Glycerol-Induced Powdery Mildew Resistance in Wheat by Regulating Plant Fatty Acid Metabolism, Plant Hormones Cross-Talk, and Pathogenesis-Related Genes
Abstract
:1. Introduction
2. Results
2.1. Bgt Resistance Test in Water-Treated and Glycerol-Treated Wheat Leaves
2.2. Transcriptome Sequencing, Assembly, and Functional Annotation
2.3. Identification of Differentially Expressed Genes (DEGs)
2.4. Expression Patterns in Response to Bgt Infection (H24 vs. H0 and G24 vs. G0)
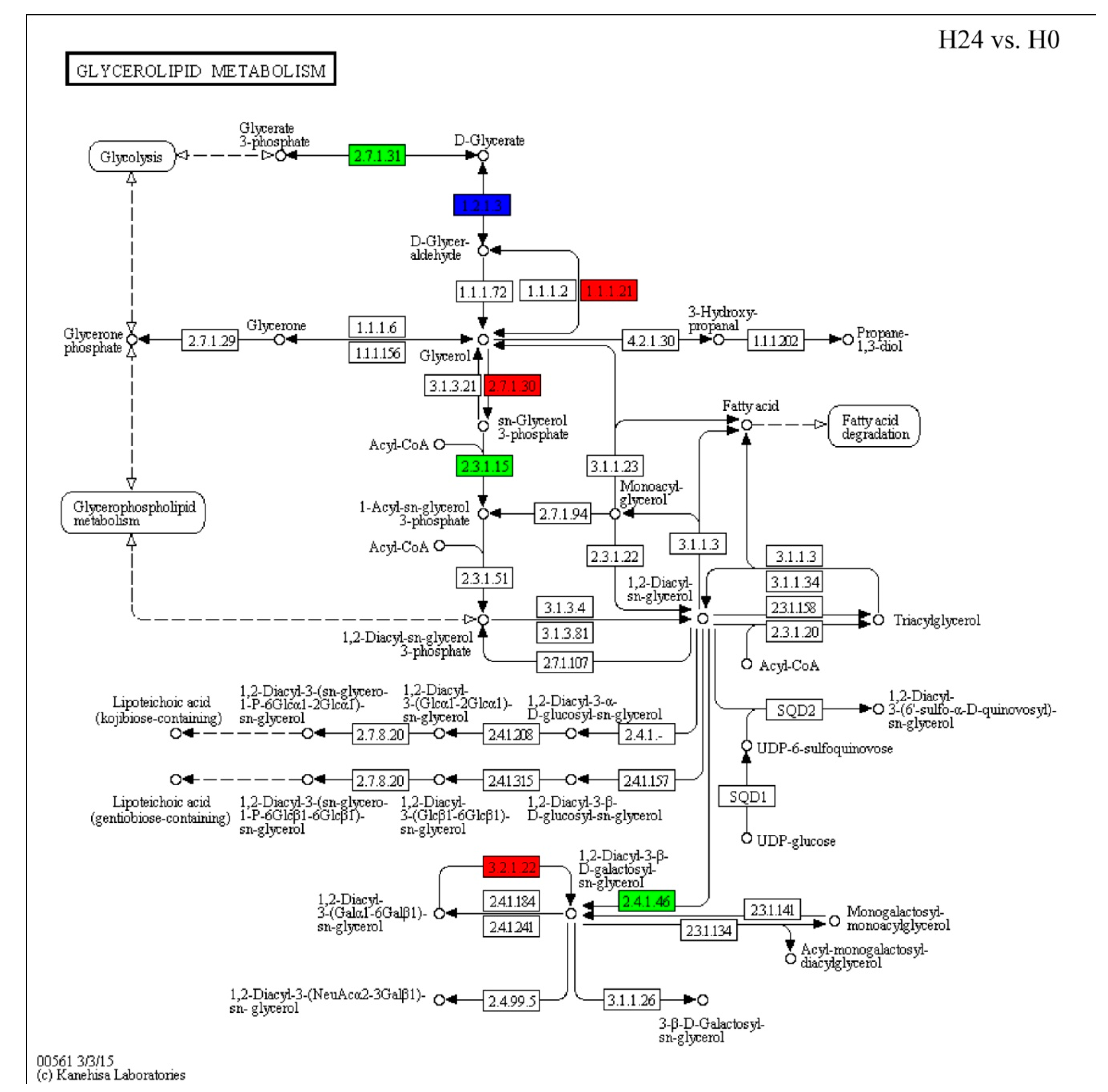
2.5. Changes in Expression Patterns of Glycerolipid Metabolism and Fatty Acid Biosynthesis Pathways in Response to Bgt Infection
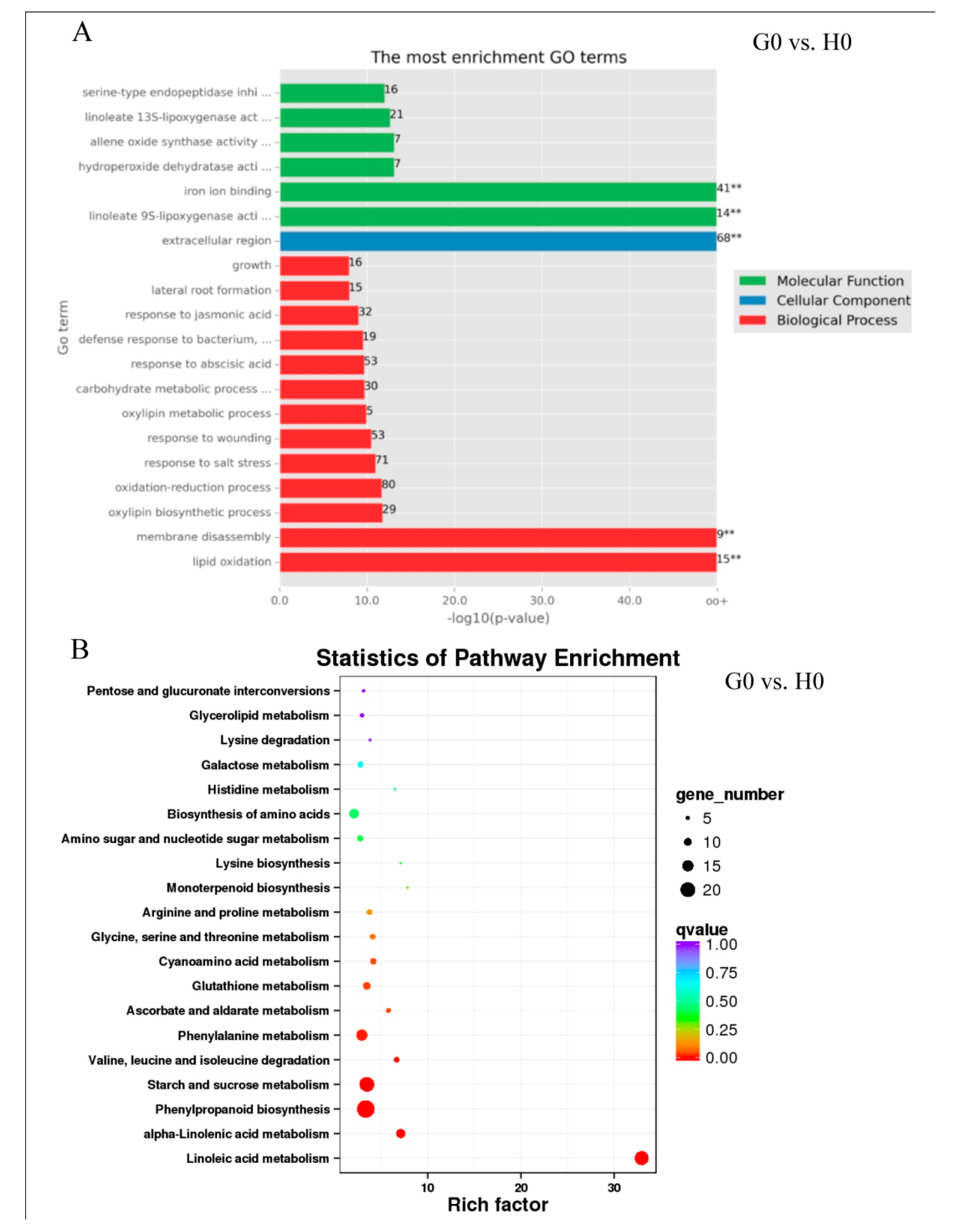
2.6. Expression Patterns in Response to Glycerol Application (G0 vs. H0)
2.7. Expression Patterns and DEGs in G24 vs. H24 Groups
2.8. Plant Hormones Cross-Talk in Response to Glycerol Application
3. Discussion
4. Material and Methods
4.1. Plant and Fungal Materials
4.2. Plants Growth and Glycerol Treatments
4.3. Pathogen Maintenance and Inoculation
4.4. Coomassie Blue Staining
4.5. RNA Extraction, cDNA Library Construction, RNA-Seq, and Data Analysis
4.6. Measurements of Endogenous Plant Hormones
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mehta, Y.R. Wheat Diseases and Their Management; Springer: Cham, Switzerland, 2014; p. 256. [Google Scholar]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323. [Google Scholar] [CrossRef] [Green Version]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Truman, W.; Bennett, M.H.; Kubigsteltig, I.; Turnbull, C.; Grant, M. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc. Natl. Acad. Sci. USA 2007, 104, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Truman, W.M.; Bennett, M.H.; Turnbull, C.G.; Grant, M.R. Arabidopsis auxin mutants are compromised in systemic acquired resistance and exhibit aberrant accumulation of various indolic compounds. Plant Physiol. 2010, 152, 1562–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Kachroo, P. Fatty Acid-derived signals in plant defense. Annu. Rev. Phytopathol. 2009, 47, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Chanda, B.; Xia, Y.; Mandal, M.K.; Yu, K.; Sekine, K.T.; Gao, Q.M.; Selote, D.; Hu, Y.; Stromberg, A.; Navarre, D.; et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 2011, 43, 421–427. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Liu, P.; Xing, H.; Li, C.; Wei, G.; Kang, Z. Glycerol-3-phosphate metabolism in wheat contributes to systemic acquired resistance against Puccinia striiformis f. sp. tritici. PLoS ONE 2013, 8, e81756. [Google Scholar] [CrossRef]
