Beyond a Measure of Liver Function—Bilirubin Acts as a Potential Cardiovascular Protector in Chronic Kidney Disease Patients
Abstract
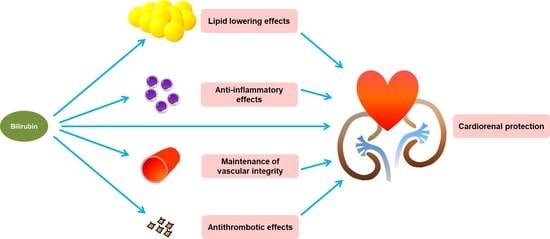
:1. Introduction
2. Potential Effects of Bilirubin against Cardiovascular Events in CKD Patients
2.1. Antioxidant Effects of Bilirubin
2.2. Immunomodulatory and Anti-Inflammatory Effects of Bilirubin
2.3. Antithrombotic Effects of Bilirubin
2.4. Lipid Lowering Effects of Bilirubin
2.5. Bilirubin and Maintenance of Vascular Integrity
2.6. Other Aspects of the Protective Action of Bilirubin
3. Role of Bilirubin in Cardioprotection in CKD Patients at Risk of CVD
3.1. Evidence Supporting the Beneficial Effects of Bilirubin on CVD
3.2. Negative or Inconclusive Results
3.3. Gene Polymorphisms Involved in Bilirubin Metabolism and Their Relationship with CKD Progression and Cardiovascular Mortality—Focused on UGT1A1 and HMOX1 Gene Polymorphisms
4. Interventions to Modulate Bilirubin Levels as a New Therapeutic Strategy for Patients with CKD and CVD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes. Trends Endocrinol. Metab. 2018, 29, 74–85. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.; Hosick, P.A.; John, K.; Stec, D.E.; Hinds, T.D., Jr. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015, 26, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutler, E.; Gelbart, T.; Demina, A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl. Acad. Sci. USA 1998, 95, 8170–8174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keppler, D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab. Dispos. 2014, 42, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D., Jr.; Burns, K.A.; Hosick, P.A.; McBeth, L.; Nestor-Kalinoski, A.; Drummond, H.A.; AlAmodi, A.A.; Hankins, M.W.; Vanden Heuvel, J.P.; Stec, D.E. Biliverdin Reductase A Attenuates Hepatic Steatosis by Inhibition of Glycogen Synthase Kinase (GSK) 3beta Phosphorylation of Serine 73 of Peroxisome Proliferator-activated Receptor (PPAR) alpha. J. Biol. Chem. 2016, 291, 25179–25191. [Google Scholar] [CrossRef] [PubMed]
- Gamaleldin, R.; Iskander, I.; Seoud, I.; Aboraya, H.; Aravkin, A.; Sampson, P.D.; Wennberg, R.P. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics 2011, 128, e925–e931. [Google Scholar] [CrossRef] [PubMed]
- Gazzin, S.; Vitek, L.; Watchko, J.; Shapiro, S.M.; Tiribelli, C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol. Med. 2016, 22, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, A.C.; Bakrania, B.; Du Toit, E.F.; Boon, A.C.; Clark, P.J.; Powell, L.W.; Wagner, K.H.; Headrick, J.P. Bilirubin acts as a multipotent guardian of cardiovascular integrity: More than just a radical idea. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H429–H447. [Google Scholar] [CrossRef]
- Wagner, K.H.; Shiels, R.G.; Lang, C.A.; Seyed Khoei, N.; Bulmer, A.C. Diagnostic criteria and contributors to Gilbert’s syndrome. Crit. Rev. Clin. Lab. Sci. 2018, 55, 129–139. [Google Scholar] [CrossRef]
- Powell, L.W.; Hemingway, E.; Billing, B.H.; Sherlock, S. Idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). A study of 42 families. N. Engl. J. Med. 1967, 277, 1108–1112. [Google Scholar] [CrossRef]
- Vitek, L.; Jirsa, M.; Brodanova, M.; Kalab, M.; Marecek, Z.; Danzig, V.; Novotny, L.; Kotal, P. Gilbert syndrome and ischemic heart disease: A protective effect of elevated bilirubin levels. Atherosclerosis 2002, 160, 449–456. [Google Scholar] [CrossRef]
