Vitamin E Is Superior to Vitamin C in Delaying Seedling Senescence and Improving Resistance in Arabidopsis Deficient in Macro-Elements
Abstract
:1. Introduction
2. Results
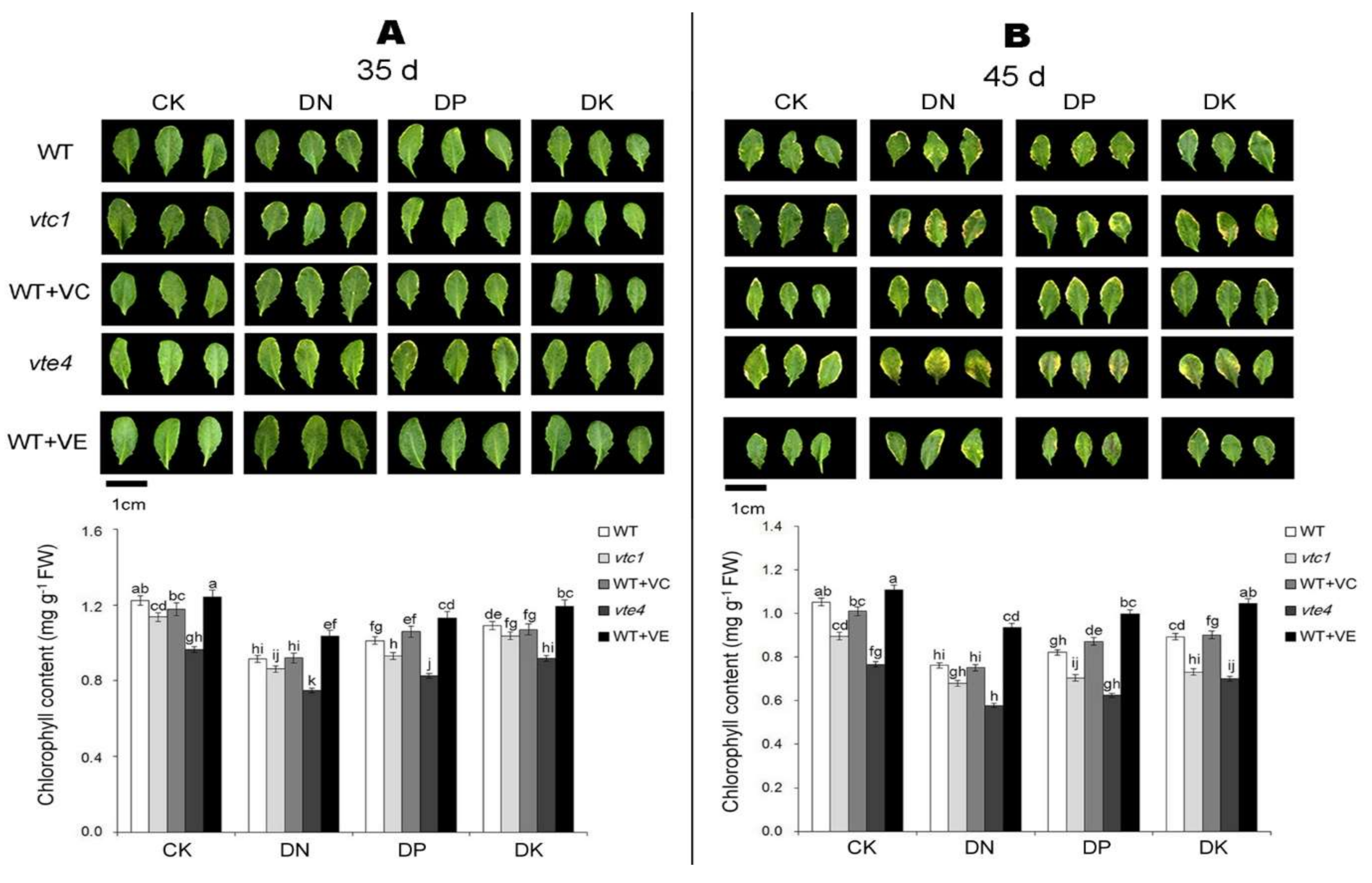
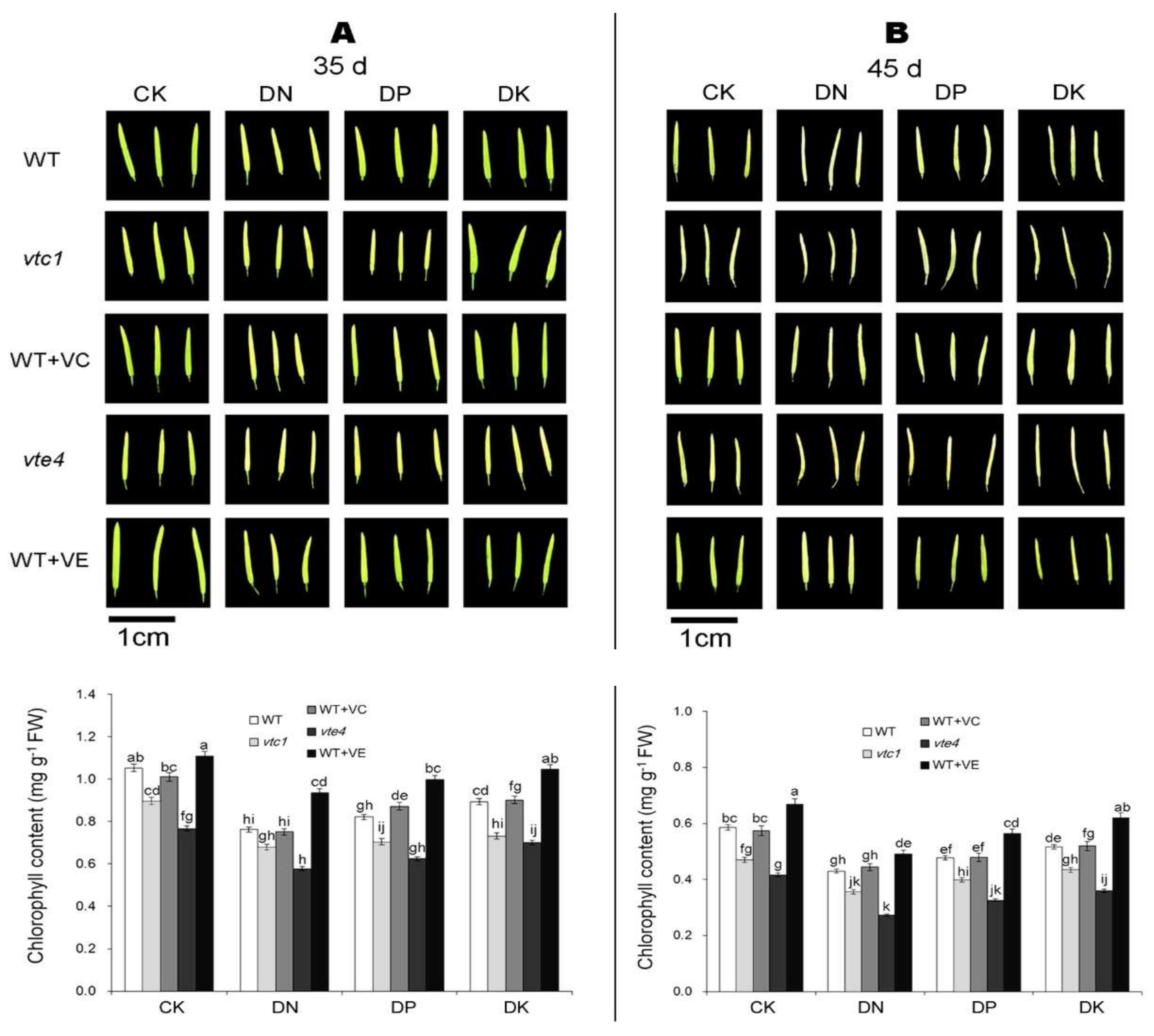
2.1. Vitamin E and Vitamin C Defer Senescence Caused by the Deficiency of Macro-Elements in Arabidopsis
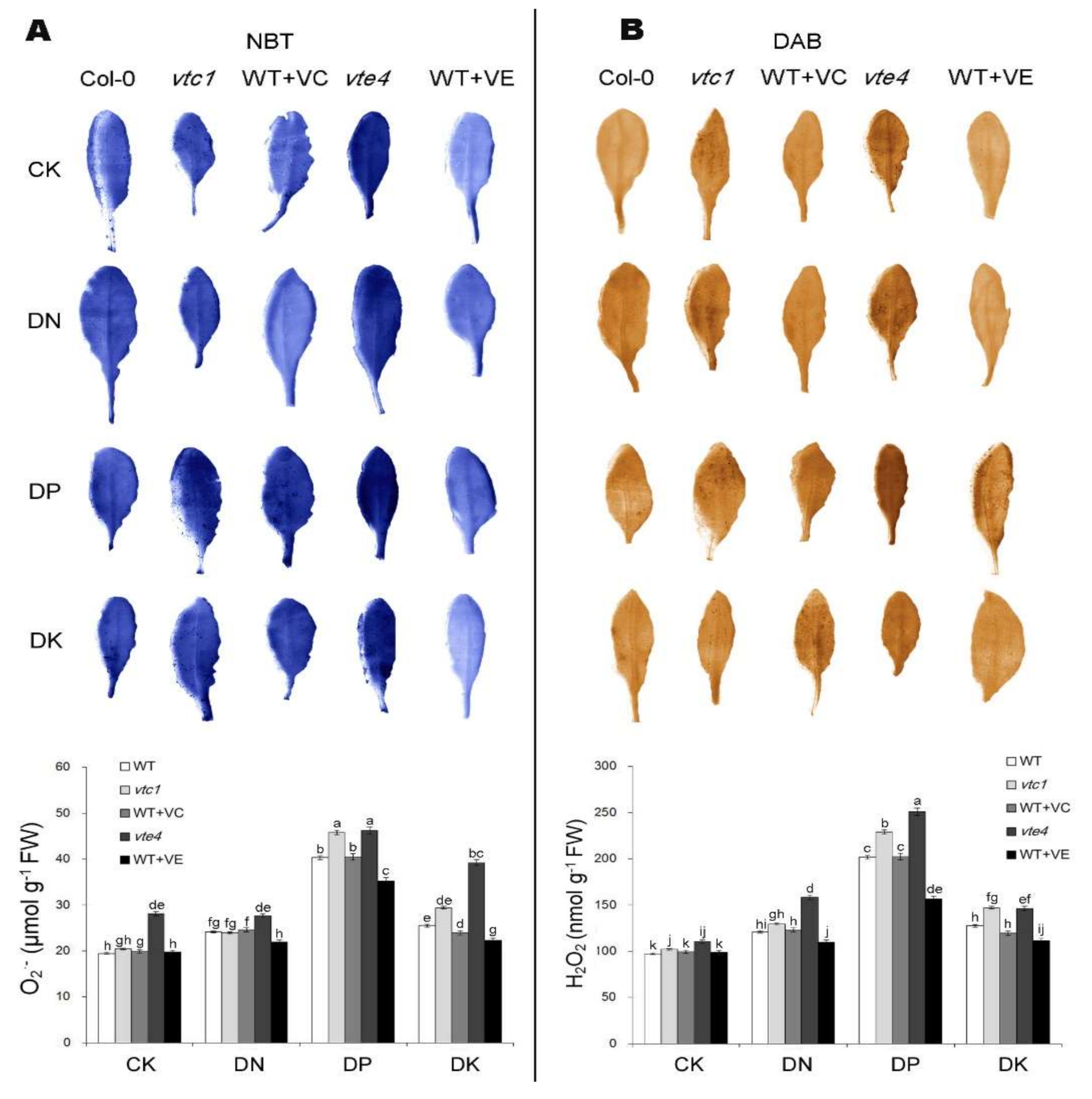
2.2. Vitamin E and Vitamin C Relieve Oxidative Stress Caused by Macro-Element Deficiency by Removing Reactive Oxygen Species (ROS)
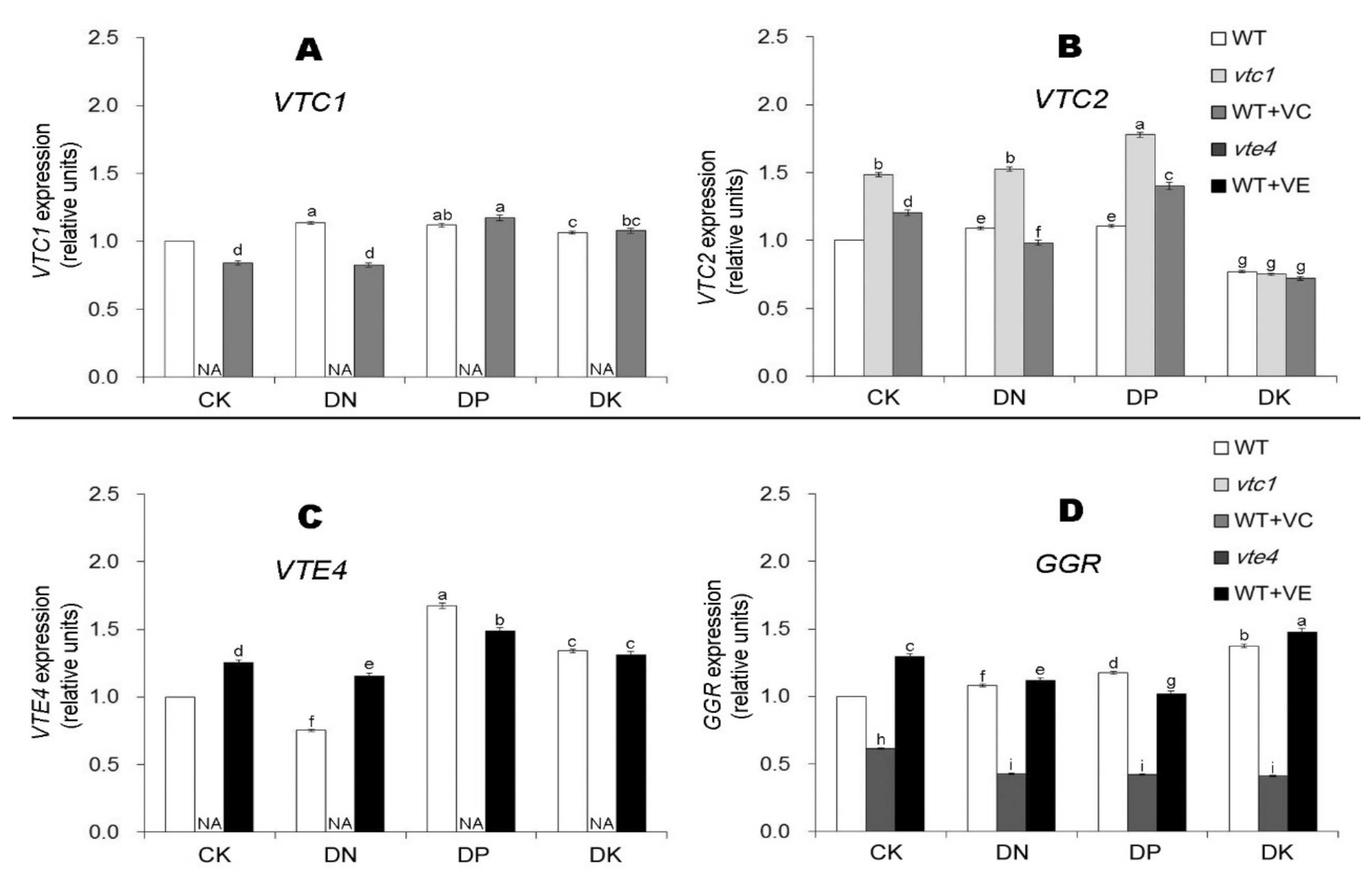
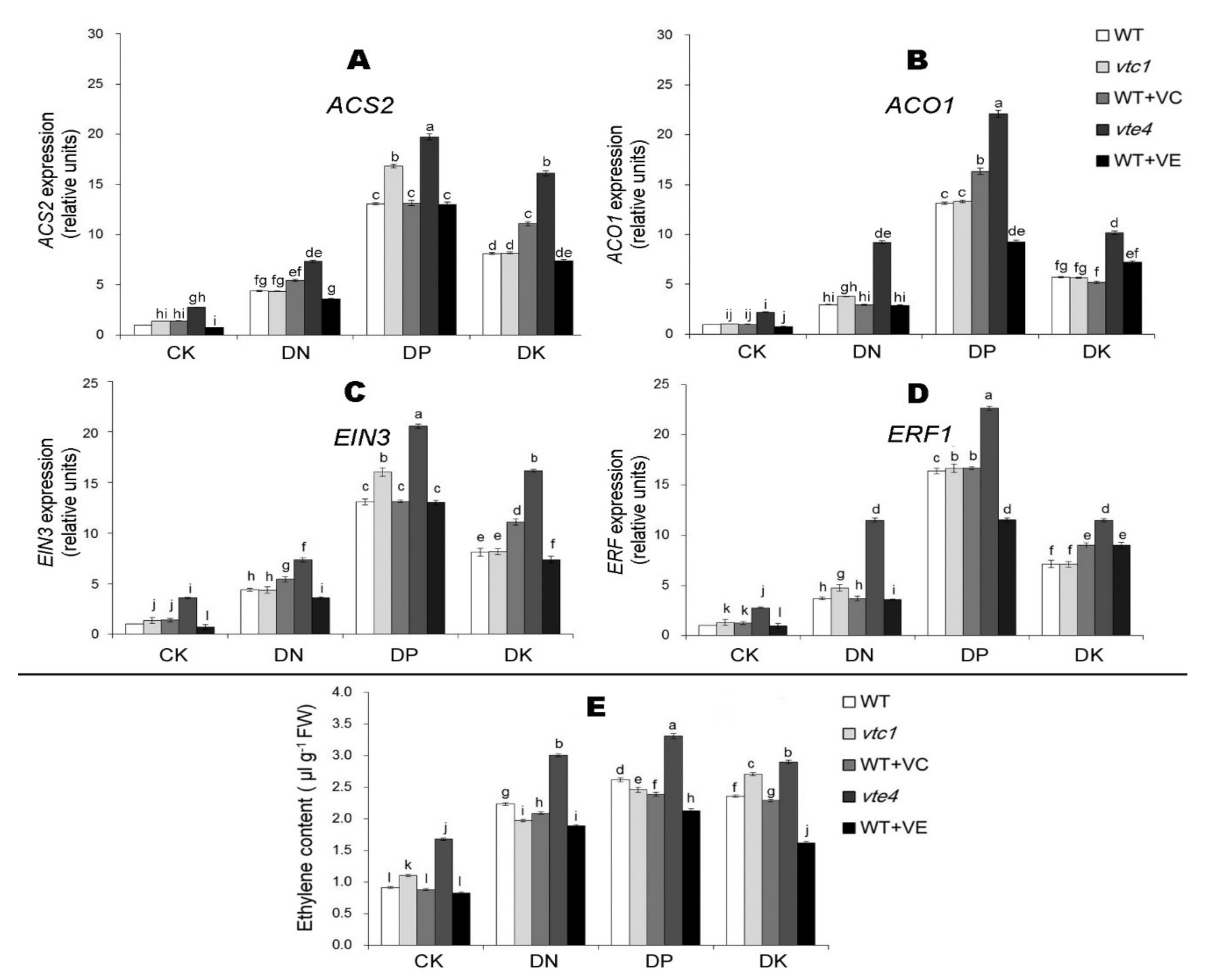
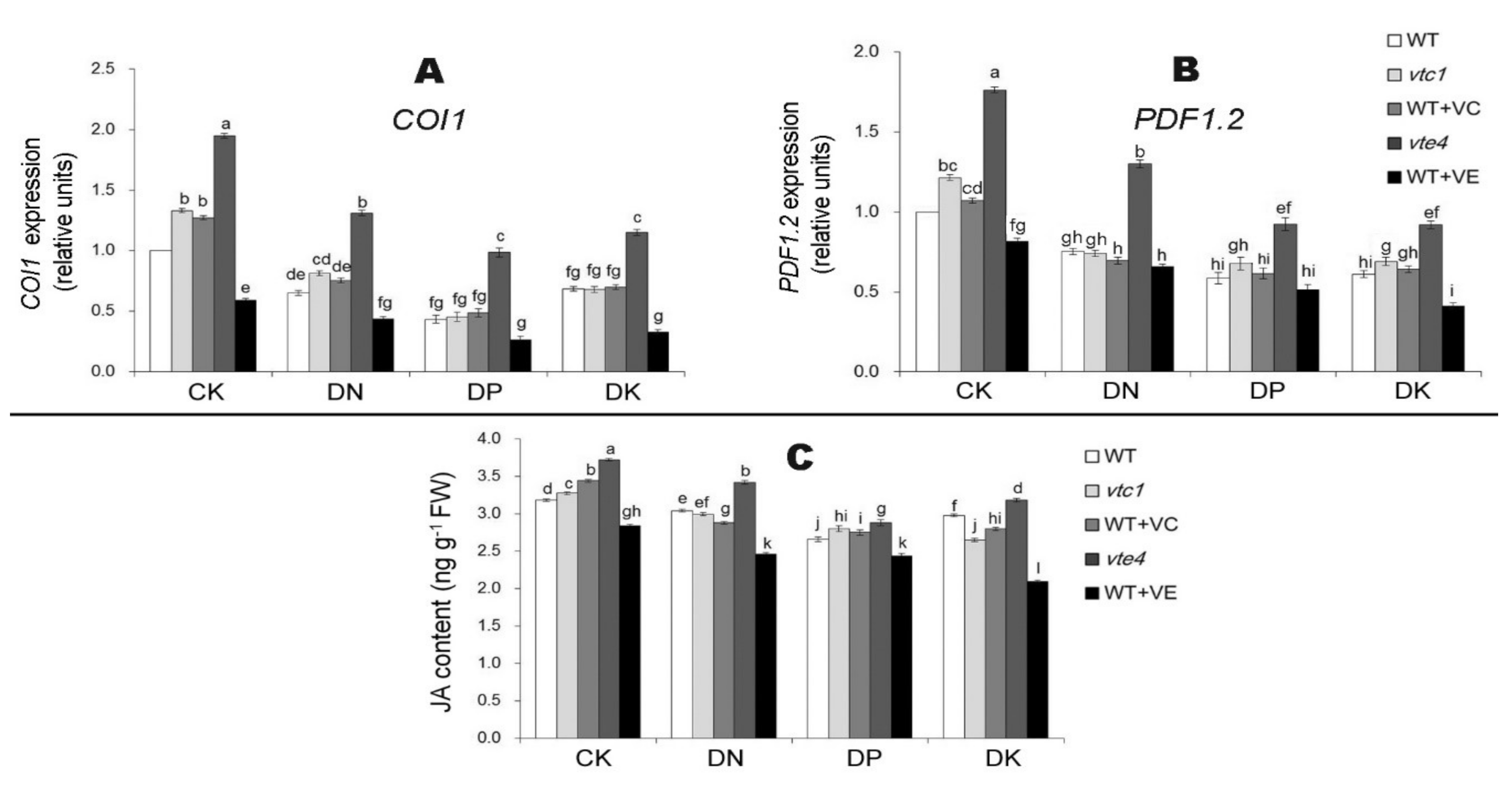
2.3. Vitamin E Inhibits the Expression of Ethylene and Jasmonic Acid under Macro-Element Deficiency
2.4. Vitamin E and Vitamin C Reverse the Yield Reduction of Arabidopsis under Macro-Element Deficiency
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Chlorophyll Contents
4.3. Determination of Ethylene Contents
4.4. Determination of Jasmonic Acid Contents
4.5. Quantitative Real-Time PCR
4.6. Oxidative Damage Measurements
4.7. Germination Rate Determination
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cechin, I.; Tdef, F. Effect of nitrogen supply on growth and photosynthesis of sunflower plants grown in the greenhouse. Plant Sci. 2004, 166, 1379–1385. [Google Scholar] [CrossRef]
- Fetene, M.; Möller, I.; Beck, E. The effect of nitrogen supply to Urtica dioica L. plants on the distribution of assimilate between shoot and roots. Plant Biol. 2015, 106, 228–234. [Google Scholar]
- Tóth, V.R.; Mészáros, I.; Veres, S.; Nagy, J. Effects of the available nitrogen on the photosynthetic activity and xanthophyll cycle pool of maize in field. J Plant Physiol. 2002, 159, 627–634. [Google Scholar] [CrossRef]
