Crystal Structure of a GH3 β-Glucosidase from the Thermophilic Fungus Chaetomium thermophilum
Abstract
:1. Introduction
2. Results
2.1. Quality of the Structure
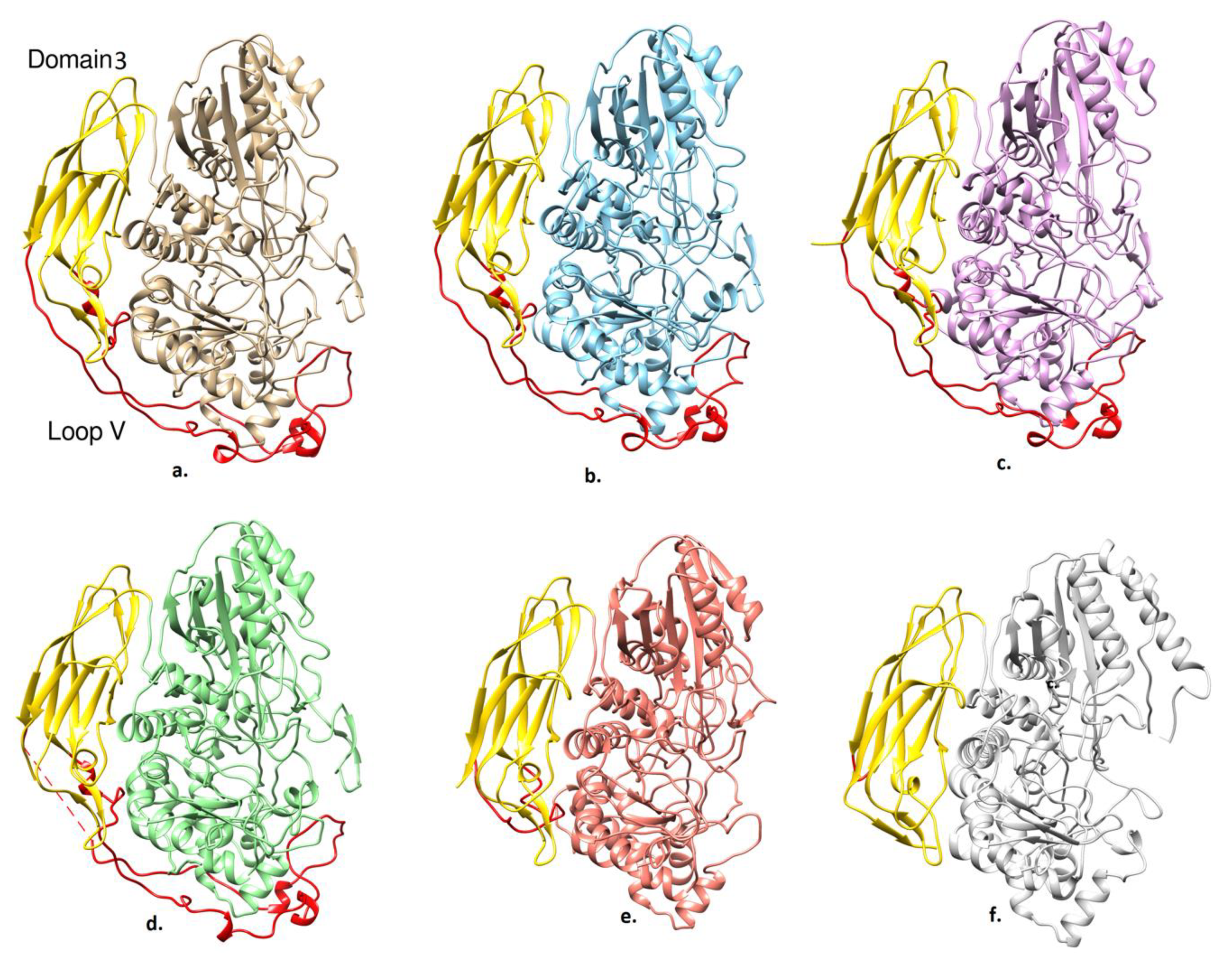
2.2. Overall Structure Comparisons
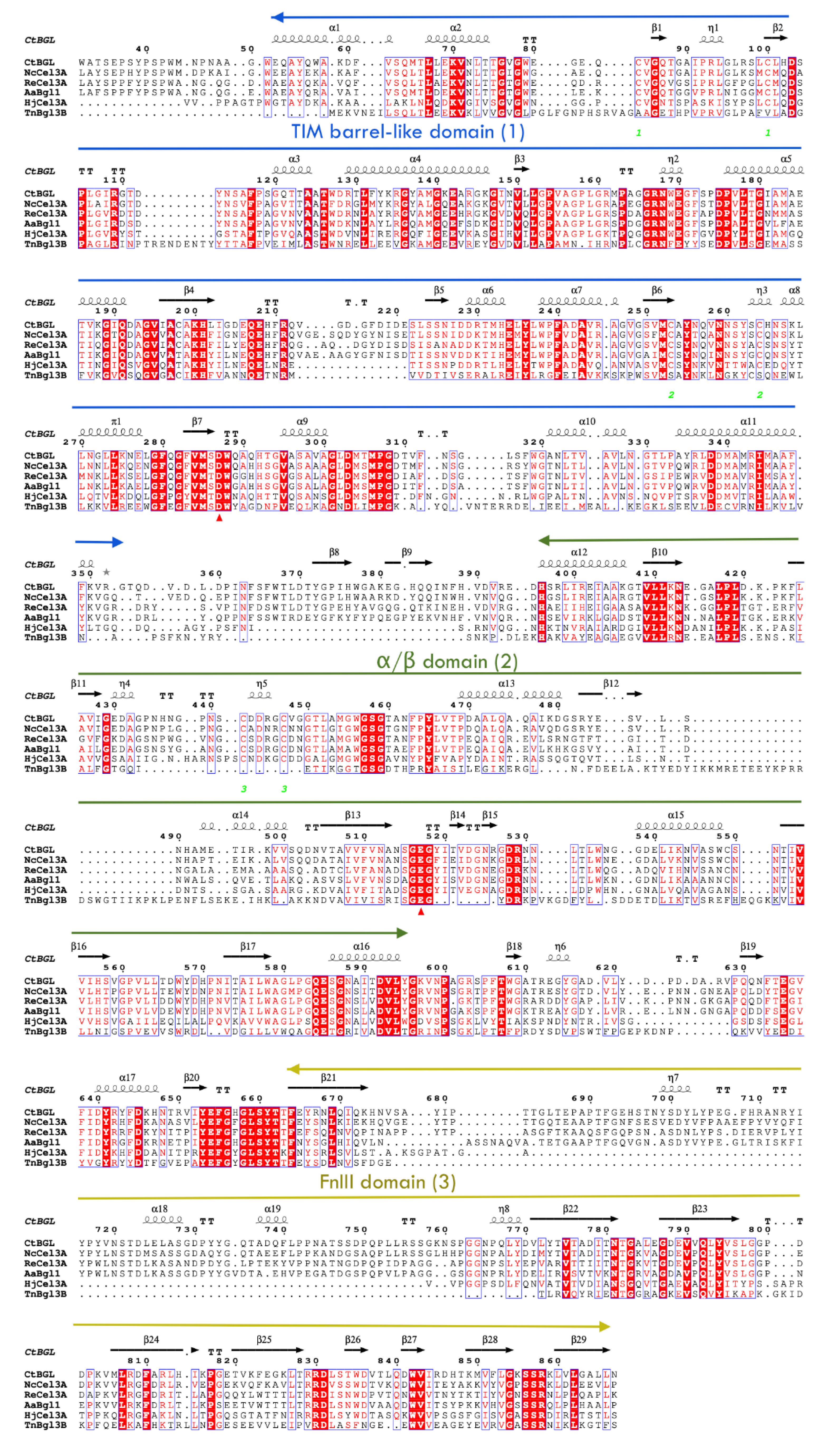
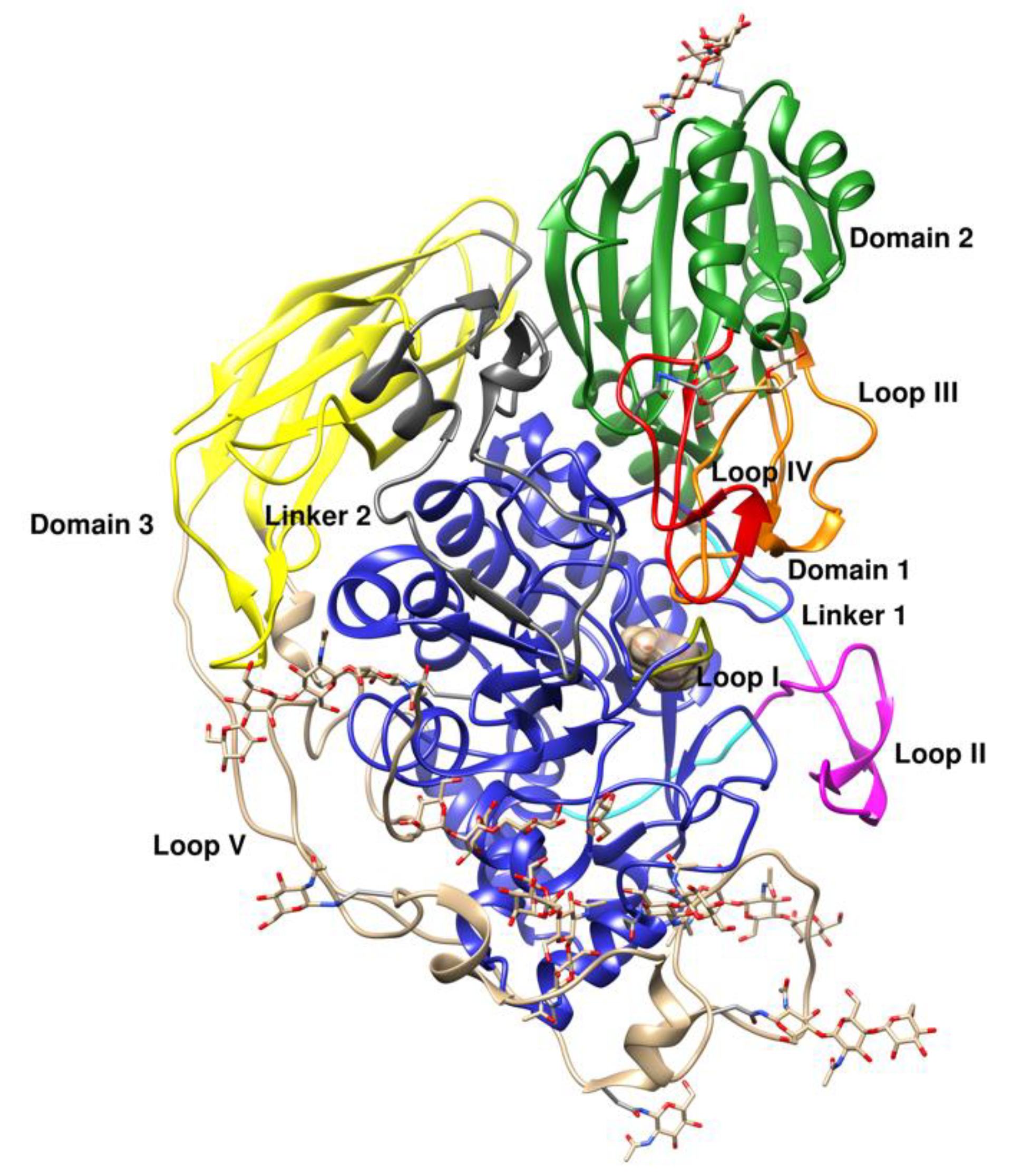
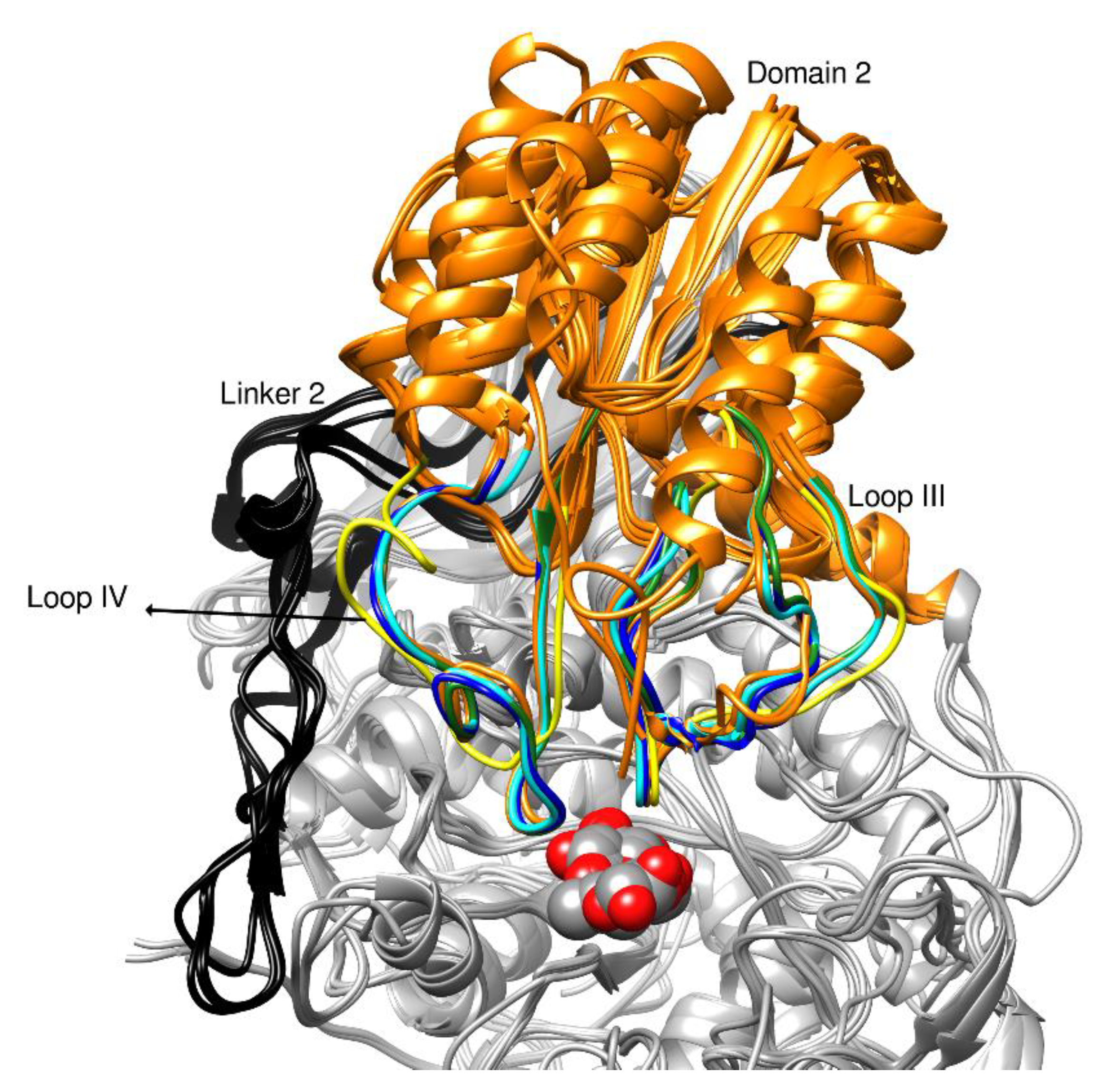
2.3. Description of the Structure
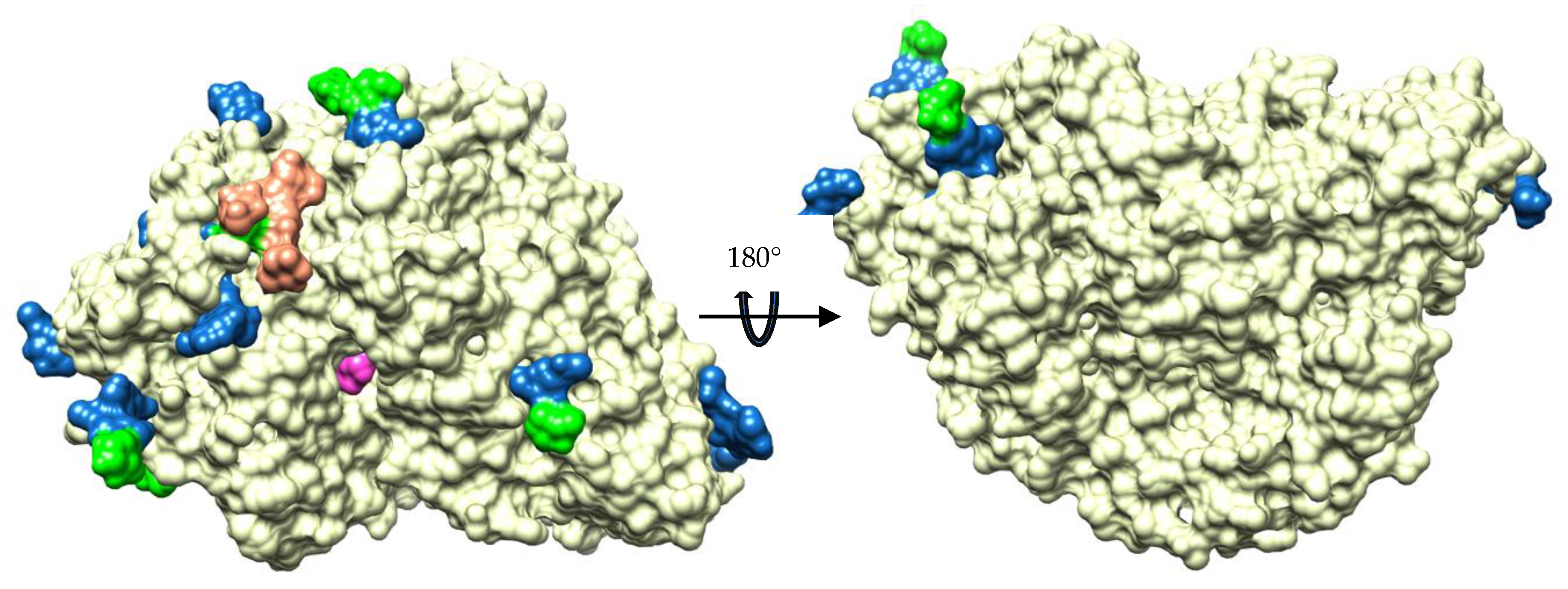
2.4. Glycosylation Sites
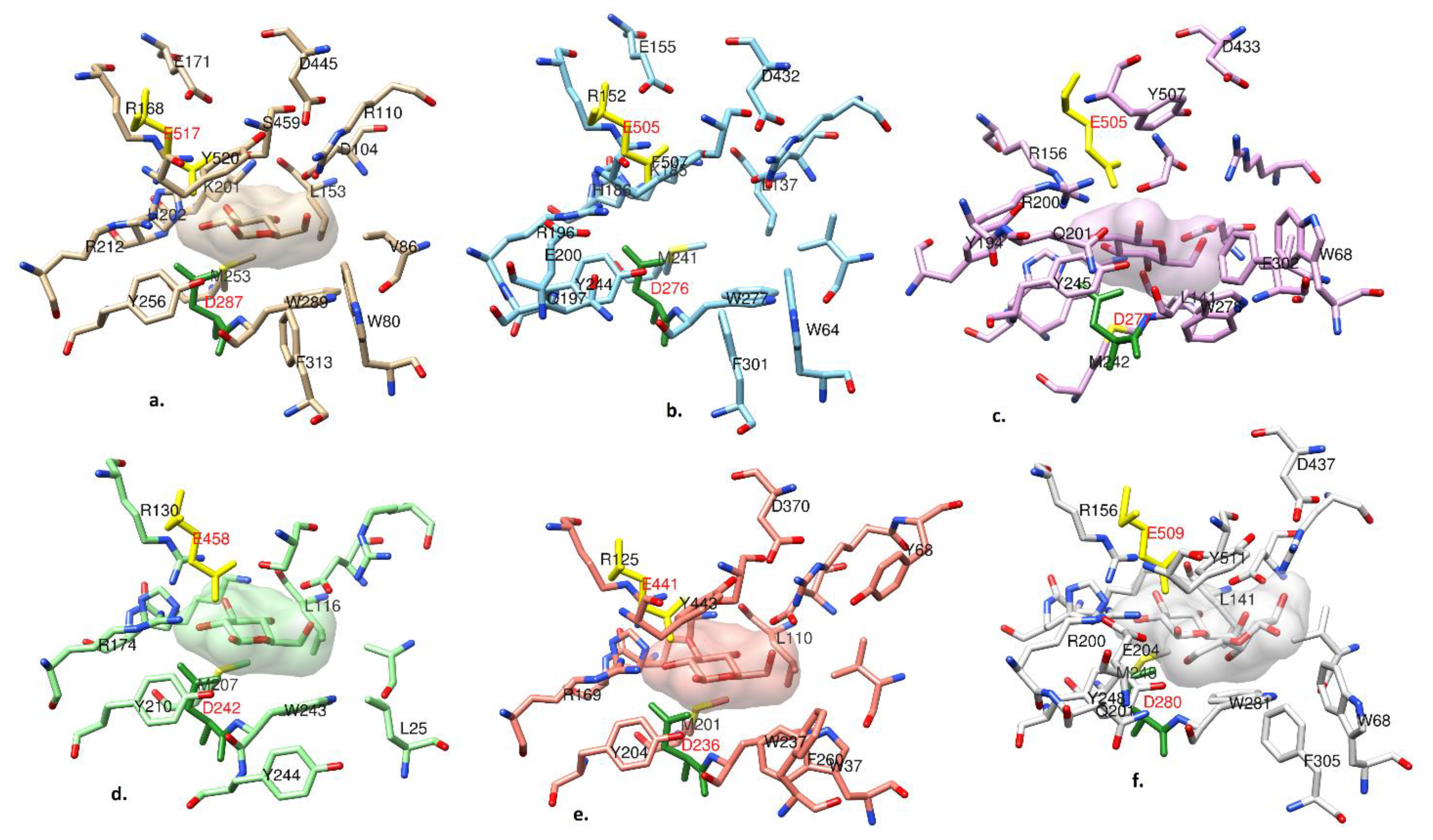
2.5. Active Site
2.6. CtBGL Thermostability
3. Materials and Methods
3.1. Protein Expression, Purification, and Crystallization
3.2. Protein Crystallization
3.3. Data Collection and Processing
3.4. Structure Determination and Refinement
3.5. Structure Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BGC | β-d-glucose |
| GH3 | Glycoside hydrolase family 3 |
| MPD | 2-methyl-2,4-pentanediol |
| PDB | Protein Data Bank |
| NCS | Non-crystallographic symmetry |
| rmsd | Root mean square deviation |
| SAS | Solvent-accessible surface |
| T1/2 | Half-life inactivation |
| Tm | Melting temperature |
References
- Davies, G.J.; Gloster, T.M.; Henrissat, B. Recent structural insights into the expanding world of carbohydrate-active enzymes. Curr. Opin. Struc. Biol. 2005, 15, 637–645. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sumitani, J.-I.; Nam, Y.-W.; Nishimaki, T.; Tani, S.; Wakagi, T.; Kawaguchi, T.; Fushinobu, S. Crystal structures of glycoside hydrolase family 3 β-glucosidase 1 from Aspergillus aculeatus. Biochem. J. 2013, 452, 211–221. