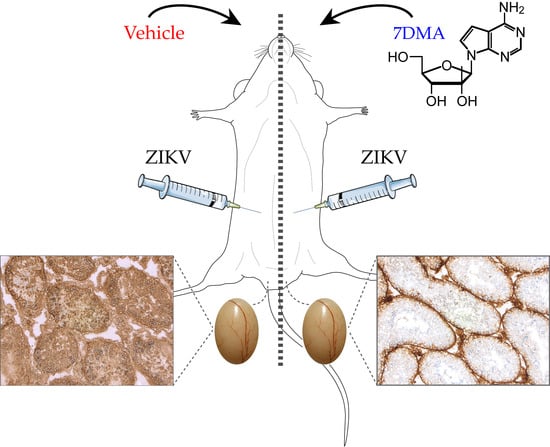
A Viral Polymerase Inhibitor Reduces Zika Virus Replication in the Reproductive Organs of Male Mice
Abstract
:1. Introduction
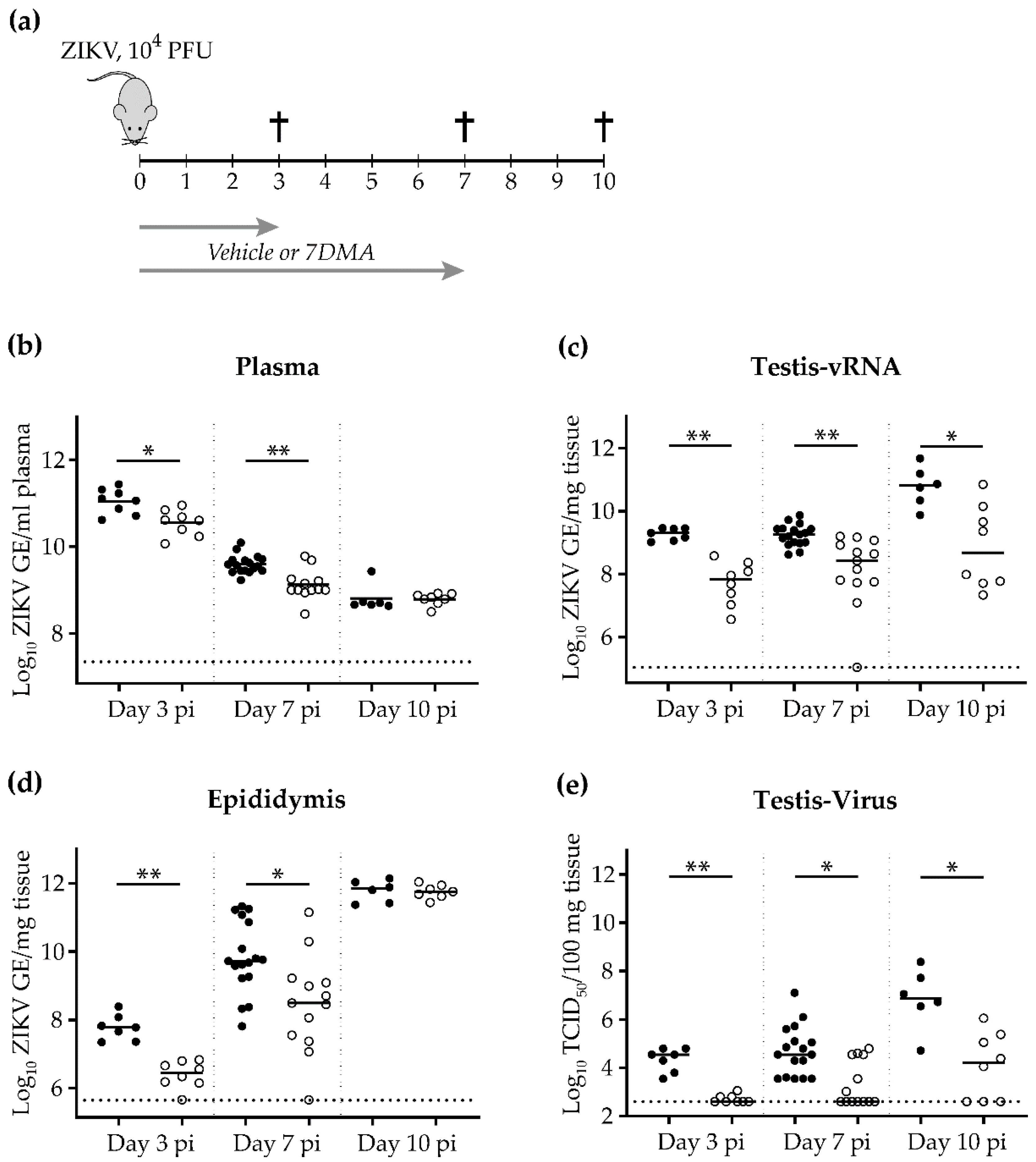
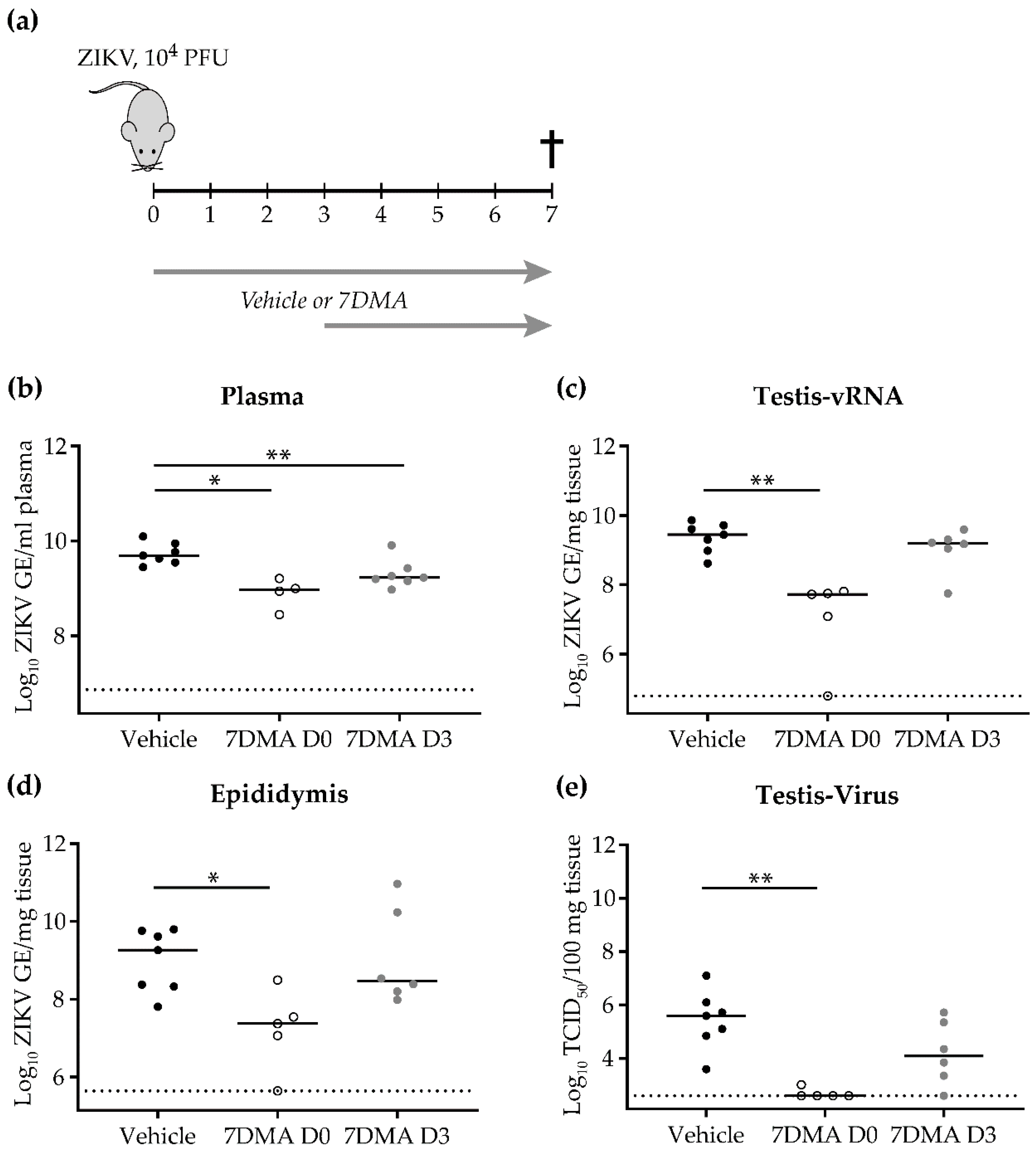
2. Results
3. Discussion
4. Materials and Methods
4.1. Cells, Virus, and Compounds
4.2. In Vivo Evaluation of 7DMA Against ZIKV Replication in AG129 Mice
4.3. Tissue RNA Isolation and Quantitative Reverse Polymerase Chain Reaction (qRT-PCR)
4.4. Virus End-Point Titration by Cell Culture Infectious Dose 50% (TCID50) Assay
4.5. Histopathology
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanluca, C.; Melo, V.C.A.D.; Mosimann, A.L.P.; Santos, G.I.V.D.; Santos, C.N.D.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [Green Version]
- PAHO/WHO—Epidemiological Alerts and Updates. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=11599:regional-zika-epidemiological-update-americas&Itemid=41691&lang=en (accessed on 2 January 2019).