- Yu, B.; Xu, C.; Benning, C. Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc. Natl. Acad. Sci. USA 2002, 99, 5732–5737. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Liu, D. Signaling components involved in plant responses to phosphate starvation. J. Integr. Plant Biol. 2008, 50, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhu, C.; Liu, Y.; Karthikeyan, A.S.; Bressan, R.A.; Kashchandra, K.G.; Liu, D. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol. 2011, 189, 1084–1095. [Google Scholar] [CrossRef]
- Amtmann, A.; Hammond, J.P.; Armengaud, P.; White, P.J. Nutrient sensing and signalling in plants: potassium and phosphorus. Adv. Bot. Res. 2005, 43, 209–257. [Google Scholar]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: new knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, C.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. Curr. Opin. Plant Biol. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, W.H. Plant sensing and signaling in response to K+-deficiency. Mol Plant. 2010, 3, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Troufflard, S.; Mullen, W.; Larson, T.R.; Graham, I.A.; Crozier, A.; Amtmann, A.; Armengaud, P. Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. J. Integr. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet. 2014, 10, e1004664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarwat, M.; Naqvi, A.R.; Ahmad, P.; Ashrafd, M.; Akram, N.A. Phytohormones and microRNAs as sensors and regulators of leaf senescence: Assigning macro roles to small molecules. Biotechnol. Adv. 2013, 31, 1153–1171. [Google Scholar] [CrossRef]