- Marconi, V.C.; Duncan, M.S.; So-Armah, K.; Re, V.L., 3rd; Lim, J.K.; Butt, A.A.; Goetz, M.B.; Rodriguez-Barradas, M.C.; Alcorn, C.W.; Lennox, J.; et al. Bilirubin Is Inversely Associated With Cardiovascular Disease Among HIV-Positive and HIV-Negative Individuals in VACS (Veterans Aging Cohort Study). J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Deetman, P.E.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.; Hillege, H.L.; van der Harst, P.; Stolk, R.P.; Navis, G.; Alizadeh, B.Z.; et al. Bilirubin as a potential causal factor in type 2 diabetes risk: A Mendelian randomization study. Diabetes 2015, 64, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fukui, M.; Okada, H.; Senmaru, T.; Asano, M.; Akabame, S.; Yamazaki, M.; Tomiyasu, K.; Oda, Y.; Hasegawa, G.; et al. Low serum bilirubin concentration is a predictor of chronic kidney disease. Atherosclerosis 2014, 234, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Wallner, M.; Molzer, C.; Gazzin, S.; Bulmer, A.C.; Tiribelli, C.; Vitek, L. Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clin. Sci. 2015, 129, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Chan, W.L.; Leu, H.B.; Huang, P.H.; Lin, S.J.; Chen, J.W. Serum bilirubin levels predict future development of metabolic syndrome in healthy middle-aged nonsmoking men. Am. J. Med. 2015, 128, 1138.e35–1138.e41. [Google Scholar] [CrossRef]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.L.; Jardine, M.; et al. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Painer, J.; Kuro, O.M.; Lanaspa, M.; Arnold, W.; Ruf, T.; Shiels, P.G.; Johnson, R.J. Novel treatment strategies for chronic kidney disease: Insights from the animal kingdom. Nat. Rev. Nephrol. 2018, 14, 265–284. [Google Scholar] [CrossRef]
- Boon, A.C.; Bulmer, A.C.; Coombes, J.S.; Fassett, R.G. Circulating bilirubin and defense against kidney disease and cardiovascular mortality: Mechanisms contributing to protection in clinical investigations. Am. J. Physiol. Renal Physiol. 2014, 307, F123–F136. [Google Scholar] [CrossRef]
- Sundararaghavan, V.L.; Binepal, S.; Stec, D.E.; Sindhwani, P.; Hinds, T.D., Jr. Bilirubin, a new therapeutic for kidney transplant? Transplant. Rev. (Orlando) 2018, 32, 234–240. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V. Chronic Kidney Disease and Disproportionally Increased Cardiovascular Damage: Does Oxidative Stress Explain the Burden? Oxid. Med. Cell. Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef]
- Xu, H.; Watanabe, M.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Anderstam, B.; Eriksson, M.; Stenvinkel, P.; Lindholm, B. Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit. Dial. Int. 2015, 35, 206–215. [Google Scholar] [CrossRef]
- Signorini, L.; Granata, S.; Lupo, A.; Zaza, G. Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Mayer, M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin. Chem. 2000, 46, 1723–1727. [Google Scholar]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.W.; Lee, E.S.; Kim, S.; Na, K.Y.; Chae, D.W.; Kim, S.; Chin, H.J. Bilirubin attenuates the renal tubular injury by inhibition of oxidative stress and apoptosis. BMC Nephrol 2013, 14, 105. [Google Scholar] [CrossRef]
- Fujii, M.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; Zheng, J.; Kobayashi, K.; Takayanagi, R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010, 78, 905–919. [Google Scholar] [CrossRef]
- Vitek, L. Bilirubin and atherosclerotic diseases. Physiol. Res. 2017, 66, S11–S20. [Google Scholar]
- Boon, A.C.; Hawkins, C.L.; Bisht, K.; Coombes, J.S.; Bakrania, B.; Wagner, K.H.; Bulmer, A.C. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic. Biol. Med. 2012, 52, 2120–2127. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [Green Version]
- Hung, S.C.; Kuo, K.L.; Wu, C.C.; Tarng, D.C. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Renal Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef]
- Bhatti, N.K.; Karimi Galougahi, K.; Paz, Y.; Nazif, T.; Moses, J.W.; Leon, M.B.; Stone, G.W.; Kirtane, A.J.; Karmpaliotis, D.; Bokhari, S.; et al. Diagnosis and Management of Cardiovascular Disease in Advanced and End-Stage Renal Disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Tsai, M.T.; Hu, F.H.; Lien, T.J.; Chen, P.J.; Huang, T.P.; Tarng, D.C. Interaction between geriatric nutritional risk index and decoy receptor 3 predicts mortality in chronic hemodialysis patients. Am. J. Nephrol. 2014, 40, 191–199. [Google Scholar] [CrossRef]
