhibitors MCL-1 and Bcl-xL: Differential impact on TRAIL resistance in Bax-deficient carcinoma. J. Cell. Biol. 2010, 188, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Krol, W. The role of dietary polyphenols in tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur. J. Cancer Prev. 2011, 20, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Chen, Z.; Kim, S.; Iqbal, S.; Chi, A.; Ritenour, C.; Wang, Y.A.; Kucuk, O.; Wu, D. Genistein enhances the efficacy of cabazitaxel chemotherapy in metastatic castration-resistant prostate cancer cells. Prostate 2013, 73, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Szliszka, E.; Król, W. The pathway of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) as a potential target in therapy of prostate cancer. Ann. Acad. Med. Siles 2013, 67, 315–321. [Google Scholar]
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Toh, J.Y.; Tan, V.M.; Lim, P.C.; Lim, S.T.; Chong, M.F. Flavonoids from fruit and vegetables: A focus on cardiovascular risk factors. Curr. Atheroscler. Rep. 2013, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamed, S.; Yokoyama, S.; Hafiyani, L.; Kalauni, S.K.; Hayakawa, Y.; Awale, S.; Saiki, I. Identification of plant extracts sensitizing breast cancer cells to TRAIL. Oncol. Rep. 2013, 29, 1991–1998. [Google Scholar] [PubMed]
- Bronikowska, J.; Szliszka, E.; Jaworska, D.; Czuba, Z.P.; Krol, W. The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 6449–6464. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Park, C.; Moon, S.K.; Kim, G.Y.; Kwon, T.K.; Lee, S.J.; Kim, W.J.; Choi, Y.H. Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs 2009, 20, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.H.; Wang, R.F.; Guo, S.Z.; Liu, B. An update on antitumor activity of naturally occurring chalcones. Evid. Based Complement. Altern. Med. 2013, 2013, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Chmiel [hops]. Available online: http://photo.podsiadly.info/2009/08/31/chmiel/ (accessed on 31 August 2009).
- Hops Blog. Available online: http://www.novascotiahopsblog.com/2013_08_01_archive.html (accessed on 29 August 2013).
- Dorn, C.; Weiss, T.S.; Heilmann, J.; Hellerbrand, C. Xanthohumol, a prenylated chalcone derived from hops, inhibits proliferation, migration and interleukin-8 expression of hepatocellular carcinoma cells. Int. J. Oncol. 2010, 36, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.C.; Miranda, C.L.; Stevens, J.F.; Deinzer, M.L.; Buhler, D.R. In vitro inhibition of human P450 enzymes by prenylated flavonoids from hops, Humulus lupulus. Xenobiotica 2000, 30, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Jaworska, D.; Kłósek, M.; Czuba, Z.P.; Król, W. Targeting death receptor TRAIL-R2 by chalcones for TRAIL-induced apoptosis in cancer cells. Int. J. Mol. Sci. 2012, 13, 15343–15359. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2009, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Park, M.A.; Heo, S.W.; Park, S.Y.; Kang, K.W.; Park, P.H.; Kim, J.A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim. Biophys. Acta 2013, 1830, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Pezzuto, J.M. Flavonoids in cancer prevention. Anticancer Agents Med. Chem. 2012, 12, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.R.; Frank, N.; Bartsch, H.; et al. Cancer chemopreventive activity of xanthohumol, a natural product derived from hop. Mol. Cancer Ther 2002, 1, 959–969. [Google Scholar] [PubMed]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Blanquer-Rosselló, M.M.; Oliver, J.; Valle, A.; Roca, P. Effect of xanthohumol and 8-prenylnaringenin on MCF-7 breast cancer cells oxidative stress and mitochondrial complexes expression. J. Cell. Biochem. 2013, 114, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Drenzek, J.G.; Seiler, N.L.; Jaskula-Sztul, R.; Rausch, M.M.; Rose, S.L. Xanthohumol decreases Notch1 expression and cell growth by cell cycle arrest and induction of apoptosis in epithelial ovarian cancer cell lines. Gynecol. Oncol. 2011, 122, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Delmulle, L.; Bellahcène, A.; Dhooge, W.; Comhaire, F.; Roelens, F.; Huvaere, K.; Heyerick, A.; Castronovo, V.; de Keukeleire, D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine 2006, 13, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mertas, A.; Paradysz, A.; Krol, W. The dietary isoflavone biochanin-a sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol. Oncol. 2013, 31, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Zydowicz, G.; Janoszka, B.; Dobosz, C.; Kowalczyk-Ziomek, G.; Krol, W. Ethanolic extract of brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2011, 38, 941–953. [Google Scholar] [PubMed]