- Yan, J.X.; Deng, Y.A.; Yu, J.; Zhang, Y.Q.; Chi, D.F. A study on JA-and BTH-induced resistance of Rosa rugosa ‘Plena’ to powdery mildew (Sphaerotheca pannosa). J. Forest Res. 2018, 29, 823–831. [Google Scholar] [CrossRef]
- Peng, H.P.; Lin, T.Y.; Wang, N.N.; Shih, M.C. Differential expression of genes encoding 1-aminocyclopropane-1-carboxylic synthase in Arabidopsis during hypoxia. Plant Mol. Biol. 2005, 58, 15–25. [Google Scholar] [CrossRef]
- Tsuchisaka, A.; Yu, G.; Jin, H.; Alonso, J.M.; Ecker, J.R.; Zhang, X.; Gao, S.; Theologis, A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 2009, 183, 979–1003. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Chung, K.M.; Woo, H.R. Three positive regulators of leaf senescence in Arabidopsis, ORE1, ORE3 and ORE9, play roles in crosstalk among multiple hormone-mediated senescence pathways. Genes Genom. 2011, 33, 373–381. [Google Scholar] [CrossRef]
- Li, Z.H.; Peng, J.Y.; Wen, X.; Guo, H.W. ETHYLENE-INSENSITIVE3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, K.; Li, Z.P.; Yang, Z.; Chen, J.Y.; Wu, S.X.; Zhu, X.Y.; Gao, S.; Gao, J.; Ren, G.D.; Kuai, B.K.; et al. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PLoS Genet. 2015, 11, e1005399. [Google Scholar] [CrossRef] [PubMed]
- Holländer-Czytko, H.; Grabowski, J.; Sandorf, I.; Weckermann, K.; Weiler, E.W. Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in Arabidopsis under stress conditions. J. Plant Physiol. 2005, 162, 767–770. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010, 179, 289–295. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Semchuk, N.M.; Lushchak, V.; Falk, J.; Krupinskab, K.; Lushchaka, V.I. Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol. Biochem. 2009, 47, 384–390. [Google Scholar] [CrossRef]