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Hansson, H.; Karkehabadi, S.; Larsson, A.; Stals, I.; Kim, S.; Sunux, S.; Fujdala, M.; Larenas, E.; Kaper, T.; et al. Structural and functional studies of the glycoside hydrolase family 3 β-glucosidase Cel3A from the moderately thermophilic fungus Rasamsonia emersonii. Acta Crystallogr. Sect. D Struct. Biol. 2016, 72, 860–870. [Google Scholar] [CrossRef]
- Karkehabadi, S.; Hansson, H.; Mikkelsen, N.E.; Kim, S.; Kaper, T.; Sandgren, M.; Gudmundsson, M. Structural studies of a glycoside hydrolase family 3 β-glucosidase from the model fungus Neurospora crassa. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 787–796. [Google Scholar] [CrossRef]
- Karkehabadi, S.; Helmich, K.E.; Kaper, T.; Hansson, H.; Mikkelsen, N.-E.; Gudmundsson, M.; Piens, K.; Fujdala, M.; Banerjee, G.; Scott-Craig, J.S.; et al. Biochemical Characterization and Crystal Structures of a Fungal Family 3 β-Glucosidase, Cel3A from Hypocrea jecorina. J. Biol. Chem. 2014, 289, 31624–31637. [Google Scholar] [CrossRef]
- Lima, M.A.; Oliveira-Neto, M.; Kadowaki, M.S.; Rosseto, F.R.; Prates, E.T.; uina, F.; Leme, A.F.; Skaf, M.S.; Polikarpov, I. Aspergillus niger β-glucosidase has a cellulase-like tadpole molecular shape: Insights into glycoside hydrolase family 3 (GH3) β-glucosidase structure and function. J. Biol. Chem. 2013, 288, 32991–33005. [Google Scholar] [CrossRef]
- Agirre, J.; Ariza, A.; Offen, W.A.; Turkenburg, J.P.; Roberts, S.M.; McNicholas, S.; Harris, P.V.; McBrayer, B.; Dohnalek, J.; Cowtan, K.D.; et al. Three-dimensional structures of two heavily N-glycosylated Aspergillus sp. family GH3 β-d-glucosidases. Acta Crystallogr. D Struct. Biol. 2016, 72, 254–265. [Google Scholar] [CrossRef]