- Kang, L.; Li, J.; Zhao, T.; Xiao, F.; Tang, X.; Thilmony, R.; He, S.; Zhou, J.M. Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc. Natl. Acad. Sci. USA 2003, 100, 3519–3524. [Google Scholar] [CrossRef] [Green Version]
- Chanda, B.; Venugopal, S.C.; Kulshrestha, S.; Navarre, D.A.; Downie, B.; Vaillancourt, L.; Kachroo, A.; Kachroo, P. Glycerol-3-phosphate levels are associated with basal resistance to the hemibiotrophic fungus Colletotrichum higginsianum in Arabidopsis. Plant Physiol. 2008, 147, 2017–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, S.C.; Chanda, B.; Vaillancourt, L.; Kachroo, A.; Kachroo, P. The common metabolite glycerol-3-phosphate is a novel regulator of plant defense signaling. Plant Signal Behav. 2009, 4, 746–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachroo, P.; Shanklin, J.; Shah, J.; Whittle, E.J.; Klessig, D.F. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 9448–9453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.J.; Shimono, M.; Maeda, S.; Inoue, H.; Mori, M.; Hasegawa, M.; Sugano, S.; Takatsuji, H. Suppression of the rice fatty-acid desaturase gene OsSSI2 enhances resistance to blast and leaf blight diseases in rice. Mol. Plant Microbe Interact. 2009, 22, 820–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.M.; Klingl, V.; von Roepenack-Lahaye, E.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife 2017, 6, 29107. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [Green Version]
- Kachroo, A.; Venugopal, S.C.; Lapchyk, L.; Falcone, D.; Hildebrand, D.; Kachroo, P. Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 5152–5157. [Google Scholar] [CrossRef] [Green Version]
- Kachroo, A.; Fu, D.Q.; Havens, W.; Navarre, D.; Kachroo, P.; Ghabrial, S.A. An oleic acid-mediated pathway induces constitutive defense signaling and enhanced resistance to multiple pathogens in soybean. Mol. Plant Microbe Interact. 2008, 21, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Smith, P.; Maximova, S.N.; Guiltinan, M.J. Application of glycerol as a foliar spray activates the defense response and enhances disease resistance of Theobroma cacao. Mol. Plant Pathol. 