Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity?
Abstract
:1. Introduction
2. Role and Structure of RBP4
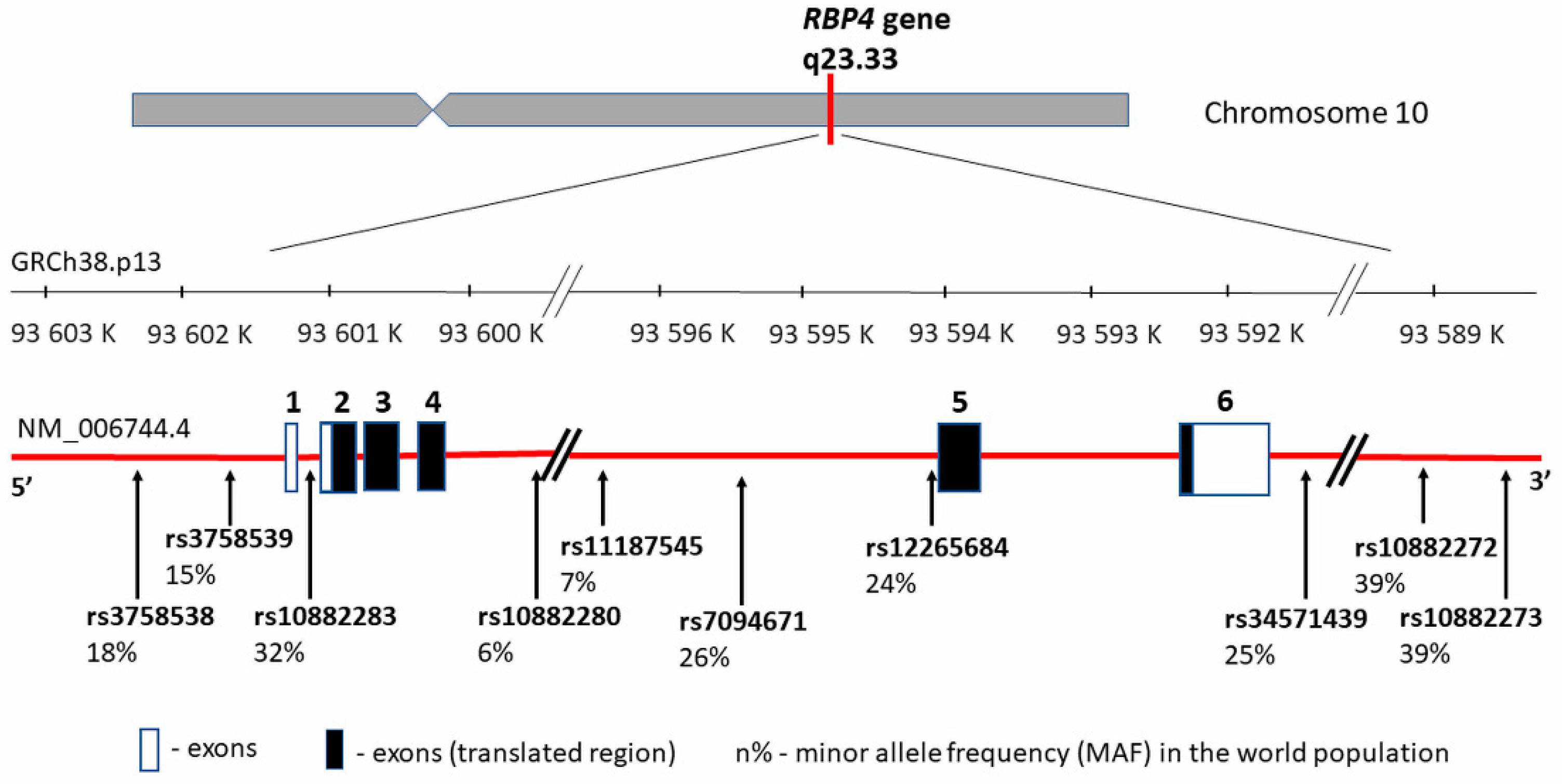
3. RBP4 Gene—Structure and Polymorphism vs. CVD in Obesity
4. RBP4, Obesity, and Metabolic Syndrome
5. RBP4 and Lipid Metabolism
6. RBP4 and the Endothelium
7. RBP4 and Intima-Media Thickness
8. Diet and Its Influence on RBP4 Levels
9. Can RBP4 Be Used as a Biomarker in CVD?
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Baigent, C.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; Backer, G.G.D.; Delgado, V.; Ference, B.A.; Graham, I.M.; Halliday, A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 11–188. [Google Scholar] [CrossRef]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo, R.-F.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- The Cost of CVD. Champion Advocates Programme. Available online: http://www.championadvocates.org/en/champion-advocates-programme/the-costs-of-cvd (accessed on 27 March 2020).
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- NCDs. Know the NCD Targets. Available online: https://www.who.int/beat-ncds/take-action/targets/en/ (accessed on 27 March 2020).