- Varghese, J.; Hrmova, M.; Fincher, G. Three-dimensional structure of a barley beta-d-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 1999, 7, 179–190. [Google Scholar] [CrossRef]
- Nakatani, Y.; Cutfield, S.M.; Cowieson, N.P.; Cutfield, J.F. Structure and activity of exo-1, 3/1, 4-β-glucanase from marine bacterium Pseudoalteromonas sp. BB1 showing a novel C-terminal domain. FEBS J. 2012, 279, 464–478. [Google Scholar] [CrossRef]
- Yoshida, E.; Hidaka, M.; Fushinobu, S.; Koyanagi, T.; Minami, H.; Tamaki, H.; Kitaoka, M.; Katayama, T.; Kumagai, H. Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 β-glucosidase from Kluyveromyces marxianus. Biochem. J. 2010, 431, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-C.; Papageorgiou, A.C. Cellulases from Thermophilic Fungi: Recent Insights and Biotechnological Potential. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S., Grube, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- de Pereira, C.J.; Marques, P.N.; Rodrigues, A.; de Oliveira, B.T.; Boscolo, M.; da Silva, R.; Gomes, E.; Martins, B.D. Thermophilic fungi as new sources for production of cellulases and xylanases with potential use in sugarcane bagasse saccharification. J. Appl. Microbiol. 2015, 118, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.; Lübeck, M.; Lübeck, P.; Ahring, B. Fungal Beta-Glucosidases: A Bottleneck in Industrial Use of Lignocellulosic Materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Topakas, E.; Paschos, T.; Taouki, I.; Christakopoulos, P. Cloning, expression and characterization of an ethanol tolerant GH3 β-glucosidase from Myceliophthora thermophila. PeerJ 2013, 1, e46. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pozzo, T.; Pasten, J.; Karlsson, E.; Logan, D.T. Structural and functional analyses of beta-glucosidase 3B from Thermotoga neapolitana: A thermostable three-domain representative of glycoside hydrolase. J. Mol. Biol. 2010, 397, 724–739. [Google Scholar] [CrossRef]
- Payne, C.M.; Jiang, W.; Shirts, M.R.; Himmel, M.E.; Crowley, M.F.; Beckham, G.T. Glycoside hydrolase processivity is directly related to oligosaccharide binding free energy. J. Am. Chem. Soc. 2013, 135, 18831–18839. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nishiyama, T.; Uchida, M. Binding Affinity of N-Glycans for Aromatic Amino Acid Residues: Implications for Novel Interactions between N-Glycans and Proteins. J. Biochem. 1999, 126, 261–265. [Google Scholar] [CrossRef]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef]
- Xu, R.; Teng, F.; Zhang, C.; Li, D. Cloning of a Gene Encoding β-Glucosidase from Chaetomium thermophilum CT2 and Its Expression in Pichia pastoris. J. Mol. Microb. Biotechnol. 2011, 20, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Brown, K.M.; Harris, P.V.; Langston, J.A.; Cherry, J.R. A Proteomics Strategy To Discover β-Glucosidases from Aspergillus fumigatus with Two-Dimensional Page In-Gel Activity Assay and Tandem Mass Spectrometry. J. Proteome Res. 2007, 6, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Naderi-Manesh, H.; Zarrabi, M.; Ranjbar, B. Effective factors in thermostability of thermophilic proteins. Biophys. Chem. 2006, 119, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, L.D.; van der Oost, J.; Koutsopoulos, S. Hyperthermophilic enzymes-stability, activity and implementation strategies for high temperature applications. FEBS J. 2007, 274, 4044–4056. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Qi, Y.; Im, W. Effects of N-glycosylation on protein conformation and dynamics: Protein Data Bank analysis and molecular dynamics simulation study. Sci. Rep. 2015, 5, 8926. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Svensson, D.; Adlercreutz, P.; Karlsson, E. A novel variant of Thermotoga neapolitana β-glucosidase B is an efficient catalyst for the synthesis of alkyl glucosides by transglycosylation. J. Biotechnol. 2007, 130, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.; Zeidner, K.; Grace, M.; Desnick, R. Human alpha-galactosidase A: Glycosylation site 3 is essential for enzyme solubility. Biochem. J. 1998, 332, 789–797. [Google Scholar] [CrossRef]
- Kayser, V.; Chennamsetty, N.; Voynov, V.; Forrer, K.; Helk, B.; Trout, B.L. Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol. J. 2011, 6, 38–44. [Google Scholar] [CrossRef]
- Meldgaard, M.; Svendsen, I. Different effects of N-glycosylation on the thermostability of highly homologous bacterial (1, 3-1, 4)-beta-glucanases secreted from yeast. Microbiology 1994, 140, 159–166. [Google Scholar] [CrossRef]
- Baba, Y.; Sumitani, J.-I.; Tani, S.; Kawaguchi, T. Characterization of Aspergillus aculeatus β-glucosidase 1 accelerating cellulose hydrolysis with Trichoderma cellulase system. AMB Express 2015, 5, 3. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, B. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Bunkoczi, G.; Read, R. Improvement of molecular-replacement models with Sculptor. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Brünger, A. Free R value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 1992, 355, 472–475. [Google Scholar] [CrossRef]