- Ismail, B.; Fagnere, C.; Limami, Y.; Ghezali, L.; Pouget, C.; Fidanzi, C.; Ouk, C.; Gueye, R.; Beneytout, J.L.; Duroux, J.L.; et al. 2′-Hydroxy-4-methylsulfonylchalcone enhances TRAIL-induced apoptosis in prostate cancer cells. Anticancer Drugs 2015, 26, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Krol, W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncol. Rep. 2011, 26, 533–541. [Google Scholar] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Paradysz, A.; Krol, W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 2010, 15, 5336–5353. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Malik, A.; Adhami, V.M.; Asim, M.; Hafeez, B.B.; Sarfaraz, S.; Mukhtar, H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 2008, 27, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Chen, Q.; Siddiqui, I.; Sarva, K.; Srivastava, R.K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3,4′,5-trihydroxystilbene): Molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.K.; Abd Malek, S.N. Xanthohumol induces growth inhibition and apoptosis in Ca Ski human cervical cancer cells. Evid. Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Delmulle, L.; Vanden Berghe, T.; Keukeleire, D.D.; Vandenabeele, P. Treatment of PC3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) inducesa caspase-independent form of cell death. Phytother. Res. 2008, 22, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Jiang, H.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010, 30, 3333–3339. [Google Scholar] [PubMed]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic Bax and Bak:A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Eskes, R.; Desagher, S.; Antonsson, B.; Martinou, J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 2000, 20, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Catz, S.D.; Johnson, J.L. Bcl-2 in prostate cancer: A mini review. Apoptosis 2003, 8, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Bucur, O.; Almasan, A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis 2005, 10, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhao, S.; Xu, L.; Lu, Y.; Lu, Z.; Chen, C.; Ni, J.; Wan, R.; Yang, L. The inhibitory effects of xanthohumol, a prenylated chalcone derived from hops, on cell growth and tumorigenesis in human pancreatic cancer. Biomed. Pharmacother. 2015, 73, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, Y.J. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J. Cell. Biochem. 2007, 100, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Zydowicz, G.; Mizgala, E.; Krol, W. Artepillin c (3,5-diprenyl- 4-hydroxycinnamic acid) sensitizes LNCaP prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2012, 41, 818–828. [Google Scholar] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Jernas, K.; Król, W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int. J. Mol. Sci. 2008, 9, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Warat, M.; Szliszka, E.; Korzonek-Szlacheta, I.; Król, W.; Czuba, Z.P. Chrysin, apigenin and acacetin inhibit tumor necrosis factor-related apoptosis-inducing ligand receptor-1 (TRAIL-R1) on activated RAW264.7 macrophages. Int. J. Mol. Sci. 2014, 15, 11510–11522. [Google Scholar] [CrossRef] [PubMed]
- Warat, M.; Sadowski, T.; Szliszka, E.; Król, W.; Czuba, Z.P. The role of selected flavonols in tumor necrosis factor-related apoptosis-inducing ligand receptor-1 (TRAIL-R1) expression on activated RAW264.7 macrophages. Molecules 2015, 20, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kostrzewa-Susłow, E.; Bronikowska, J.; Jaworska, D.; Janeczko, T.; Czuba, Z.P.; Krol, W. Synthetic flavanones augment the anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 11693–11711. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem. Biophys Res. 2005, 333, 833–838. [Google Scholar] [CrossRef] [PubMed]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłósek, M.; Mertas, A.; Król, W.; Jaworska, D.; Szymszal, J.; Szliszka, E. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Prostate Cancer Cells after Treatment with Xanthohumol—A Natural Compound Present in Humulus lupulus L. Int. J. Mol. Sci. 2016, 17, 837. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060837
Kłósek M, Mertas A, Król W, Jaworska D, Szymszal J, Szliszka E. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Prostate Cancer Cells after Treatment with Xanthohumol—A Natural Compound Present in Humulus lupulus L. International Journal of Molecular Sciences. 2016; 17(6):837. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060837
Chicago/Turabian StyleKłósek, Małgorzata, Anna Mertas, Wojciech Król, Dagmara Jaworska, Jan Szymszal, and Ewelina Szliszka. 2016. "Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Prostate Cancer Cells after Treatment with Xanthohumol—A Natural Compound Present in Humulus lupulus L." International Journal of Molecular Sciences 17, no. 6: 837. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060837