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135. [Google Scholar] [CrossRef]
- Krzysztoszek, J.; Laudańska, I.-K.; Bronikowski, M. Assessment of epidemiological obesity among adults in EU countries. Ann. Agric. Environ. Med. 2019, 26, 341–349. [Google Scholar] [CrossRef]
- Rychter, A.M.; Ratajczak, A.E.; Zawada, A.; Dobrowolska, A.; Krela, I.-K. Non-Systematic Review of Diet and Nutritional Risk Factors of Cardiovascular Disease in Obesity. Nutrients 2020, 12, 814. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, R.W. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front. Biosci. 2019, 24, 890–934. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Xu, A.; Hui, X.; Zhou, P.; Li, X.; Zhong, H.; Tang, W.; Huang, G.; Zhou, Z. Circulating Lipocalin-2 and Retinol-Binding Protein 4 Are Associated with Intima-Media Thickness and Subclinical Atherosclerosis in Patients with Type 2 Diabetes. PLoS ONE 2013, 8, e66607. [Google Scholar] [CrossRef] [Green Version]
- Mattu, H.S.; Randeva, H.S. Role of adipokines in cardiovascular disease. J. Endocrinol. 2013, 216, T17–T36. [Google Scholar] [CrossRef] [Green Version]
- Shibata, R.; Ouchi, N.; Ohashi, K.; Murohara, T. The role of adipokines in cardiovascular disease. J. Cardiol. 2017, 70, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Molica, F.; Morel, S.; Kwak, B.R.; Rohner-Jeanrenaud, F.; Steffens, S. Adipokines at the crossroad between obesity and cardiovascular disease. Thromb. Haemost. 2015, 113, 553–566. [Google Scholar] [CrossRef]
- Ntaios, G.; Gatselis, N.K.; Makaritsis, K.; Dalekos, G.N. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013, 227, 216–221. [Google Scholar] [CrossRef]
- Korek, E.; Krauss, H. Nowe adipokiny o potencjalnym znaczeniu w patogenezie otyłości i zaburzeń metabolicznych. Postepy Hig. Med. Dosw. 2015, 69, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Yu, K.; Wheeler, W.; Zhang, H.; Weinstein, S.J.; Major, J.M.; Cornelis, M.C.; Männistö, S.; Hazra, A.; Hsing, A.W.; et al. Genome-wide association study of circulating retinol levels. Hum. Mol. Genet. 2011, 20, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Majerczyk, M.; Olszanecka-Glinianowicz, M.; Puzianowska, M.; Chudek, J. Białko wiążące retinol typu 4 (RBP4) jako czynnik i marker uszkodzenia naczyń związany z insulinoopornością. Postepy Hig. Med. Dosw. 2016, 70, 1267–1275. [Google Scholar]
- Lee, J.-W.; Lee, H.-R.; Shim, J.-Y.; Im, J.-A.; Lee, D.-C. Abdominal Visceral Fat Reduction Is Associated with Favorable Changes of Serum Retinol Binding Protein-4 in Nondiabetic Subjects. Endocr. J. 2008, 55, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J. Intern. Med. 2007, 262, 496–503. [Google Scholar] [CrossRef]
- Su, Y.; Huang, Y.; Jiang, Y.; Zhu, M. The Association between Serum Retinol-Binding Protein 4 Levels and Cardiovascular Events in Patients with Chronic Kidney Disease. Lab. Med. 2020, lmz104. [Google Scholar] [CrossRef] [Green Version]
- Bobbert, T.; Raila, J.; Schwarz, F.; Mai, K.; Henze, A.; Pfeiffer, A.F.H.; Schweigert, F.J.; Spranger, J. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis 2010, 213, 549–551. [Google Scholar] [CrossRef]
- Olsen, T.; Blomhoff, R. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Adv. Nutr. 2020, 11, 644–666. [Google Scholar] [CrossRef]
- Generoso, G.; Bittencourt, M.S. Vitamin A: An enhanced vision of the relationship between apolipoproteins and cardiovascular risk? Atherosclerosis 2017, 265, 256–257. [Google Scholar] [CrossRef]
- Takebayashi, K.; Suetsugu, M.; Wakabayashi, S.; Aso, Y.; Inukai, T. Retinol Binding Protein-4 Levels and Clinical Features of Type 2 Diabetes Patients. J. Clin. Endocrinol. Metab. 2007, 92, 2712–2719. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, I.; Straczkowski, M.; Adamska, A.; Nikolajuk, A.; Karczewska, M.-K.; Otziomek, E.; Górska, M. Serum retinol binding protein 4 is related to insulin resistance and nonoxidative glucose metabolism in lean and obese women with normal glucose tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 2786–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.; Bañuls, C.; Bellod, L.; Rovira, S.-L.; Morillas, C.; Solá, E.; Víctor, V.M.; Hernández-Mijares, A. Association of Serum Retinol Binding Protein 4 with Atherogenic Dyslipidemia in Morbid Obese Patients. PLoS ONE 2013, 8, e78670. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Blüher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.-A.; Smith, U.; et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Sundström, J.; Melhus, H.; Michaëlsson, K.; Berne, C.; Vasan, R.S.; Risérus, U.; Blomhoff, R.; Lind, L.; Ärnlöv, J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis 2009, 206, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Santini, E.; Madec, S.; Rossi, C.; Muscelli, E. Retinol-binding protein-4 in women with untreated essential hypertension. Am. J. Hypertens. 2009, 22, 1001–1006. [Google Scholar] [CrossRef] [Green Version]