- Machowska, A.; Carrero, J.J.; Lindholm, B.; Stenvinkel, P. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl. Res. 2016, 167, 204–213. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Hassan, F.; Thomas, F.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Treatment of rheumatoid arthritis with biologic agents lowers the risk of incident chronic kidney disease. Kidney Int. 2018, 93, 1207–1216. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, P.; Lu, J.; Xiong, W.; Oger, J.; Tetzlaff, W.; Cynader, M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2008, 181, 1887–1897. [Google Scholar] [CrossRef]
- Basiglio, C.L.; Arriaga, S.M.; Pelusa, H.F.; Almara, A.M.; Roma, M.G.; Mottino, A.D. Protective role of unconjugated bilirubin on complement-mediated hepatocytolysis. Biochim. Biophys. Acta 2007, 1770, 1003–1010. [Google Scholar] [CrossRef]
- Keshavan, P.; Deem, T.L.; Schwemberger, S.J.; Babcock, G.F.; Cook-Mills, J.M.; Zucker, S.D. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J. Immunol. 2005, 174, 3709–3718. [Google Scholar] [CrossRef]
- Rocuts, F.; Zhang, X.; Yan, J.; Yue, Y.; Thomas, M.; Bach, F.H.; Czismadia, E.; Wang, H. Bilirubin promotes de novo generation of T regulatory cells. Cell Transplant. 2010, 19, 443–451. [Google Scholar] [CrossRef]
- Deetman, P.E.; Zelle, D.M.; Homan van der Heide, J.J.; Navis, G.J.; Gans, R.O.; Bakker, S.J. Plasma bilirubin and late graft failure in renal transplant recipients. Transpl. Int. 2012, 25, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Ribic, C.; Crowther, M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematology Am. Soc. Hematol. Educ. Program 2016, 2016, 188–195. [Google Scholar] [CrossRef]
- Kolachalama, V.B.; Shashar, M.; Alousi, F.; Shivanna, S.; Rijal, K.; Belghasem, M.E. Uremic Solute-Aryl Hydrocarbon Receptor-Tissue Factor Axis Associates with Thrombosis after Vascular Injury in Humans. J. Am. Soc. Nephrol. 2018, 29, 1063–1072. [Google Scholar] [CrossRef]
- Ocak, G.; Verduijn, M.; Vossen, C.Y.; Lijfering, W.M.; Dekker, F.W.; Rosendaal, F.R.; Gansevoort, R.T.; Mahmoodi, B.K. Chronic kidney disease stages 1-3 increase the risk of venous thrombosis. J. Thromb. Haemost. 2010, 8, 2428–2435. [Google Scholar] [CrossRef] [Green Version]
- Kitching, A.R.; Hutton, H.L. The Players: Cells Involved in Glomerular Disease. Clin. J. Am. Soc. Nephrol. 2016, 11, 1664–1674. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.P. Platelets in liver and renal disease. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 251–255. [Google Scholar] [CrossRef]
- Donadio, J.V., Jr.; Anderson, C.F.; Mitchell, J.C., 3rd; Holley, K.E.; Ilstrup, D.M.; Fuster, V.; Chesebro, J.H. Membranoproliferative glomerulonephritis. A prospective clinical trial of platelet-inhibitor therapy. N. Engl. J. Med. 1984, 310, 1421–1426. [Google Scholar] [CrossRef]
- Lozano, I.; Rondan, J.; Vegas, J.M.; Segovia, E. Chronic Kidney Disease and Antiplatelet Therapy: A Worrying Gap Between Evidence Based Medicine and Clinical Practice. JACC Cardiovasc. Interv. 2018, 11, 319–320. [Google Scholar] [CrossRef]
- Lee, K.H.; Li, S.Y.; Liu, J.S.; Huang, C.T.; Chen, Y.Y.; Lin, Y.P.; Hsu, C.C.; Tarng, D.C. Association of warfarin with congestive heart failure and peripheral artery occlusive disease in hemodialysis patients with atrial fibrillation. J. Chin. Med. Assoc. 2017, 80, 277–282. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chen, Y.Y.; Chang, W.J.; Li, S.Y. Warfarin accelerated vascular calcification and worsened cardiac dysfunction in remnant kidney mice. J. Chin. Med. Assoc. 2018, 81, 324–330. [Google Scholar] [CrossRef]