- Ioos, S.; Mallet, H.P.; Goffart, I.L.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika virus epidemiology and recent epidemics. Méd. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Eur. Surveill. 2014, 19, 20720. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastere, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.G.H.; do Carmo, G.M.I.; Henriques, C.M.P.; Coelho, G.E.; Araújo de França, G.V. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef] [Green Version]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martínez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barré Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.L.; Horovitz, D.D.G.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.R.; Neri, J.I.; Neto, J.M.d.P.; Wanderley, H.Y.C.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Rapid Risk Assessment: Microcephaly in Brazil Potentially linked to the Zika virus Epidemic. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf (accessed on 27 September 2018).
- Boyer, S.; Calvez, E.; Chouin-Carneiro, T.; Diallo, D.; Failloux, A.B.; Anna-Bella, F. An overview of mosquito vectors of Zika virus. Microbes Infect. 2018, 20, 646–660. [Google Scholar] [CrossRef]
- Stassen, L.; Armitage, C.W.; Van Der Heide, D.J.; Beagley, K.W.; Frentiu, F.D. Zika Virus in the Male Reproductive Tract. Viruses 2018, 10, 198. [Google Scholar] [CrossRef]
- Maxian, O.; Neufeld, A.; Talis, E.J.; Childs, L.M.; Blackwood, J.C. Zika virus dynamics: When does sexual transmission matter? Epidemics 2017, 21, 48–55. [Google Scholar] [CrossRef]
- Gao, D.; Lou, Y.; He, D.; Porco, T.C.; Kuang, Y.; Chowell, G.; Ruan, S. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci. Rep. 2016, 6, 28070. [Google Scholar] [CrossRef] [Green Version]
- Wilder-Smith, A.; Chang, C.R.; Leong, W.Y. Zika in travellers 1947–2017: A systematic review. J. Travel Med. 2018, 25, tay044. [Google Scholar] [CrossRef]
- Althaus, C.L.; Low, N. How Relevant Is Sexual Transmission of Zika Virus? PLoS Med. 2016, 13, e1002157. [Google Scholar] [CrossRef]
- CDC.Zika Virus: Sexual Transmission and Prevention. 2018. Available online: https://www.cdc.gov/zika/prevention/sexual-transmission-prevention.html (accessed on 28 September 2018).