- Graham, T.E.; Wason, C.J.; Blüher, M.; Kahn, B.B. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 2007, 50, 814–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjo, K.M.; Farjo, R.A.; Halsey, S.; Moiseyev, G.; Ma, J.-X. Retinol-Binding Protein 4 Induces Inflammation in Human Endothelial Cells by an NADPH Oxidase- and Nuclear Factor Kappa B-Dependent and Retinol-Independent Mechanism. Mol. Cell. Biol. 2012, 32, 5103–5115. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-Z.; Lu, X.-Z.; Liu, J.-B.; Chen, L. Serum Retinol-Binding Protein 4 Levels in Patients with Diabetic Retinopathy. J. Int. Med. Res. 2010, 38, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Zabetian-Targhi, F.; Mahmoudi, M.J.; Rezaei, N.; Mahmoudi, M. Retinol Binding Protein 4 in Relation to Diet, Inflammation, Immunity, and Cardiovascular Diseases. Adv. Nutr. 2015, 6, 748–762. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Yin, S.; Lin, D.; Liu, Y.; Chen, N.; Bai, X.; Ke, Q.; Shen, J.; You, L.; Lin, X.; et al. Association of Serum Retinol-Binding Protein 4 Levels and the Risk of Incident Type 2 Diabetes in Subjects With Prediabetes. Diabetes Care 2019, 42, 1574–1581. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chu, N.F.; Hung, Y.-J.; Chang, J.-B.; He, C.-T.; Hsiao, F.-C.; Hsieh, C.-H. The Association of Retinol-Binding Protein 4 With Metabolic Syndrome and Obesity in Adolescents: The Effects of Gender and Sex Hormones. Clin. Pediatr. (Phila.) 2013, 52, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Moreno, J.M.; Ricart, W. Circulating Retinol-Binding Protein-4 Concentration Might Reflect Insulin Resistance-Associated Iron Overload. Diabetes 2008, 57, 1918–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo-Gallego, R.; Lacalle, L.; Pérez-Calahorra, S.; Marco-Benedí, V.; Recasens, V.; Padrón, N.; Lamiquiz-Moneo, I.; Baila-Rueda, L.; Jarauta, E.; Calmarza, P.; et al. Efficacy of repeated phlebotomies in hypertriglyceridemia and iron overload: A prospective, randomized, controlled trial. J. Clin. Lipidol. 2018, 12, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Wang, H.; Liu, X.; Li, Y.; Su, Y.; Gao, X.; Jiang, W. Serum Retinol-binding Protein 4 is Elevated and Positively Associated with Insulin Resistance in Postmenopausal Women. Endocr. J. 2009, 56, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Zhu, Y.; Yan, C.; Wng, Y.; Zhang, Z. Retinol binding protein 4 correlates with and is an early predictor of carotid atherosclerosis in type 2 diabetes mellitus patients. J. Biomed. Res. 2015, 29, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Won, J.C.; Park, C.-Y.; Oh, S.W.; Park, S.W. Increased plasma levels of retinol-binding protein 4 with visceral obesity is associated with cardiovascular risk factors: Visceral obesity, RBP4 and CVD risk. J. Diabetes Investig. 2012, 3, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, M.; Heshmat, R.; Tabatabaei-Malazy, O.; Sharifi, F.; Badamchizadeh, Z.; Alatab, S.; Omidfar, K.; Fakhrzadeh, H.; Larijani, B. The association of carotid intima media thickness with retinol binding protein-4 and total and high molecular weight adiponectin in type 2 diabetic patients. J. Diabetes Metab. Disord. 2012, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.-H.; Lam, H.-C.; Lee, J.-K.; Lu, C.-C.; Sun, C.-C.; Cheng, H.-J.; Wang, M.-C.; Chuang, M.-J. Elevated serum retinol-binding protein 4 concentrations are associated with chronic kidney disease but not with the higher carotid intima-media thickness in type 2 diabetic subjects. Endocr. J. 2011, 58, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, K.; Yan, J.; Wang, L.; Wang, Y.; Shen, X.; Sun, H.; Liu, L.; Zhao, C.; He, H.; et al. Serum retinol-binding protein 4 as a predictor of cardiovascular events in elderly patients with chronic heart failure. ESC Heart Fail. 2020, 7, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Bachmayer, C.; Kemmer, A.; Ehrmann, N.; Hasenberg, T.; Lammert, A.; Hammes, H.-P. Adipokines and endothelial dysfunction in obesity WHO°III. Microvasc. Res. 2013, 89, 129–133. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Carrasco-Luna, J.; Allepuz, P.; Codoñer-Alejos, A.; Guillem, V. Association of RBP4 genetic variants with childhood obesity and cardiovascular risk factors: SNPs of RBP4 and cardiovascular risk. Pediatr. Diabetes 2016, 17, 576–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shajarian, M.; Rafiee, L.; Naji-Esfahani, H.; Haghjooy-Javanmard, S.; Nizal, S. Association of RBP4 gene variants with adverse lipid profile and obesity. Gene 2015, 561, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, R.; Blangero, J.; Almasy, L.; Dyer, T.D.; Williams, K.L.; Leach, R.J.; O’Connell, P.; Stern, M.P. Linkage of Type 2 Diabetes Mellitus and of Age at Onset to a Genetic Location on Chromosome 10q in Mexican Americans. Am. J. Hum. Genet. 1999, 64, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meigs, J.B.; Panhuysen, C.I.M.; Myers, R.H.; Wilson, P.W.F.; Cupples, L.A. A Genome-Wide Scan for Loci Linked to Plasma Levels of Glucose and HbA1c in a Community-Based Sample of Caucasian Pedigrees: The Framingham Offspring Study. Diabetes 2002, 51, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Shea, J.L.; Loredo-Osti, J.C.; Sun, G. Association of RBP4 Gene Variants and Serum HDL Cholesterol Levels in the Newfoundland Population. Obesity 2010, 18, 1393–1397. [Google Scholar] [CrossRef]