- Kundur, A.R.; Singh, I.; Bulmer, A.C. Bilirubin, platelet activation and heart disease: A missing link to cardiovascular protection in Gilbert’s syndrome? Atherosclerosis 2015, 239, 73–84. [Google Scholar] [CrossRef]
- Kundur, A.R.; Santhakumar, A.B. Mildly elevated unconjugated bilirubin is associated with reduced platelet activation-related thrombogenesis and inflammation in Gilbert’s syndrome. Platelets 2017, 28, 779–785. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Thushara, R.M.; Sundaram, M.S.; Hemshekhar, M.; Paul, M.; Thirunavukkarasu, C.; Basappa; Nagaraju, G.; Raghavan, S.C.; Girish, K.S.; et al. Unconjugated Bilirubin exerts Pro-Apoptotic Effect on Platelets via p38-MAPK activation. Sci. Rep. 2015, 5, 15045. [Google Scholar] [CrossRef] [Green Version]
- Bulbul, M.C.; Dagel, T.; Afsar, B.; Ulusu, N.N.; Kuwabara, M.; Covic, A.; Kanbay, M. Disorders of Lipid Metabolism in Chronic Kidney Disease. Blood Purif. 2018, 46, 144–152. [Google Scholar] [CrossRef]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
- Wanner, C.; Krane, V.; Marz, W.; Olschewski, M.; Mann, J.F.; Ruf, G.; Ritz, E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005, 353, 238–248. [Google Scholar] [CrossRef]
- Fellstrom, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.W.; Chevaile, A.; Cobbe, S.M.; Gronhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Haynes, R.; Lewis, D.; Emberson, J.; Reith, C.; Agodoa, L.; Cass, A.; Craig, J.C.; de Zeeuw, D.; Feldt-Rasmussen, B.; Fellstrom, B.; et al. Effects of lowering LDL cholesterol on progression of kidney disease. J. Am. Soc. Nephrol. 2014, 25, 1825–1833. [Google Scholar] [CrossRef]
- Oda, E. A decrease in total bilirubin predicted hyper-LDL cholesterolemia in a health screening population. Atherosclerosis 2014, 235, 334–338. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Verkade, H.J.; Wagner, K.H. Bilirubin and beyond: A review of lipid status in Gilbert’s syndrome and its relevance to cardiovascular disease protection. Prog. Lipid Res. 2013, 52, 193–205. [Google Scholar] [CrossRef]
- Dietrich, C. Antioxidant Functions of the Aryl Hydrocarbon Receptor. Stem Cells Int. 2016, 2016, 7943495. [Google Scholar] [CrossRef]
- Phelan, D.; Winter, G.M.; Rogers, W.J.; Lam, J.C.; Denison, M.S. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch. Biochem. Biophys. 1998, 357, 155–163. [Google Scholar] [CrossRef]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D., Jr. Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Hosick, P.A.; Chen, S.; Tukey, R.H.; Hankins, M.W.; Nestor-Kalinoski, A.; Stec, D.E. Mice with hyperbilirubinemia due to Gilbert’s syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARalpha. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E244–E252. [Google Scholar] [CrossRef]
- De Boer, J.F.; Schonewille, M.; Dikkers, A.; Koehorst, M.; Havinga, R.; Kuipers, F.; Tietge, U.J.; Groen, A.K. Transintestinal and Biliary Cholesterol Secretion Both Contribute to Macrophage Reverse Cholesterol Transport in Rats-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 643–646. [Google Scholar] [CrossRef]
- Martens, C.R.; Kirkman, D.L.; Edwards, D.G. The Vascular Endothelium in Chronic Kidney Disease: A Novel Target for Aerobic Exercise. Exerc. Sport Sci. Rev. 2016, 44, 12–19. [Google Scholar] [CrossRef]
- Perry, H.M.; Okusa, M.D. Endothelial Dysfunction in Renal Interstitial Fibrosis. Nephron 2016, 134, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipphardt, M.; Song, J.W.; Matsumoto, K.; Dadafarin, S.; Dihazi, H.; Muller, G.; Goligorsky, M.S. The third path of tubulointerstitial fibrosis: Aberrant endothelial secretome. Kidney Int. 2017, 92, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Gullu, H.; Erdogan, D.; Tok, D.; Topcu, S.; Caliskan, M.; Ulus, T.; Muderrisoglu, H. High serum bilirubin concentrations preserve coronary flow reserve and coronary microvascular functions. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Amor, A.J.; Ortega, E.; Perea, V.; Cofan, M.; Sala-Vila, A.; Nunez, I.; Gilabert, R.; Ros, E. Relationship Between Total Serum Bilirubin Levels and Carotid and Femoral Atherosclerosis in Familial Dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Kihara, Y.; Chayama, K.; et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation 2012, 126, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.E.; Idelman, G.; Konaniah, E.S.; Zucker, S.D. Bilirubin Prevents Atherosclerotic Lesion Formation in Low-Density Lipoprotein Receptor-Deficient Mice by Inhibiting Endothelial VCAM-1 and ICAM-1 Signaling. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, T.; Chaudhary, R.; Garg, S.K.; Sandhu, G.S.; Mittal, V.; Gupta, R.; Bodin, R.; Sule, S. Bilirubin in coronary artery disease: Cytotoxic or protective? World J. Gastrointest. Pharmacol. Ther. 2016, 7, 469–476. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Kaur, H.; Hughes, M.N.; Green, C.J.; Naughton, P.; Foresti, R.; Motterlini, R. Interaction of bilirubin and biliverdin with reactive nitrogen species. FEBS Lett. 2003, 543, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L. Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis. Cardiovasc. Res. 2014, 103, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graca-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef]
- Peyton, K.J.; Shebib, A.R.; Azam, M.A.; Liu, X.M.; Tulis, D.A.; Durante, W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front. Pharmacol. 2012, 3, 48. [Google Scholar] [CrossRef]
- Stoeckius, M.; Erat, A.; Fujikawa, T.; Hiromura, M.; Koulova, A.; Otterbein, L.; Bianchi, C.; Tobiasch, E.; Dagon, Y.; Sellke, F.W.; et al. Essential roles of Raf/extracellular signal-regulated kinase/mitogen-activated protein kinase pathway, YY1, and Ca2+ influx in growth arrest of human vascular smooth muscle cells by bilirubin. J. Biol. Chem. 2012, 287, 15418–15426. [Google Scholar] [CrossRef]
- Zhang, M.; Malik, A.B.; Rehman, J. Endothelial progenitor cells and vascular repair. Curr. Opin. Hematol. 2014, 21, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Ozkok, A.; Yildiz, A. Endothelial Progenitor Cells and Kidney Diseases. Kidney Blood Press. Res. 2018, 43, 701–718. [Google Scholar] [CrossRef] [Green Version]
- Jabarpour, M.; Siavashi, V.; Asadian, S.; Babaei, H.; Jafari, S.M.; Nassiri, S.M. Hyperbilirubinemia-induced pro-angiogenic activity of infantile endothelial progenitor cells. Microvasc. Res. 2018, 118, 49–56. [Google Scholar] [CrossRef]
- Ikeda, Y.; Hamano, H.; Satoh, A.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Kihira, Y.; Ishizawa, K.; Aihara, K.; Tsuchiya, K.; Tamaki, T. Bilirubin exerts pro-angiogenic property through Akt-eNOS-dependent pathway. Hypertens. Res. 2015, 38, 733–740. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.H.; Hwang, J.H.; Kim, Y.C.; Kim, J.H.; Lim, C.S.; Kim, Y.S.; Yang, S.H.; Lee, J.P. Elevated bilirubin levels are associated with a better renal prognosis and ameliorate kidney fibrosis. PLoS ONE 2017, 12, e0172434. [Google Scholar] [CrossRef]
- Liu, J.; Dong, H.; Zhang, Y.; Cao, M.; Song, L.; Pan, Q.; Bulmer, A.; Adams, D.B.; Dong, X.; Wang, H. Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARgamma Levels. Sci. Rep. 2015, 5, 9886. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Antioxidant bilirubin works in multiple ways to reduce risk for obesity and its health complications. Open Heart 2018, 5, e000914. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A. Bilirubin: A potential biomarker and therapeutic target for diabetic nephropathy. Diabetes 2014, 63, 2613–2616. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Renal Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef] [Green Version]