2015, 16, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, N.; Zhao, C.; Li, F.; Geng, M.; Wang, Y.; Liu, W.; Xie, C.; Sun, Q. Application of glycerol for induced powdery mildew resistance in Triticum aestivum L. Front. Physiol. 2016, 7, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Wang, X.; Peng, H.; Yao, Y.; Xie, C.; Han, Y.; Ni, Z.; Sun, Q. Transcriptome comparison of susceptible and resistant wheat in response to powdery mildew infection. Genom. Proteom. Bioinform. 2012, 10, 94–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhang, H.; Mandal, S.N.; Wang, C.; Chen, C.; Ji, W. Quantitative proteomics reveals the central changes of wheat in response to powdery mildew. J. Proteom. 2016, 130, 108–119. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Wang, C.; Liu, M.; Li, H.; Fu, Y.; Wang, Y.; Nie, Y.; Liu, X.; Ji, W. Large-scale transcriptome comparison reveals distinct gene activations in wheat responding to stripe rust and powdery mildew. BMC Genom. 2014, 15, 898. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.K.; Lee, S.; Chung, E.; Park, J.M.; Yu, S.H.; Ryu, C.M.; Choi, D. Insight into Types I and II nonhost resistance using expression patterns of defense-related genes in tobacco. Planta 2006, 223, 1101–1107. [Google Scholar] [CrossRef]
- Song, N.; Hu, Z.; Li, Y.; Li, C.; Peng, F.; Yao, Y.; Peng, H.; Ni, Z.; Xie, C.; Sun, Q. Overexpression of a wheat stearoyl-ACP desaturase (SACPD) gene TaSSI2 in Arabidopsis ssi2 mutant compromise its resistance to powdery mildew. Gene 2013, 524, 220–227. [Google Scholar] [CrossRef]
- Blümke, A.; Falter, C.; Herrfurth, C.; Sode, B.; Bode, R.; Schäfer, W.; Feussner, I.; Voigt, C.A. Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol. 2014, 165, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Park, O.K. Lipases associated with plant defense against pathogens. Plant Sci. 2019, 279, 51–58. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid-and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Rajendran, S.R.C.K.; Gaur, M.; Sajeesh, P.K.; Kumar, A. Plant defense signaling and responses against necrotrophic fungal pathogens. J. Plant Growth Regul. 2016, 35, 1159–1174. [Google Scholar] [CrossRef]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef] [Green Version]