- Kovacs, P.; Geyer, M.; Berndt, J.; Kloting, N.; Graham, T.E.; Bottcher, Y.; Enigk, B.; Tonjes, A.; Schleinitz, D.; Schon, M.R.; et al. Effects of Genetic Variation in the Human Retinol Binding Protein-4 Gene (RBP4) on Insulin Resistance and Fat Depot Specific mRNA Expression. Diabetes 2007, 56, 3095–3100. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, H.; Loos, R.J.F.; Qi, Q.; Hu, F.B.; Liu, Y.; Lin, X. RBP4 variants are significantly associated with plasma RBP4 levels and hypertriglyceridemia risk in Chinese Hans. J. Lipid Res. 2009, 50, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, K.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Paus, T.; Pausova, Z. Dietary Vitamin A and Visceral Adiposity: A Modulating Role of the Retinol-Binding Protein 4 Gene. J. Nutr. Nutr. 2015, 8, 164–173. [Google Scholar] [CrossRef]
- Wan, K.; Zhao, J.; Deng, Y.; Chen, X.; Zhang, Q.; Zeng, Z.; Zhang, L.; Chen, Y. A Genetic Polymorphism in RBP4 Is Associated with Coronary Artery Disease. Int. J. Mol. Sci. 2014, 15, 22309–22319. [Google Scholar] [CrossRef] [Green Version]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Korek, E.; Gibas-Dorna, M.; Chęcińska-Maciejewska, Z.; Krauss, H.; Łagiedo-Żelazowska, M.; Kołodziejczak, B.; Bogdański, P. Serum RBP4 positively correlates with triglyceride level but not with BMI, fat mass and insulin resistance in healthy obese and non-obese individuals. Biomarkers 2018, 23, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Wessel, H.; Saeed, A.; Heegsma, J.; Connelly, M.A.; Faber, K.N.; Dullaart, R.P.F. Plasma Levels of Retinol Binding Protein 4 Relate to Large VLDL and Small LDL Particles in Subjects with and without Type 2 Diabetes. J. Clin. Med. 2019, 8, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majerczyk, M.; Kocełak, P.; Choręza, P.; Arabzada, H.; Owczarek, A.J.; Bożentowicz-Wikarek, M.; Brzozowska, A.; Szybalska, A.; Puzianowska-Kuźnicka, M.; Grodzicki, T.; et al. Components of metabolic syndrome in relation to plasma levels of retinol binding protein 4 (RBP4) in a cohort of people aged 65 years and older. J. Endocrinol. Investig. 2018, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Wang, D.; Khan, U.I.; Zeb, I.; Manson, J.E.; Miller, V.; Hodis, H.N.; Budoff, M.J.; Merriam, G.R.; Harman, M.S.; et al. Associations between retinol-binding protein 4 and cardiometabolic risk factors and subclinical atherosclerosis in recently postmenopausal women: Cross-sectional analyses from the KEEPS study. Cardiovasc. Diabetol. 2012, 11, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Bachmayer, C.; Lammert, A.; Hasenberg, T.; Hammes, H.-P. Healthy Obese and Post Bariatric Patients - Metabolic and Vascular Patterns. Exp. Clin. Endocrinol. Diabetes 2013, 121, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Schindler, K.; Prager, G.; Bohdjalian, A.; Luger, A.; Wolzt, M.; Ludvik, B. Serum Retinol-Binding Protein 4 Is Reduced after Weight Loss in Morbidly Obese Subjects. J. Clin. Endocrinol. Metab. 2007, 92, 1168–1171. [Google Scholar] [CrossRef] [Green Version]
- Tschoner, A.; Sturm, W.; Engl, J.; Kaser, S.; Laimer, M.; Laimer, E.; Weiss, H.; Patsch, J.R.; Ebenbichler, C.F. Retinol-binding Protein 4, Visceral Fat, and the Metabolic Syndrome: Effects of Weight Loss. Obesity 2008, 16, 2439–2444. [Google Scholar] [CrossRef]
- Klöting, N.; Graham, T.E.; Berndt, J.; Kralisch, S.; Kovacs, P.; Wason, C.J.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M.; et al. Serum Retinol-Binding Protein Is More Highly Expressed in Visceral than in Subcutaneous Adipose Tissue and Is a Marker of Intra-abdominal Fat Mass. Cell Metab. 2007, 6, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Im, J.-A.; Lee, H.-R.; Shim, J.-Y.; Youn, B.-S.; Lee, D.-C. Visceral Adiposity Is Associated with Serum Retinol Binding Protein-4 Levels in Healthy Women*. Obesity 2007, 15, 2225–2232. [Google Scholar] [CrossRef] [Green Version]
- Zachariah, J.P.; Quiroz, R.; Nelson, K.P.; Teng, Z.; Keaney, J.F.; Sullivan, L.M.; Vasan, R.S. Prospective Relation of Circulating Adipokines to Incident Metabolic Syndrome: The Framingham Heart Study. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Karamfilova, V.; Gateva, A.; Alexiev, A.; Zheleva, N.; Velikova, T.; Ivanova-Boyanova, R.; Ivanova, R.; Cherkezov, N.; Kamenov, Z.; Mateva, L. The association between retinol-binding protein 4 and prediabetes in obese patients with nonalcoholic fatty liver disease. Arch. Physiol. Biochem. 2019, 63, 1–6. [Google Scholar] [CrossRef]