- Agirre, J. Strategies for carbohydrate model building, refinement and validation. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2256–2268. [Google Scholar] [CrossRef]
- Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Huang, C.C.; Ferrin, T.E. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinform. 2006, 7, 339. [Google Scholar]
- Krissinel, E. Macromolecular complexes in crystals and solutions. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
| Data Collection | |
| Beamline | EMBL-DESY X13 |
| Wavelength (Å) | 0.8123 |
| Data Processing | |
| Space group | P41212 |
| Unit cell dimensions a, b, c (Å) | 121.9, 121.9, 264.9 |
| No. of molecules/asymmetric units | 1 |
| Mosaicity (°) | 0.070 |
| Resolution range (Å) | 20.0–2.99 (3.11–2.99) * |
| Total measurements/unique reflections | 222508 (22974)/41051 (4513) |
| Completeness (%) | 99.3 (98.6) |
| <I/σ(I)> | 7.8 (1.4) |
| Rmeas | 0.287 (1.452) |
| Rpim | 0.122 (0.846) |
| CC1/2 | 0.978 (0.492) |
| Wilson B-factor (Å2) | 46.2 |
| Refinement | |
| Reflections (working/test) | 38861/2047 |
| Rcryst/Rfree | 0.201/0.249 |
| No. of protein atoms | 6457 |
| Ligand atoms | 12 |
| No. of Sugar Atoms | 355 |
| No. of Water Molecules | 6 |
| Geometry | |
| rmsd in bond lengths (Å) | 0.007 |
| rmsd in bond angles (°) | 0.94 |
| Most favorable regions (%) | 92.8 |
| Additional allowed regions (%) | 6.2 |
| Outliers (%) | 1.0 |