- Zalewski, K.; Lahuta, L.B.; Martysiak-Żurowska, D.; Okorski, A.; Nitkiewicz, B.; Zielonka, Ł. The effect of exogenous application of methyl jasmonate on the lipid and carbohydrate content and composition of winter triticale (Triticosecale Wittm.) grain and the severity of fungal infections in triticale plants and grain. J. Agric. Food Chem. 2019, 67, 5932–5939. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Song, M.; Wei, Z.; Tong, J.; Zhang, L.; Xiao, L.; Ma, Z.; Wang, Y. A jacalin-related lectin-like gene in wheat is a component of the plant defence system. J. Exp. Bot. 2011, 62, 5471–5483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Yoshida, Y.; Major, I.T.; Wang, K.; Sugimoto, K.; Kapali, G.; Havko, N.E.; Benning, C.; Howe, G.A. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 10768–10777. [Google Scholar] [CrossRef] [Green Version]
- Ishiga, Y.; Ishiga, T.; Uppalapati, S.R.; Mysore, K.S. Jasmonate ZIM-domain (JAZ) protein regulates host and nonhost pathogen-induced cell death in tomato and Nicotiana benthamiana. PLoS ONE 2013, 8, e75728. [Google Scholar] [CrossRef]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid–amido synthetase GH3-8 suppresses expansin expression and promotes salicylate-and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Liu, H.; Li, Y.; Yu, H.; Li, X.; Xiao, J.; Wang, S. Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice. Plant Physiol. 2011, 155, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef] [Green Version]
- Fousia, S.; Tsafouros, A.; Roussos, P.A.; Tjamos, S.E. Increased resistance to Verticillium dahliae in Arabidopsis plants defective in auxin signalling. Plant Pathol. 2018, 67, 1749–1757. [Google Scholar] [CrossRef]
- Fan, S.; Chang, Y.; Liu, G.; Shang, S.; Tian, L.; Shi, H. Molecular functional analysis of auxin/indole-3-acetic acid proteins (Aux/IAAs) in plant disease resistance in cassava. Physiol. Plant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bi, W.; Jing, G.; Yu, X.; Wang, H.; Liu, D. Systemic acquired resistance, NPR1, and pathogenesis-related genes in wheat and barley. J. Integr. Agric. 2018, 17, 2468–2477. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.; Usha, K.; Singh, B. Pathogenesis related (PR) proteins in plant defense mechanism. Sci. Against Microb. Pathog. 2011, 2, 1043–1054. [Google Scholar]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Yi, G.X.; Wang, B.M.; Deng, A.X.; Nan, T.G.; Li, Z.H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef]