- Vink, R.G.; Roumans, N.J.; Mariman, E.C.; van Baak, M.A. Dietary weight loss-induced changes in RBP4, FFA, and ACE predict weight regain in people with overweight and obesity. Physiol. Rep. 2017, 5, e13450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.E.; Kim, D.H.; Lee, J.H.; Park, J.S.; Kang, E.S.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Retinol-binding protein-4 is associated with endothelial dysfunction in adults with newly diagnosed type 2 diabetes mellitus. Atherosclerosis 2009, 204, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M. CANTOS: A breakthrough that proves the inflammatory hypothesis of atherosclerosis. Glob. Cardiol. Sci. Pract. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Li, D.; Sun, R.; Xia, M. Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol. Metab. Syndr. 2014, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Takebayashi, K.; Sohma, R.; Aso, Y.; Inukai, T. Effects of retinol binding protein-4 on vascular endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 58–64. [Google Scholar] [CrossRef]
- Norseen, J.; Hosooka, T.; Hammarstedt, A.; Yore, M.M.; Kant, S.; Aryal, P.; Kiernan, U.A.; Phillips, D.A.; Maruyama, H.; Kraus, B.J.; et al. Retinol-Binding Protein 4 Inhibits Insulin Signaling in Adipocytes by Inducing Proinflammatory Cytokines in Macrophages through a c-Jun N-Terminal Kinase- and Toll-Like Receptor 4-Dependent and Retinol-Independent Mechanism. Mol. Cell. Biol. 2012, 32, 2010–2019. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Martin, A.; Hays, F.; Johnson, J.; Farjo, R.A.; Farjo, K.M. Serum retinol-binding protein-induced endothelial inflammation. Mol. Vis. 2017, 23, 185–197. [Google Scholar]
- Berry, D.C.; Jin, H.; Majumdar, A.; Noy, N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. USA 2011, 108, 4340–4345. [Google Scholar] [CrossRef] [Green Version]
- Dessein, P.H.; Tsang, L.; Norton, G.R.; Woodiwiss, A.J.; Solomon, A. Retinol Binding Protein 4 Concentrations Relate to Enhanced Atherosclerosis in Obese Patients with Rheumatoid Arthritis. PLoS ONE 2014, 9, e92739. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Kwanbunjan, K.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Wechjakwen, N.; Puduang, S.; Auyyuenyong, R.; Henkel, I.; Schweigert, F.J. Association of retinol binding protein 4 and transthyretin with triglyceride levels and insulin resistance in rural thais with high type 2 diabetes risk. BMC Endocr. Disord. 2018, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Xia, N.; Zhang, W.; Huang, J.; Ren, Z.; Zhu, L.; Zhang, Z.; Yang, L. Serum retinol-binding protein 4 is associated with insulin resistance in patients with early and untreated rheumatoid arthritis. Joint Bone Spine 2019, 86, 335–341. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Pérez-Matos, M.C.; Ricardo-Silgado, M.L.; Morales-Álvarez, M.C.; Mantilla-Rivas, J.O.; Pinzón-Cortés, J.A.; Pérez-Mayorga, M.; Arévalo-García, M.L.; Tolosa-González, G.; Mendivil, C.O. Comparison of plasma pigment epithelium-derived factor (PEDF), retinol binding protein 4 (RBP-4), chitinase-3-like protein 1 (YKL-40) and brain-derived neurotrophic factor (BDNF) for the identification of insulin resistance. J. Diabetes Complicat. 2017, 31, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Esangbedo, I.C.; Xu, L.; Fu, J.; Li, L.; Feng, D.; Han, L.; Xiao, X.; Li, M.; Mi, J.; et al. Childhood retinol-binding protein 4 (RBP4) levels predicting the 10-year risk of insulin resistance and metabolic syndrome: The BCAMS study. Cardiovasc. Diabetol. 2018, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Adams-Huet, B.; Duong, F.; Smith, G. Relationship between Retinol-Binding Protein-4/Adiponectin and Leptin/Adiponectin Ratios with Insulin Resistance and Inflammation. Metab. Syndr. Relat. Disord. 2014, 12, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Słomka, T.; Drelich, A.-Z.; Roman, T.; Kuczyńska, M.; Trojanowski, P.; Jargiełło, T. Grubość kompleksu intima-media oraz występowanie blaszki miażdżycowej w tętnicach szyjnych w populacji lubelskiej w odniesieniu do stylu życia badanych. Postępy Nauk Medycznych 2017, 4, 177–182. [Google Scholar]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B. Carotid-Wall Intima–Media Thickness and Cardiovascular Events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, D.H.; Bots, M.L. Imaging of atherosclerosis: Carotid intima-media thickness. Eur. Heart J. 2010, 31, 1682–1689. [Google Scholar] [CrossRef] [Green Version]