- Khan, T.A.; Mazid, M.; Mohammad, F. Potential of ascorbic acid against oxidative burst in plants under biotic stress: A Review. J. Ind. Res. Tech. 2012, 2, 72–80. [Google Scholar]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, Z.L.; Jbara, M. A vitamins for enhancing plant resistance. Planta 2016, 244, 529–546. [Google Scholar] [CrossRef]
- Upadhyaya, C.P.; Akula, N.; Young, K.E.; Chun, S.C.; Kim, D.H.; Park, S.W. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol. Lett. 2010, 32, 321–330. [Google Scholar]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aglio, E.; Mhamdi, A. What are the roles for dehydroascorbate reductases and glutathione in sustaining ascorbate accumulation? Plant Physiol. 2020, 183, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhou, Y.; Wang, Z.; Sun, X.; Tang, K. Engineering tocopherol biosynthetic pathway in Arabidopsis leaves and its effect on antioxidant metabolism. Plant Sci. 2010, 178, 312–320. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Coman, D.; Beck, G.M.; Barja, M.V.; Colinas, M.; Graf, A.; Welsch, R.; Rütimann, P.; Bühlmann, P.; Bigler, L.; et al. Arabidopsis GERANYLGERANYL DIPHOSPHATE SYNTHASE 11 is a hub isozyme required for the production of most photosynthesis-related isoprenoids. New Phytol. 2015, 209, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Shpilyov, A.V.; Zinchenko, V.V.; Shestakov, S.V.; Grimm, B.; Lokstein, H. Inactivation of the geranylgeranyl reductase (ChlP) gene in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerget. 