| DEG Set | All DEGs | Upregulated | Downregulated |
|---|---|---|---|
| H24 vs. H0 | 5616 | 3489 | 2127 |
| G24 vs. G0 | 2494 | 1723 | 771 |
| G0 vs. H0 | 414 | 376 | 38 |
| G24 vs. H24 | 463 | 308 | 155 |
| Gene #ID | H0 | G0 | H24 | G24 | Annotation | |
|---|---|---|---|---|---|---|
| 1.13.11.12 | Traes_5BS_060785740 | 32.93 | 123.12 | 46.68 | 97.83 | Lipoxygenase 2.1 |
| Traes_5DS_E8892706A | 93.92 | 330.19 | 155.71 | 290.37 | ||
| Traes_6DS_7CA5A8F12 | 41.62 | 73.37 | 30.51 | 38.32 | Lipoxygenase 2.3 | |
| 4.2.1.92 | Traes_4AS_41FB87D39 | 2.37 | 21.67 | 5.56 | 24.73 | Allene oxide synthase 2 |
| Traes_4AS_9F1B2A7DD | 1.66 | 14.56 | 1.00 | 7.24 | ||
| Traes_4BL_523D155E21 | 2.63 | 43.79 | 6.79 | 49.98 | ||
| Traes_4BL_C01E043B6 | 1.10 | 10.83 | 1.46 | 7.70 | ||
| Traes_4BL_DD6DD7487 | 17.33 | 119.91 | 38.96 | 170.11 | ||
| Traes_4BL_EC6D20026 | 5.55 | 115.08 | 29.68 | 122.97 | ||
| Traes_4DL_B3E978E9F | 1.76 | 14.96 | 1.02 | 6.22 | ||
| 1.3.1.42 | Traes_6DL_94DCF0B70 | 0.29 | 1.18 | 2.48 | 2.35 | 12-oxophytodienoate reductase |
| Traes_7BS_62CC4CA59 | 0.63 | 6.32 | 0.19 | 2.01 | ||
| 1.13.11.58 | Traes_2DL_17913EE21 | 109.35 | 358.45 | 275.74 | 312.34 | Linoleate 9S-lipoxygenase 1 |
| Traes_2DL_CE85DC5C0 | 35.10 | 133.18 | 93.19 | 117.98 | ||
| Traes_2DL_D4BCDAA76 | 24.12 | 70.55 | 41.85 | 65.25 | ||
| Traes_4BS_63DD9D036 | 2.13 | 5.62 | 5.75 | 10.62 | ||
| Traes_4BS_71CB57A0D | 4.33 | 15.90 | 8.60 | 17.88 | ||
| Traes_4BS_939C79184 | 1.45 | 9.81 | 4.19 | 9.49 | ||
| Traes_4BS_9DDF3D7C6 | 2.05 | 6.42 | 5.43 | 9.57 | ||
| Traes_4DS_7868A8C2E | 2.13 | 11.58 | 7.16 | 18.30 | ||
| Traes_2DL_B5B62EE11 | 31.16 | 95.42 | 65.15 | 85.79 | Probable linoleate 9S-Lipoxygenase 5 | |
| Traes_2BL_77148B8D8 | 18.72 | 76.36 | 38.65 | 61.36 | ||
| Traes_2DL_FE7B99D58 | 3700 | 21095 | 16544 | 15919 | ||
| Traes_6AS_9557563D1 | 8.17 | 70.75 | 6.10 | 88.52 | Putative linoleate 9S-Lipoxygenase 3 | |
| Traes_6BS_B26FD03C8 | 7.08 | 62.70 | 4.01 | 41.90 | ||
| Triticum_aestivumLinn_newGene_11732 | 1.44 | 13.99 | 0.68 | 17.84 | ||
| Traes_5BL_304FAFA26 | 0.09 | 1.78 | 0.15 | 3.64 | Linoleate 9S-lipoxygenase 2 | |
| Traes_2AL_5BAB26827 | 27.39 | 75.74 | 78.10 | 76.00 | Seed linoleate 9S-lipoxygenase-3 | |
| 1.11.2.3 | Traes_2AL_6A8D574C4 | 0.32 | 3.42 | 0.25 | 2.85 | Peroxygenase |