- Makimura, H.; Wei, J.; Dolan-Looby, S.E.; Ricchiuti, V.; Grinspoon, S. Retinol-binding protein levels are increased in association with gonadotropin levels in healthy women. Metabolism 2009, 58, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Oyama, J.; Murohara, T.; Kitakaze, M.; Ishizu, T.; Sato, Y.; Kitagawa, K.; Kamiya, H.; Ajioka, M.; Ishihara, M.; Dai, K.; et al. The Effect of Sitagliptin on Carotid Artery Atherosclerosis in Type 2 Diabetes: The PROLOGUE Randomized Controlled Trial. PLoS Med. 2016, 13, e1002051. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Katakami, N.; Yoshii, H.; Onuma, T.; Kaneto, H.; Osonoi, T.; Shiraiwa, T.; Kosugi, K.; Umayahara, Y.; Yamamoto, T.; et al. Alogliptin, a Dipeptidyl Peptidase 4 Inhibitor, Prevents the Progression of Carotid Atherosclerosis in Patients with Type 2 Diabetes: The Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care 2016, 39, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irace, C.; Casciaro, F.; Scavelli, F.B.; Oliverio, R.; Cutruzzolà, A.; Cortese, C.; Gnasso, A. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc. Diabetol. 2018, 17, 52. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Amato, A.; Al-Busaidi, N.; Al-Rasadi, K.; Soresi, M.; Banach, M.; Montalto, G.; et al. Liraglutide improves carotid intima-media thickness in patients with type 2 diabetes and non-alcoholic fatty liver disease: An 8-month prospective pilot study. Expert Opin. Biol. Ther. 2015, 15, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Chandalia, M.; Patti, A.; Di Bartolo, V.; Rizvi, A.A.; Montalto, G.; Abate, N. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc. Diabetol. 2014, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Burchardt, P.; Zawada, A.; Kaczmarek, J.; Marcinkaniec, J.; Wysocki, H.; Wierusz-Wysocka, B.; Grzymisławski, M.; Rzeźniczak, J.; Zozulińska-Ziółkiewicz, D.; Naskręt, D. Association between adjunctive metformin therapy in young type 1 diabetes patients with excess body fat and reduction of carotid intima–media thickness. Pol. Arch. Intern. Med. 2016, 126, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Lundby-Christensen, L.; Tarnow, L.; Boesgaard, T.W.; S Lund, S.; Wiinberg, N.; Perrild, H.; Krarup, T.; Snorgaard, O.; Gade-Rasmussen, B.; Thorsteinsson, B.; et al. Metformin versus placebo in combination with insulin analogues in patients with type 2 diabetes mellitus—The randomised, blinded Copenhagen Insulin and Metformin Therapy (CIMT) trial. BMJ Open 2016, 6, e008376. [Google Scholar] [CrossRef] [Green Version]
- Karakas, S.E.; Banaszewska, B.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A. Free fatty acid binding protein-4 and retinol binding protein-4 in polycystic ovary syndrome: Response to simvastatin and metformin therapies. Gynecol. Endocrinol. 2013, 29, 483–487. [Google Scholar] [CrossRef]
- Abbas, N.A.T.; El. Salem, A. Metformin, sitagliptin, and liraglutide modulate serum retinol-binding protein-4 level and adipocytokine production in type 2 diabetes mellitus rat model. Can. J. Physiol. Pharmacol. 2018, 96, 1226–1231. [Google Scholar] [CrossRef]
- Xiao, M.-L.; Lin, J.-S.; Li, Y.-H.; Liu, M.; Deng, Y.-Y.; Wang, C.-Y.; Chen, Y.-M. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutr. 2020, 23, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Abete, I.; Martínez, J.A. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine 2009, 36, 445–451. [Google Scholar] [CrossRef]
- Baden, M.Y.; Satija, A.; Hu, F.B.; Huang, T. Change in Plant-Based Diet Quality Is Associated with Changes in Plasma Adiposity-Associated Biomarker Concentrations in Women. J. Nutr. 2019, 149, 676–686. [Google Scholar] [CrossRef]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: A meta-analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tobias, D.K.; Hruby, A.; Rifai, N.; Tworoger, S.S.; Hu, F.B. An Increase in Dietary Quality Is Associated with Favorable Plasma Biomarkers of the Brain-Adipose Axis in Apparently Healthy US Women. J. Nutr. 2016, 146, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, B.J.; Neidlinger, T.R.; Van Loan, M.; Keim, N.L. Effect of low-calorie diets on plasma retinol-binding protein concentrations in overweight women. J. Nutr. Biochem. 1990, 1, 484–486. [Google Scholar] [CrossRef]
- Mateo, R.-G.; Lamiquiz, I.-M.; Perez, S.-C.; Marco, V.-B.; Bea, A.M.; Baila, L.-R.; Laclaustra, M.; Peñalvo, J.L.; Civeira, F.; Cenarro, A. Different protein composition of low-calorie diet differently impacts adipokine profile irrespective of weight loss in overweight and obese women. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 133–142. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate Restriction has a More Favorable Impact on the Metabolic Syndrome than a Low Fat Diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Daneshzad, E.; Farsad, A.-N.; Heshmati, J.; Mirzaei, K.; Maghbooli, Z.; Keshavarz, S.-A. The association between dietary antioxidants and adipokines level among obese women. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1369–1373. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, S.