- Qaisiya, M.; Coda Zabetta, C.D.; Bellarosa, C.; Tiribelli, C. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell. Signal. 2014, 26, 512–520. [Google Scholar] [CrossRef]
- Kim, S.D.; Antenos, M.; Squires, E.J.; Kirby, G.M. Cytochrome P450 2A5 and bilirubin: Mechanisms of gene regulation and cytoprotection. Toxicol. Appl. Pharmacol. 2013, 270, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Hamoud, A.R.; Weaver, L.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin in the Liver-Gut Signaling Axis. Trends Endocrinol. Metab. 2018, 29, 140–150. [Google Scholar] [CrossRef]
- Sakoh, T.; Nakayama, M.; Tanaka, S.; Yoshitomi, R.; Ura, Y.; Nishimoto, H.; Fukui, A.; Shikuwa, Y.; Tsuruya, K.; Kitazono, T. Association of serum total bilirubin with renal outcome in Japanese patients with stages 3-5 chronic kidney disease. Metab. Clin. Exp. 2015, 64, 1096–1102. [Google Scholar] [CrossRef]
- Wu, B.; Bell, K.; Stanford, A.; Kern, D.M.; Tunceli, O.; Vupputuri, S.; Kalsekar, I.; Willey, V. Understanding CKD among patients with T2DM: Prevalence, temporal trends, and treatment patterns-NHANES 2007-2012. BMJ Open Diabetes Res Care 2016, 4, e000154. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, X.; Bi, Y.; Yang, Y. Effect of bilirubin concentration on the risk of diabetic complications: A meta-analysis of epidemiologic studies. Sci. Rep. 2017, 7, 41681. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.J.; Lee, Y.J.; Park, B.J.; Hong, K.W.; Jung, D.H. Total serum bilirubin and 8-year incident type 2 diabetes mellitus: The Korean Genome and Epidemiology Study. Diabetes Metab. 2018, 44, 346–353. [Google Scholar] [CrossRef]
- Okada, H.; Fukui, M.; Tanaka, M.; Matsumoto, S.; Kobayashi, K.; Iwase, H.; Tomiyasu, K.; Nakano, K.; Hasegawa, G.; Nakamura, N. Low serum bilirubin concentration is a novel risk factor for the development of albuminuria in patients with type 2 diabetes. Metab. Clin. Exp. 2014, 63, 409–414. [Google Scholar] [CrossRef]
- Riphagen, I.J.; Deetman, P.E.; Bakker, S.J.; Navis, G.; Cooper, M.E.; Lewis, J.B.; de Zeeuw, D.; Lambers Heerspink, H.J. Bilirubin and progression of nephropathy in type 2 diabetes: A post hoc analysis of RENAAL with independent replication in IDNT. Diabetes 2014, 63, 2845–2853. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hung, S.C.; Tarng, D.C. Serum bilirubin links UGT1A1*28 polymorphism and predicts long-term cardiovascular events and mortality in chronic hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 567–574. [Google Scholar] [CrossRef]
- Fukui, M.; Tanaka, M.; Yamazaki, M.; Hasegawa, G.; Nishimura, M.; Iwamoto, N.; Ono, T.; Imai, S.; Nakamura, N. Low serum bilirubin concentration in haemodialysis patients with Type 2 diabetes. Diabet. Med. 2011, 28, 96–99. [Google Scholar] [CrossRef]
- Do Sameiro-Faria, M.; Kohlova, M.; Ribeiro, S.; Rocha-Pereira, P.; Teixeira, L.; Nascimento, H.; Reis, F.; Miranda, V.; Bronze-da-Rocha, E.; Quintanilha, A.; et al. Potential cardiovascular risk protection of bilirubin in end-stage renal disease patients under hemodialysis. BioMed Res. Int. 2014, 2014, 175286. [Google Scholar] [CrossRef]
- Zelenka, J.; Dvorak, A. Hyperbilirubinemia Protects against Aging-Associated Inflammation and Metabolic Deterioration. Oxid. Med. Cell. Longev. 2016, 2016, 6190609. [Google Scholar] [CrossRef]
- Tosevska, A.; Moelzer, C.; Wallner, M.; Janosec, M.; Schwarz, U.; Kern, C.; Marculescu, R.; Doberer, D.; Weckwerth, W.; Wagner, K.H. Longer telomeres in chronic, moderate, unconjugated hyperbilirubinaemia: Insights from a human study on Gilbert’s Syndrome. Sci. Rep. 2016, 6, 22300. [Google Scholar] [CrossRef]