| Traes_2BL_5880DAAC3 | 0.11 | 1.31 | 0.00 | 0.41 | ||
| Traes_2DL_00618F315 | 0.09 | 1.91 | 0.10 | 0.83 | ||
| AUX/IAA | Triticum_aestivumLinn_newGene_126 | 0.70 | 1.47 | 0.87 | 1.62 | Auxin-responsive protein IAA18 |
| PP2C | Traes_4BL_3403452C0 | 0.61 | 2.36 | 0.30 | 0.88 | Serine/threonine protein phosphatase 2C 30 |
| JAZ | Triticum_aestivumLinn_newGene_18579 | 0.50 | 1.32 | 0.52 | 0.92 | Protein TIFY 10B |
| HSP | Traes_2AS_67EFE0FAE | 0.24 | 1.53 | 0.29 | 0.79 | Heat shock protein 90 |
| Traes_2DS_3B16D8173 | 0.09 | 1.58 | 0.42 | 0.83 | ||
| Traes_1AL_51CED3DBF | 4.02 | 7.93 | 5.77 | 8.92 | Heat shock cognate 70 kDa protein 4 | |
| Traes_1AS_5DE9A16CD | 0.86 | 1.90 | 2.53 | 2.77 | Heat shock 70 kDa protein 4L | |
| Traes_3B_B67388A96 | 0.33 | 1.89 | 0.35 | 0.87 | Heat shock cognate 70 kDa protein | |
| Traes_3B_FB10B725B | 0.54 | 2.53 | 0.46 | 1.36 | Heat shock cognate 70 kDa protein | |
| Traes_5BL_C318204D2 | 1.58 | 3.14 | 2.16 | 3.18 | Hsp70 nucleotide exchange factor fes1-like | |
| PR-1 | Traes_3DL_85AC9E60D | 0.71 | 22.84 | 152.38 | 109.17 | Glucan endo-1,3-beta-glucosidase GII |
| Traes_3DL_F5930F58D | 2.13 | 36.99 | 344.10 | 210.39 | ||
| Traes_3DL_48F92563F | 5.79 | 18.03 | 258.95 | 136.05 | Glucan endo-1,3-beta-glucosidase GIII | |
| Triticum_aestivumLinn_newGene_5203 | 0.92 | 2.32 | 35.78 | 22.19 | beta-1,3-glucanase precursor | |
| Traes_3AL_BCFD5F303 | 0.54 | 16.40 | 152.58 | 103.74 | beta-1,3-glucanase | |
| Traes_3B_9F3320C78 | 0.12 | 3.59 | 38.21 | 26.41 | Glucan endo-1,3-beta-D-glucosidase | |
| Traes_5DL_ED441B7EB | 1.62 | 3.54 | 3.34 | 5.37 | Cell wall beta-glucosidase | |
| Triticum_aestivumLinn_newGene_1652 | 0.13 | 1.50 | 35.07 | 17.75 | Glucan endo-1,3-beta-glucosidase 13 | |
| Traes_1BL_A6F7A9A54 | 0.60 | 1.39 | 0.45 | 1.03 | Glucan endo-1,3-beta-glucosidase 14 | |
| PR-3 | Traes_7AL_FAE816A85 | 3.68 | 29.00 | 60.31 | 80.11 | Chitinase 1 |
| Traes_7DL_24AA71860 | 1.55 | 11.67 | 28.87 | 35.39 | ||
| Traes_1AL_E96C0662D | 8.97 | 15.92 | 297.95 | 169.09 | Chitinase 8 | |
| Traes_5BL_DE0C53CE2 | 1.42 | 18.76 | 27.60 | 25.49 | ||
| Traes_5DL_EEF38A7E4 | 0.23 | 3.51 | 6.41 | 13.68 | ||
| Callose synthase | Traes_3DL_9021F9E75 | 0.51 | 1.12 | 1.11 | 1.07 | Callose synthase 12 |
| Traes_6BL_2D48C932A | 0.13 | 0.41 | 0.42 | 0.16 | Callose synthase 3 | |
| Traes_7DS_B481462CF | 0.56 | 1.19 | 1.16 | 1.06 | Callose synthase 10 | |