-D.; Chen, C.; Wang, W. Involvement of RBP4 in Diabetic Atherosclerosis and the Role of Vitamin D Intervention. J. Diabetes Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; del-Río-Navarro, B.E.; Leija-Martinez, J.; Torres-Alcantara, S.; Ruiz-Bedolla, E.; Hernández-Cadena, L.; Barraza-Villarreal, A.; Romero-Nava, R.; Sanchéz-Muñoz, F.; Villafaña, S.; et al. Effect of omega-3 fatty acids supplementation combined with lifestyle intervention on adipokines and biomarkers of endothelial dysfunction in obese adolescents with hypertriglyceridemia. J. Nutr. Biochem. 2019, 64, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Puchau, B.; Bressan, J.; Martínez, J.A. Association of retinol-binding protein-4 with dietary selenium intake and other lifestyle features in young healthy women. Nutrition 2009, 25, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Choi, S.H.; Jeong, I.-K.; Kim, J.H.; Moon, M.K.; Park, K.S.; Lee, H.K.; Kim, Y.-B.; Jang, H.C. Insulin-Sensitizing Effects of Exercise on Adiponectin and Retinol-Binding Protein-4 Concentrations in Young and Middle-Aged Women. J. Clin. Endocrinol. Metab. 2008, 93, 2263–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkharfy, K.M.; Al-Daghri, N.M.; Vanhoutte, P.M.; Krishnaswamy, S.; Xu, A. Serum retinol-binding protein 4 as a marker for cardiovascular disease in women. PLoS ONE 2012, 7, e48612. [Google Scholar] [CrossRef] [Green Version]
- Pala, L.; Monami, M.; Ciani, S.; Dicembrini, I.; Pasqua, A.; Pezzatini, A.; Francesconi, P.; Cresci, B.; Mannucci, E.; Rotella, C.M. Adipokines as Possible New Predictors of Cardiovascular Diseases: A Case Control Study. J. Nutr. Metab. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, E.; Soares, H.; Guimarães, H.; Vitorino, R.; Ferreira, R.; Henriques, T.-C. Prediction of cardiovascular risk in preterm neonates through urinary proteomics: An exploratory study. Porto Biomed. J. 2017, 2, 287–292. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.-W.; Yun, J.W.; Chung, I.-S.; Cho, H.C.; Song, S.-E.; Im, S.-S.; Song, D.-K. Proteomics approach to identify serum biomarkers associated with the progression of diabetes in Korean patients with abdominal obesity. PLoS ONE 2019, 14, e0222032. [Google Scholar] [CrossRef]
- Patterson, C.C.; Blankenberg, S.; Ben, Y.-S.; Heslop, L.; Bayer, A.; Lowe, G.; Zeller, T.; Gallacher, J.; Young, I.; Yarnell, J. Which biomarkers are predictive specifically for cardiovascular or for non-cardiovascular mortality in men? Evidence from the Caerphilly Prospective Study (CaPS). Int. J. Cardiol. 2015, 201, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ding, M.; Chiuve, S.E.; Rimm, E.B.; Franks, P.W.; Meigs, J.B.; Hu, F.B.; Sun, Q. Plasma Levels of Fatty Acid-Binding Protein 4, Retinol-Binding Protein 4, High-Molecular Weight Adiponectin, and Cardiovascular Mortality among Men with Type 2 Diabetes: A 22-Year Prospective Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2259–2267. [Google Scholar] [CrossRef] [Green Version]




| Authors | Study Population | Groups, Sex (Group Size, n) | Age (Years) | BMI (kg/m2) | CV Risk Assessment Method | CV Risk (Group Size, n) | RBP4 Measurement Method (Unit) | RBP4 Specimen | Serum RBP4 Range | Relation between RBP4 and CV Risk |
|---|---|---|---|---|---|---|---|---|---|---|
| Feng et al. 2015 [48] | T2DM | 498 F; 578 M (1076) | 62.80 ± 13.60 | 27.50 ± 4.20 | cIMT (mm) | G 1 (332): no abnormalities | ELISA (mg/L) | serum | G 1 32.10 ± 10.3 | + |
| 27.90 ± 3.40 | G 2 (386): ≥ 1 | G 2 38.20 ± 8.30 | ||||||||
| 27.60 ± 3.60 | G 3 (358): ≥ 1.5 | G 3 46.90 ± 7.60 | ||||||||
| Xiao et al. 2013 [19] | T2DM | 140 F; 144 M (284) | 35.00–70.00 | 25.10 ± 2.80 | cIMT (mm) fIMT (mm) iIMT (mm) | subAS (78) cIMT 0.94 ± 0.34 fIMT 0.97 ± 0.33 iIMT 1.13 ± 0.28 | ELISA with monoclonal antibodies (mg/L) | serum | 37.1 (32.3–40.8) | + |
| 24.50 ± 2.80 | Non-subAS cIMT 0.70 ± 0.11 fIMT 0.70 ± 0.11 iIMT 0.76 ± 0.10 | 23.2 (20.1–29.2) | + | |||||||
| Won et al. 2012 [49] | Healthy | 175 F; 116 M (291) | 40.00 ± 11.00 | 27.00 ± 2.60 | The Framingham Risk Score | MetS (57) Framingham risk: 2.0, 0.0 to >30.0 Framingham score: 9.0, −7.0 to 17.0 | EIA (µg/mL) | plasma | MetS 65.1 ± 26.8 | + |
| 23.60 ± 3.00 | Non-MetS (234) Framingham risk: 0.5, 0.0 to 20.0 Framingham score: 3.0, −9.0 to 18.0 | Non-MetS 52.2 ± 20.0 | ||||||||
| Su et al. 2020 [29] | CKD | 58 F; 111 M (169) | 59.50–78.00 | 27.40 ± 2.90 | CV events (fatal and nonfatal) | (total 80) CV events: 41 CV mortality: 10 | ELISA (mg/L) | serum | >33.86 | + (higher rates of CV events than RBP4 < 33.86) |
| 25.90 ± 2.10 | (total 89) CV events: 11 CV mortality: 4 | <33.86 | + | |||||||
| Solini et al. 2009 [38] | HYP | 35 F | 47.40 ± 5.00 | 25.00 ± 1.60 | cIMT (mm) | 0.54 ± 0.15 | ELISA (µg/mL) | plasma | Median value 38.75 | + |
| CTL | 35 F | 46.90 ± 6.30 | 25.70 ± 1.40 | 0.5 ± 0.13 | Median value 10.00 | None | ||||
| Mansouri et al. 2012 [50] | T2DM | 53 F; 48 M (101) | 53.60 ± 8.40 | 27.70 ± 4.10 | cIMT (mm) | 0.8 ± 0.2 | ELISA (µg/mL) | serum | 71.9 ± 35.6 | None |