- Chmielewski, P.; Strzelec, B.; Chmielowiec, J.; Chmielowiec, K.; Borysławski, K. Association of serum bilirubin with longevity: Evidence from a retrospective longitudinal study and cross-sectional data. Anthropol. Rev. 2017, 80, 335. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liang, M.; Wang, G.; Li, J.; Zhang, Y.; Huo, Y.; Cui, Y.; Xu, X.; Qin, X. Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease. Clin. Epidemiol. 2018, 10, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Su, H.H.; Kao, C.M.; Lin, Y.C.; Lin, Y.C.; Kao, C.C.; Chen, H.H.; Hsu, C.C.; Chen, K.C.; Peng, C.C.; Wu, M.S. Relationship between serum total bilirubin levels and mortality in uremia patients undergoing long-term hemodialysis: A nationwide cohort study. Atherosclerosis 2017, 265, 155–161. [Google Scholar] [CrossRef]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Mishra, J.; Mori, K.; Ma, Q.; Kelly, C.; Barasch, J.; Devarajan, P. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004, 24, 307–315. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Hosohata, K.; Ando, H.; Fujiwara, Y.; Fujimura, A. Vanin-1: A potential biomarker for nephrotoxicant-induced renal injury. Toxicology 2011, 290, 82–88. [Google Scholar] [CrossRef]
- Van Slambrouck, C.M.; Salem, F.; Meehan, S.M.; Chang, A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013, 84, 192–197. [Google Scholar] [CrossRef]
- Aniort, J.; Poyet, A.; Kemeny, J.L.; Philipponnet, C.; Heng, A.E. Bile Cast Nephropathy Caused by Obstructive Cholestasis. Am. J. Kidney Dis. 2017, 69, 143–146. [Google Scholar] [CrossRef]
- Memon, N.; Weinberger, B.I.; Hegyi, T.; Aleksunes, L.M. Inherited disorders of bilirubin clearance. Pediatr. Res. 2016, 79, 378–386. [Google Scholar] [CrossRef]
- Lin, J.P.; Vitek, L.; Schwertner, H.A. Serum bilirubin and genes controlling bilirubin concentrations as biomarkers for cardiovascular disease. Clin. Chem. 2010, 56, 1535–1543. [Google Scholar] [CrossRef]
- Chen, Y.H.; Kuo, K.L.; Hung, S.C.; Hsu, C.C.; Chen, Y.H.; Tarng, D.C. Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease. J. Am. Soc. Nephrol. 2014, 25, 2669–2677. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hung, S.C.; Tarng, D.C. Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 1756–1763. [Google Scholar] [CrossRef]
- Kimpara, T.; Takeda, A.; Watanabe, K.; Itoyama, Y.; Ikawa, S.; Watanabe, M.; Arai, H.; Sasaki, H.; Higuchi, S.; Okita, N.; et al. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum. Genet. 1997, 100, 145–147. [Google Scholar] [CrossRef]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme Oxygenase-1 in Kidney Health and Disease. Antioxid. Redox Signal. 2016, 25, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Lee, Y.H.; Kim, S.H.; Jung, K.S.; Kwon, O.; Kim, B.S.; Nam, C.M.; Park, C.S.; Lee, B.W.; Kang, E.S.; et al. Association Between Heme Oxygenase-1 Promoter Polymorphisms and the Development of Albuminuria in Type 2 Diabetes: A Case-Control Study. Medicine (Baltim.) 2015, 94, e1825. [Google Scholar] [CrossRef]
- Ozaki, K.S.; Marques, G.M.; Nogueira, E.; Feitoza, R.Q.; Cenedeze, M.A.; Franco, M.F.; Mazzali, M.; Soares, M.P.; Pacheco-Silva, A.; Camara, N.O. Improved renal function after kidney transplantation is associated with heme oxygenase-1 polymorphism. Clin. Transplant. 2008, 22, 609–616. [Google Scholar] [CrossRef]
- Saraf, S.L.; Zhang, X.; Shah, B.; Kanias, T.; Gudehithlu, K.P.; Kittles, R.; Machado, R.F.; Arruda, J.A.; Gladwin, M.T.; Singh, A.K.; et al. Genetic variants and cell-free hemoglobin processing in sickle cell nephropathy. Haematologica 2015, 100, 1275–1284. [Google Scholar] [CrossRef]
- Bosma, P.; Chowdhury, J.R.; Jansen, P.H. Genetic inheritance of Gilbert’s syndrome. Lancet 1995, 346, 314–315. [Google Scholar] [CrossRef]
- Lin, J.P.; O’Donnell, C.J.; Schwaiger, J.P.; Cupples, L.A.; Lingenhel, A.; Hunt, S.C.; Yang, S.; Kronenberg, F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 2006, 114, 1476–1481. [Google Scholar] [CrossRef]