2005, 1706, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants. J. Plant Nutr. Soil Sci. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Sattler, S.E.; Cheng, Z.; DellaPenna, D. From Arabidopsis to agriculture: Engineering improved Vitamin E content in soybean. Trends Plant Sci. 2004, 9, 365–367. [Google Scholar] [CrossRef]
- Sattler, S.E.; Mène-Saffrané, L.; Farmer, E.E.; Krischke, M.; Mueller, M.J.; DellaPenna, D. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 2006, 18, 3706–3720. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil. 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Saur, A.; Hennig, P.; Tschiersch, H.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Tocopherol deficiency in transgenic tobacco (Nicotiana tabacum L.) plants leads to accelerated senescence. Plant Cell Environ. 2009, 32, 144–157. [Google Scholar] [CrossRef]
- Caretto, S.; Nisi, R.; Paradiso, A.; Gara, L.D. Tocopherol production in plant cell cultures. Mol. Nutr. Food Res. 2010, 54, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.A.; Munné-Bosch, S. Redox signaling and stress tolerance in plants: A focus on Vitamin E. Ann. N. Y. Acad. Sci. 2015, 1340, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Saleemi, S.; Razzaq, H.; Yaqub, A.; Shakoor, S.; Qureshi, R. Investigating the scavenging of reactive oxygen species by antioxidants via theoretical and experimental methods. J. Photochem. Photobiol. B. 2018, 180, 268–275. [Google Scholar] [CrossRef]