| Bobbert et al. 2010 [30] | T2DM and non-T2DM | 52 F; 44 M (96) | 55.00 ± 1.30 | 30.80 ± 0.70 | cIMT (mm) | 0.72 ± 0.02 | ELISA (µmol/L) | serum | 1.89 ± 0.05 | + |
| Chu et al. 2011 [51] | T2DM with CKD | 86 (sex NM) | 70.00 ± 11.00 | 26.20 ± 6.20 | cIMT (mm) | 0.75 ± 0.16 | ELISA (µg/mL) | serum | 44.8 ± 6.4 | None |
| T2DM without CKD | 153 (sex NM) | 60.00 ± 12.00 | 26.30 ± 5.90 | 0.69 ± 0.14 | 39.5 ± 4.9 | None | ||||
| Li et al. 2020 [52] | CHF | 227 F; 707 M (934) | ≥60 | 22.49–26.67 | MACE (Multivariable Cox regression) | - | ELISA (µg/mL) | serum | 46.66 ± 12.38 | + (log RBP4 associated with 1.6 times higher risk of MACE) |
| Bachmayer et al. 2013 [53] | Patients with obesity | 65 F; 27 M (92) | 43.00 ± 10.00 | 50.00 ± 7.00 | Endothelial dysfunction: CRAE (µm); CRVE (µm); AVR | CRAE 178 ± 19 | ELISA (ng/mL) | NM | 24,773 ± 14,025 | None |
| CRVE 221 ± 24 | None | |||||||||
| AVR 0.81 ± 0.09 | None |
| Variant | Genetic Location | Study Group | Pathophysiology Association | Reference | |
|---|---|---|---|---|---|
| n (Total) | Diagnosis | ||||
| rs10882280 | g.6681G > T c.355+837G > T (intronic) | 1422 F; 414 M (1836) | healthy (metabolic, cardiovascular, or endocrine disease excluded) | Higher high-density lipoprotein level associated with minor allele T (p = 0.043) and C (p = 0.042), respectively | Shea et al. 2010 [58] |
| rs11187545 | g.8889T > C c.355+3045T > C (intronic) | ||||
| rs10882283 | g.5030T > G c. −55T > G (5’ UTR variant) | 457 F; 477 M (934); 716 CTL | T2DM | G-allele associated with a higher body-mass index and waist-to-hip ratio values (p < 0.05). | Kovacs et al. 2007 [59] |
| rs10882273 | g.27484T > C c.*1539T > C (3′ UTR variant) | 457 F; 477 M (934); 716 CTL | T2DM | C-allele associated with an increased BMI, plasma insulin, and circulating free fatty acid concentrations (p < 0.05) | Kovacs et al. 2007 [59] |
| 1787 F;1423 M (3210) | Chinese Hans population 50–70 years old | Higher body-mass index values. Higher insulin and free fatty acids levels. Association with plasma RBP4 levels (p = 0.005). | Wu et al. 2009 [60] | ||
| rs10882272 | g.26761T > C c.*816T > C (3′ UTR variant) | 593 F; 454 M (947) | French-Canadian founder population 12–18 years old | Association with circulating retinol levels. Modulation between vitamin A intake and abdominal adiposity. | Goodwin et al. 2015 [61] |
| 5 006 | Caucasian cohorts from Finland, USA, and Italy | Association with circulating retinol levels. | Mondul et al. 2011 [25] | ||
| rs3758538 | g.3944A > C c.697–1781A > C (upstream transcript variant) | 97 with obesity; 83 normal-weight | Spanish Caucasian children | Association with triglycerides levels and plasma RBP4 levels. C allele associated with obesity and higher BMI z-score. | Codõner-Franch et al. 2016 [54] |
| 1787 F; 1423 M (3210) | Chinese Hans population 50–70 years old | Association with hypertriglyceridemia and plasma RBP4 levels. | Wu et al. 2009 [60] | ||
| rs3758539 | g.4406G > A c.697-2243G > A (upstream transcript variant) | 97 cases 83 CTL | Obesity, Spanish Caucasian children | Association with triglycerides levels in children. | Codõner-Franch et al. 2016 [54] |
| 66 F; 63 M (129) 192 CTL | Obesity, cohort from Iran | Association with an increased susceptibility for obesity and an increased BMI. | Shajarian et al. 2015 [55] | ||
| rs12265684 | g.12177G > A c.356-25G > A (intronic) | 97 cases 83 CTL | Obesity, Spanish Caucasian children | Association with triglycerides levels and blood pressure. | Codõner-Franch et al. 2016 [54] |
| rs34571439 | g.14684T > G c.697-12521A > C (upstream transcript variant) | Association with triglycerides and plasma RBP4 levels as well as plasma C-reactive protein values. | |||
| rs7094671 | g.10377C > T c.356-1825C > T (intronic) | 297 M; 217 M CTL | CAD, Chinese patients | G allele associated with a higher risk of CAD | Wan et al. 2014 [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychter, A.M.; Skrzypczak-Zielińska, M.; Zielińska, A.; Eder, P.; Souto, E.B.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? Int. J. Mol. Sci. 2020, 21, 5229. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155229
Rychter AM, Skrzypczak-Zielińska M, Zielińska A, Eder P, Souto EB, Zawada A, Ratajczak AE, Dobrowolska A, Krela-Kaźmierczak I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? International Journal of Molecular Sciences. 2020; 21(15):5229. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155229
Chicago/Turabian StyleRychter, Anna Maria, Marzena Skrzypczak-Zielińska, Aleksandra Zielińska, Piotr Eder, Eliana B. Souto, Agnieszka Zawada, Alicja Ewa Ratajczak, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2020. "Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity?" International Journal of Molecular Sciences 21, no. 15: 5229. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155229