| Clashscore | 10.4 |
| Average B-Factors (Å2) | |
| Protein | 50.4 |
| Ligand | 63.8 |
| Sugars | 72.4 |
| Water molecules | 32.8 |
| Pyranose Conformations (Total/Percentage) | |
| Lowest energy conformations | 23/85.2 |
| Highest energy conformations | 4/14.8 |
| Residue | Corresponding Glycan Structure |
|---|---|
| Asn72 | Man-β1,4–GlcNAc-β1,4–GlcNAc |
| Asn259 | Man-β1,4–Man-β1,4–GlcNAc-β1,4–GlcNAc |
| Asn322 | GlcNAc-β1,4–GlcNAc |
| Asn329 | Man-α1,2–Man-α1,6–(α1,3-Man)-α1,6–Man-(α1,3-Man)Man-β1,4–GlcNAc-β1,4–GlcNAc |
| Asn504 | GlcNAc |
| Asn531 | Man-β1,3–GlcNAc |
| Asn572 | GlcNAc-β1,4–GlcNAc |
| Asn698 | GlcNAc |
| Asn720 | Man-β1,4–GlcNAc-β1,4–GlcNAc |
| Asn744 | GlcNAc |
| CtBGL | NcCel3A(5nbs) | AaBgl1 (4iif) | ReCel3A (5ju6) | HjCel3A (3zyz) # |
|---|---|---|---|---|
| Asn72 (+) | Asn57 (+) | Asn61 (+) | Asn61 (+) | Gly30 (−) |
| Asn259 (+) | Asn248 (+) | Asn252 (+) | Asn249 (+) | Asn208 (+) |
| Asn322 (+) | Asn311 (+) | Asn315 (+) | Asn312 (+) | Ala270 (−) |
| Asn329 (+) | Asn318 (+) | Asn322 (+) | Asn319 (+) | Ser277 (−) |
| Asn504 (+) | Asp492 (−) | Ala496 (−) | Ala492(−) | Lys428 (−) |
| Asn531 (+) | Asn519 (+) | Asn523 (+) | Asn519 (+) | Asn455 (−) |
| Asn572 (+) | Asn560 (+) | Asn564 (+) | Asn560 (+) | Gln496 (−) |
| Asn698 (+) | Ser687 (−) | Asn690 (+) | Asn685 (+) | |
| Asn720 (+) | Asn710 (+) | Asn712 (+) | Asn707 (+) | |
| Asn744(+) | Lys734 (−) | Gly736 (−) | Asn731 (+) |
| CtBGL | AfβG (5FJI) | AaBgl1 (4IIF) | ReCel3A (5JU6) | TnBgl3B (2X40) | HjCel3A (3ZYZ) | |
|---|---|---|---|---|---|---|
| Thermal stability characteristics | T1/2 65 °C after 55 min | 19 h at 65 °C [23] | T1/2 62 °C after 30 min [31] | Tm 87.3 °C [4] | Unfolding temperature 86–89 °C [27] | Tm 77.6 °C [4] |