- Lingenhel, A.; Kollerits, B.; Schwaiger, J.P.; Hunt, S.C.; Gress, R.; Hopkins, P.N.; Schoenborn, V.; Heid, I.M.; Kronenberg, F. Serum bilirubin levels, UGT1A1 polymorphisms and risk for coronary artery disease. Exp. Gerontol. 2008, 43, 1102–1107. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.J.; Chen, M.J.; Liao, Y.L.; Liao, T.N. Polymorphisms of the uridine-diphosphoglucuronosyltransferase 1A1 gene and coronary artery disease. Cell. Mol. Biol. Lett. 2008, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dekker, D.; Dorresteijn, M.J.; Welzen, M.E.B.; Timman, S.; Pickkers, P.; Burger, D.M. Parenteral bilirubin in healthy volunteers: A reintroduction in translational research. Br. J. Clin. Pharmacol. 2018, 84, 268–279. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Tian, X.Y.; Liu, L.; Wong, W.T.; Zhang, Y.; Han, Q.B.; Ho, H.M.; Wang, N.; Wong, S.L.; et al. Unconjugated bilirubin mediates heme oxygenase-1-induced vascular benefits in diabetic mice. Diabetes 2015, 64, 1564–1575. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Stec, D.E. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 2018, 72, 788–795. [Google Scholar] [CrossRef]
- Lerner-Marmarosh, N.; Miralem, T.; Gibbs, P.E.; Maines, M.D. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 6870–6875. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, P.E.; Miralem, T.; Lerner-Marmarosh, N.; Maines, M.D. Nanoparticle Delivered Human Biliverdin Reductase-Based Peptide Increases Glucose Uptake by Activating IRK/Akt/GSK3 Axis: The Peptide Is Effective in the Cell and Wild-Type and Diabetic Ob/Ob Mice. J. Diabetes Res. 2016, 2016, 4712053. [Google Scholar] [CrossRef]
- Hu, Z.; Pei, G.; Wang, P.; Yang, J.; Zhu, F.; Guo, Y.; Wang, M.; Yao, Y.; Zeng, R.; Liao, W.; et al. Biliverdin Reductase A (BVRA) Mediates Macrophage Expression of Interleukin-10 in Injured Kidney. Int. J. Mol. Sci. 2015, 16, 22621–22635. [Google Scholar] [CrossRef] [Green Version]
- Oda, S.; Fujiwara, R.; Kutsuno, Y.; Fukami, T.; Itoh, T.; Yokoi, T.; Nakajima, M. Targeted screen for human UDP-glucuronosyltransferases inhibitors and the evaluation of potential drug-drug interactions with zafirlukast. Drug Metab. Dispos. 2015, 43, 812–818. [Google Scholar] [CrossRef]
- LaFleur, J.; Bress, A.P.; Rosenblatt, L.; Crook, J.; Sax, P.E.; Myers, J.; Ritchings, C. Cardiovascular outcomes among HIV-infected veterans receiving atazanavir. AIDS 2017, 31, 2095–2106. [Google Scholar] [CrossRef]
- Dekker, D.; Dorresteijn, M.J.; Pijnenburg, M.; Heemskerk, S.; Rasing-Hoogveld, A.; Burger, D.M.; Wagener, F.A.; Smits, P. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 458–463. [Google Scholar] [CrossRef]
- Cheng, X.; Lv, X.; Qu, H.; Li, D.; Hu, M.; Guo, W.; Ge, G.; Dong, R. Comparison of the inhibition potentials of icotinib and erlotinib against human UDP-glucuronosyltransferase 1A1. Acta Pharm. Sin. B 2017, 7, 657–664. [Google Scholar] [CrossRef]
- Ben-Amotz, R.; Bonagura, J.; Velayutham, M.; Hamlin, R.; Burns, P.; Adin, C. Intraperitoneal bilirubin administration decreases infarct area in a rat coronary ischemia/reperfusion model. Front. Physiol. 2014, 5, 53. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-T.; Tarng, D.-C. Beyond a Measure of Liver Function—Bilirubin Acts as a Potential Cardiovascular Protector in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2019, 20, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010117
Tsai M-T, Tarng D-C. Beyond a Measure of Liver Function—Bilirubin Acts as a Potential Cardiovascular Protector in Chronic Kidney Disease Patients. International Journal of Molecular Sciences. 2019; 20(1):117. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010117
Chicago/Turabian StyleTsai, Ming-Tsun, and Der-Cherng Tarng. 2019. "Beyond a Measure of Liver Function—Bilirubin Acts as a Potential Cardiovascular Protector in Chronic Kidney Disease Patients" International Journal of Molecular Sciences 20, no. 1: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010117