- Siu, A.W.; Reiter, R.J.; To, C.H. The efficacy of vitamin E and melatonin as antioxidants against lipid peroxidation in rat retinal homogenates. J. Pineal. Res. 1998, 24, 239–244. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xu, X.C.; Liu, T.; Yuan, S. Mitochondrion-permeable antioxidants to treat ROS-burst-mediated acute diseases. Oxid. Med. Cell. Longev. 2016, 2016, 6859523. [Google Scholar] [CrossRef] [Green Version]
- Nozomi, N.; Ryouichi, T.; Soichirou, S. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell 2005, 17, 233–240. [Google Scholar]
- Zhong, Y.Q.; Chen, C.; Nawaz, M.A.; Jiao, Y.Y.; Zheng, Z.H.; Shi, X.F.; Xie, W.Y.; Yu, Y.G.; Guo, J.; Zhu, S.H.; et al. Using rootstock to increase watermelon fruit yield and quality at low-K supply: A comprehensive analysis from agronomic, physiological and transcriptional perspective. Sci. Hortic. 2018, 241, 144–151. [Google Scholar] [CrossRef]
- Obed, I.; Hernández-Pérez, L.A.; Aguilar, V.; Alia-Tejacal, I.; Andrew, D.C.; Donita, L.C. Tomato fruit yield, quality, and nutrient status in response to potassium: Calcium balance and electrical conductivity in the nutrient solution. J. Soil Sci. Plant Nutr. 2020, 20, 484–492. [Google Scholar]
- Edward, G.; Lionel, J.M.; Constantin, J.; Sylvain, P.; Michael, D. Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum (L.). Environ. Exp. Bot. 2010, 67, 451–459. [Google Scholar]
- Zhong, S.; Zhao, M.; Shi, T.; Shi, H.; An, F.; Zhao, Q.; Guo, H. EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 2009, 106, 21431–21436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.W.; Wang, J.; Li, S.H.; Kakan, X.; Zhou, Y.; Miao, Y.; Wang, F.F.; Qin, H.; Huang, R.F. Ascorbic acid integrates the antagonistic modulation of ethylene and abscisic acid in the accumulation of reactive oxygen species. Plant Physiol. 