| Asp + Glu (−) | 93 | 95 | 92 | 87 | 113 | 55 |
| Arg + Lys (+) | 66 | 67 | 65 | 59 | 93 | 51 |
| Pro: Gly | 0.51 | 0.55 | 0.51 | 0.61 | 0.58 | 0.52 |
| Val (%) | 7.0 | 7.9 | 8.0 | 7.5 | 9.2 | 8.8 |
| Amino acid residues | 867 | 844 | 841 | 857 | 721 | 714 |
| SAS (Å2) | 28,502.9 | 28,092.1 | 28,074.9 | 28,481.7 | 26,430.0 | 22,551.0 |
| Intra-chain salt Bridges | 46 | 49 | 42 | 44 | 39 | 34 |
| H-bonding interactions | 12 ∞ | 22 | 25 | 22 | 23 | 8 |
| Interface area, Å2 | 1544.2 ∞ | 1324.3 | 1445.3 | 1322.5 | 1253.1 | 699.3 |
| Helical content (%) | 21.2 | 21.8 | 21.8 | 20.5 | 26.5 | 23.7 |
| Sugars | 27 | 45 (chain A) | 44 % | 50 (chain C) | NA $ | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsin, I.; Poudel, N.; Li, D.-C.; Papageorgiou, A.C. Crystal Structure of a GH3 β-Glucosidase from the Thermophilic Fungus Chaetomium thermophilum. Int. J. Mol. Sci. 2019, 20, 5962. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20235962
Mohsin I, Poudel N, Li D-C, Papageorgiou AC. Crystal Structure of a GH3 β-Glucosidase from the Thermophilic Fungus Chaetomium thermophilum. International Journal of Molecular Sciences. 2019; 20(23):5962. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20235962
Chicago/Turabian StyleMohsin, Imran, Nirmal Poudel, Duo-Chuan Li, and Anastassios C. Papageorgiou. 2019. "Crystal Structure of a GH3 β-Glucosidase from the Thermophilic Fungus Chaetomium thermophilum" International Journal of Molecular Sciences 20, no. 23: 5962. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20235962