- Zmurko, J.; Marques, R.E.; Schols, D.; Verbeken, E.; Kaptein, S.J.; Neyts, J. The Viral Polymerase Inhibitor 7-Deaza-2′-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl. Trop. Dis. 2016, 10, e0004695. [Google Scholar] [CrossRef]
- Kawiecki, A.B.; Mayton, E.H.; Dutuze, M.F.; Goupil, B.A.; Langohr, I.M.; Del Piero, F.; Christofferson, R.C. Tissue tropisms, infection kinetics, histologic lesions, and antibody response of the MR766 strain of Zika virus in a murine model. Virol. J. 2017, 14, 82. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.L.; Azar, S.R.; Tesh, R.B.; Vasilakis, N.; Paessler, S.; Langsjoen, R.M.; Auguste, A.J.; Weaver, S.C.; Muruato, A.E.; Hanley, K.A. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Van Boheemen, S.; Tas, A.; Anvar, S.Y.; Van Grootveld, R.; Albulescu, I.C.; Bauer, M.P.; Feltkamp, M.C.; Bredenbeek, P.J.; Van Hemert, M.J. Quasispecies composition and evolution of a typical Zika virus clinical isolate from Suriname. Sci. Rep. 2017, 7, 2368. [Google Scholar] [CrossRef]
- LaZear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2016, 167, 1511–1524. [Google Scholar] [CrossRef]
- Uraki, R.; Hwang, J.; Jurado, K.A.; Householder, S.; Yockey, L.J.; Hastings, A.K.; Homer, R.J.; Iwasaki, A.; Fikrig, E. Zika virus causes testicular atrophy. Sci. Adv. 2017, 3, e1602899. [Google Scholar] [CrossRef] [Green Version]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.J.J.; Bowen, R.A.; Brault, A.C. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef]
- Robinson, C.L.; Chong, A.C.N.; Ashbrook, A.W.; Jeng, G.; Jin, J.; Chen, H.; Tang, E.I.; Martin, L.A.; Kim, R.S.; Kenyon, R.M.; et al. Male germ cells support long-term propagation of Zika virus. Nat. Commun. 2018, 9, 2090. [Google Scholar] [CrossRef] [Green Version]
- Froeschl, G.; Huber, K.; Von Sonnenburg, F.; Nothdurft, H.-D.; Bretzel, G.; Hoelscher, M.; Zoeller, L.; Trottmann, M.; Pan-Montojo, F.; Dobler, G.; et al. Long-term kinetics of Zika virus RNA and antibodies in body fluids of a vasectomized traveller returning from Martinique: a case report. BMC Infect. Dis. 2017, 17, 269. [Google Scholar] [CrossRef]
- Arsuaga, M.; Bujalance, S.G.; Díaz-Menéndez, M.; Vázquez, A.; Arribas, J.R. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect. Dis. 2016, 16, 1107. [Google Scholar] [CrossRef]
- Spencer, J.L.; Lahon, A.; Tran, L.L.; Arya, R.P.; Kneubehl, A.R.; Vogt, M.B.; Xavier, D.; Rowley, D.R.; Kimata, J.T.; Rico-Hesse, R.R. Replication of Zika Virus in Human Prostate Cells: A Potential Source of Sexually Transmitted Virus. J. Infect. Dis. 2018, 217, 538–547. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, S.; Delang, L.; Verbeken, E.; Neyts, J.; Kaptein, S.J.F. A Viral Polymerase Inhibitor Reduces Zika Virus Replication in the Reproductive Organs of Male Mice. Int. J. Mol. Sci. 2019, 20, 2122. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092122
Jacobs S, Delang L, Verbeken E, Neyts J, Kaptein SJF. A Viral Polymerase Inhibitor Reduces Zika Virus Replication in the Reproductive Organs of Male Mice. International Journal of Molecular Sciences. 2019; 20(9):2122. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092122
Chicago/Turabian StyleJacobs, Sofie, Leen Delang, Eric Verbeken, Johan Neyts, and Suzanne J.F. Kaptein. 2019. "A Viral Polymerase Inhibitor Reduces Zika Virus Replication in the Reproductive Organs of Male Mice" International Journal of Molecular Sciences 20, no. 9: 2122. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092122