2019, 179, 1861–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cela, J.; Falk, J.; Munné-Bosch, S. Ethylene signaling may be involved in the regulation of tocopherol biosynthesis in Arabidopsis thaliana. FEBS Lett. 2009, 583, 992–996. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedmann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Datta, R.; Kumar, D.; Sultana, A.; Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiol. 2015, 169, 2963–2981. [Google Scholar]
- Kristl, J.; Veber, M.; Krajničič, B.; Orešnik, K.; Slekovec, M. Determination of jasmonic acid in Lemna minor (L.) by liquid chromatography with fluorescence detection. Anal Bioanal. Chem. 2005, 383, 886–893. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, Z.W.; Zheng, C.; Zhao, Z.Y.; Wang, Y.; Feng, L.Y.; Niu, G.; Wang, C.Q.; Wang, J.H.; Feng, H.; et al. Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proc. Natl. Acad. Sci. USA 2016, 113, 7661–7666. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Suorsa, M.; Järvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjärvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2002, 151, 59–66. [Google Scholar] [CrossRef]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dormann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [Green Version]


| Gene | Locus | The Forward Primer Sequences | The Reverse Primer Sequences |
|---|---|---|---|
| VTC1 | At2g39770 | GGCAACCCCGTGACTACATAAC | CCAATCAAACATCCTTCCCCAA |
| VTC2 | At4g26850 | GGTCGTCACTTGAAGAAGAGGC | GGGAAGAACTGAACTTGGGCAT |
| VTE4 | At1g64970 | AGCAGCACCCTCTTCTCTCACA | CCCAAATCTCTTCCCACAAACC |
| GGR | At4g38460 | ATGGTGGAGCAGAGAAGGGAAT | AGGTGGTAGCGAAGATGAATGG |