| Peroxidase | Traes_2AS_457604359 | 0.44 | 4.44 | 67.41 | 50.64 | Peroxidase 2 |
| Traes_2BS_EAB2C09D0 | 0.14 | 1.88 | 35.62 | 36.93 | ||
| Traes_2DS_708F03DA3 | 20.25 | 50.88 | 301.61 | 183.45 | ||
| Traes_3DL_B38DFDDFF | 0.39 | 11.67 | 2.54 | 22.57 | ||
| Traes_2DS_D76AB139C | 0.25 | 1.08 | 141.26 | 60.39 | Peroxidase 1 | |
| Traes_6BS_0BDACE205 | 20.88 | 42.56 | 197.18 | 181.62 | Peroxidase 6 | |
| Traes_5BL_36EBD512B | 0.20 | 0.94 | 0.77 | 1.91 | Peroxidase 35 | |
| Disease-related gene | Traes_2BL_19C5224BE | 0.41 | 2.35 | 0.41 | 1.24 | Pathogenesis-related protein 1 |
| Traes_2BL_B657F7F3A | 0.00 | 1.43 | 0.12 | 0.77 | ||
| Traes_4AS_C5AE1BBDD | 2.14 | 5.27 | 16.46 | 8.89 | ||
| Traes_4DL_5C688784F | 3.18 | 9.00 | 22.61 | 16.89 | ||
| Traes_6DL_F504536D5 | 4.64 | 9.13 | 17.02 | 24.47 | Putative disease resistance RGA4 | |
| Traes_7BS_94EB3B3D6 | 11.54 | 42.41 | 484.99 | 343.31 | Pathogenesis-related protein PRMS | |
| Triticum_aestivumLinn_newGene_11833 | 2.82 | 5.11 | 6.73 | 9.12 | Putative disease resistance RPM1 | |
| Triticum_aestivumLinn_newGene_22934 | 0.37 | 1.80 | 1.28 | 3.06 | Putative LRR receptor-like serine/threonine-protein kinase |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qiu, L.; Liu, X.; Zhang, Q.; Zhuansun, X.; Fahima, T.; Krugman, T.; Sun, Q.; Xie, C. Glycerol-Induced Powdery Mildew Resistance in Wheat by Regulating Plant Fatty Acid Metabolism, Plant Hormones Cross-Talk, and Pathogenesis-Related Genes. Int. J. Mol. Sci. 2020, 21, 673. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020673
Li Y, Qiu L, Liu X, Zhang Q, Zhuansun X, Fahima T, Krugman T, Sun Q, Xie C. Glycerol-Induced Powdery Mildew Resistance in Wheat by Regulating Plant Fatty Acid Metabolism, Plant Hormones Cross-Talk, and Pathogenesis-Related Genes. International Journal of Molecular Sciences. 2020; 21(2):673. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020673
Chicago/Turabian StyleLi, Yinghui, Lina Qiu, Xinye Liu, Qiang Zhang, Xiangxi Zhuansun, Tzion Fahima, Tamar Krugman, Qixin Sun, and Chaojie Xie. 2020. "Glycerol-Induced Powdery Mildew Resistance in Wheat by Regulating Plant Fatty Acid Metabolism, Plant Hormones Cross-Talk, and Pathogenesis-Related Genes" International Journal of Molecular Sciences 21, no. 2: 673. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020673