| ACS2 | At1g01480 | GTGTCTCCTGGCTCTTCCTTCC | GCCGTCAAAAACAACCCTAATG |
| ACO1 | At2g19590 | TCCTGAGCTTATGAGAGGGCTG | AATGGTATTGTTCTTGGATGGC |
| EIN3 | At3g20770 | ACAACAATAACAGTAGCGGCAACA | AGCGATAGAGACAGAGAGACCCAG |
| ERF1 | At3g23240 | GCAGTCCACGCAACAAACCTA | CTTGAACTCTCTCCGCCGAAA |
| PDF1.2 | At5g44420 | CTTGTTCTCTTTGCTGCTTTCG | CATGATCCATGTTTGGCTCCTT |
| COL1 | At5g15850 | AATGGCTTCTCGATTGGGGAT | TGGAGGGTAAGGTGGTTGGTC |
| ACT7 | At5g09810 | ATCCCTCAGCACCTTCCAAC | ACCCGATACTTAAATAATTGTCTCAT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-W.; Yang, X.-Y.; Zheng, X.-J.; Fu, Y.-F.; Lan, T.; Tang, X.-Y.; Wang, C.-Q.; Chen, G.-D.; Zeng, J.; Yuan, S. Vitamin E Is Superior to Vitamin C in Delaying Seedling Senescence and Improving Resistance in Arabidopsis Deficient in Macro-Elements. Int. J. Mol. Sci. 2020, 21, 7429. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197429
Zhang Z-W, Yang X-Y, Zheng X-J, Fu Y-F, Lan T, Tang X-Y, Wang C-Q, Chen G-D, Zeng J, Yuan S. Vitamin E Is Superior to Vitamin C in Delaying Seedling Senescence and Improving Resistance in Arabidopsis Deficient in Macro-Elements. International Journal of Molecular Sciences. 2020; 21(19):7429. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197429
Chicago/Turabian StyleZhang, Zhong-Wei, Xin-Yue Yang, Xiao-Jian Zheng, Yu-Fan Fu, Ting Lan, Xiao-Yan Tang, Chang-Quan Wang, Guang-Deng Chen, Jian Zeng, and Shu Yuan. 2020. "Vitamin E Is Superior to Vitamin C in Delaying Seedling Senescence and Improving Resistance in Arabidopsis Deficient in Macro-Elements" International Journal of Molecular Sciences 21, no. 19: 7429. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197429





