3.2.1. Potential Energy Change
In
Section 3.1.4, we observe water transport from the 200 ns simulations. Now, the following question arises: what is the driving force for the water transport? Since this is an NVT simulation, the appropriate free energy for the system is the Helmholtz free energy
, where
,
, and
are the internal energy, temperature, and entropy, respectively [
6]. Therefore, to systematically understand the Helmholtz free energy change Δ
A, we can consider the energetic contribution related to the first term
and the entropic contribution related to the second term
. However, from
Figure 2 and
Figure 3, we understand that the energetic contribution is dominant over the entropic contribution because if the entropic change is dominant, water molecules should be transferred to Compartment 2 due to the translational entropy and the mixing entropy with osmolytes. Therefore, we focus on the internal energy change Δ
E, which is the sum of the potential energy change and kinetic energy change. Furthermore, since we study the system under a constant temperature, we expect that the change due to the kinetic energy is not significant. Thus, we expect that the potential energy change is responsible for the water transport. We calculate the potential energy change to determine if the change is correlated with the water transport, to explain the driving force for the transport.
As shown in
Figure 6, we calculate the potential energy change for the cases of
ISWO = 10, 20, and 50, where water transport is observed. In this calculation, for convenience, for each value of
ISWO, we set the average potential energy over the time interval between 100 ns and 200 ns for the
NO = 10 case to zero. The potential energies for the other cases are calculated based on this reference. As we expect, when water transport occurs, the potential energy change is significant and is highly correlated with the change in
N1, which implies that the potential energy change due to the mixing of water molecules and osmolytes is the major driving force for water transport driven by osmosis.
3.2.2. Kinetically Stable States
From
Figure 4, we observe kinetically stable states in terms of the numbers of water molecules in Compartment 1, the CNT and Compartment 2. Specifically, we find three main stable states, some of which, we believe, are global equilibrium states. In other words, the initial state in which water molecules are only in Compartment 1 transitions to one of three states: in one state, water occupies only Compartment 1 and the CNT (State I); in another state, water occupies only the CNT and Compartment 2 (State II); and in the other state, water occupies only Compartment 2 (State III). Here, State I can be considered a metastable state in the presence of osmolytes. Specifically, when we compare a separated state in which water molecules are in Compartment 1 and osmolytes are in Compartment 2 (State I) with a mixed state in which water molecules and osmolytes are in Compartment 2 (State II and State III), the mixed state is more energetically favorable than State I because of the strong interaction between water molecules and osmolytes. Therefore, the observation of State I is probably due to the kinetic barrier for the transition to State II or State III. In
Figure 4, the only exception to the above three states is the stable state in the case of
NO = 10 and
ISWO = 20, in which water molecules exist in all regions. We will discuss this state later, which we believe is another metastable state.
When the interaction between osmolytes and water molecules is weak, i.e.,
ISWO = 0.1, 1, and 5, the systems reach State I, and transfer to Compartment 2 does not occur. Additionally, when the interaction is intermediate, i.e.,
ISWO = 10, and the number of osmolytes is not sufficient to induce water transport (
NO = 10, 25, and 50), the system also reaches State I. However, when
NO = 75, 100, and 125, the stable states are State II. Moreover, when the interaction is strong, i.e.,
ISWO = 20, 50, even a small number of osmolytes can induce water transport and the states also become State II. Specifically, the cases with
NO = 25, 50, 75, and 100 for
ISWO = 20 and
NO = 10, 25, 50, and 75 for
ISWO = 50 show State II as a stable state. Here, interestingly, the addition of more osmolytes induces complete transfer of water molecules to Compartment 2, which means that the stable states are State III. Specifically, the cases with
NO = 125 for
ISWO = 20 and
NO = 100 and 125 for
ISWO = 50 show State III as a stable state. The observations for the stable states can be summarized in
Table 1.
Table 1 shows that when the interaction strength
ISWO and number of osmolytes
NO increase, the kinetically stable state shifts from State I to State II to State III. For example, from the right-most column (
NO = 125) in
Table 1, we easily note that State I, State II, and State III appear in order when
ISWO increases. Similarly, if we examine the states along the row of
ISWO = 10 (the states with the same
ISWO value of 10) in the table, we find that as
NO increases, State I and State II appear in order. Here, one interesting question is whether we can observe State III if we increase the number of osmolytes. To address this question, we could insert more osmolytes into Compartment 2. However, since the compartment space is limited in terms of the number of osmolytes, we use an alternative method in which we increase the ratio of the number of osmolytes to the number of water molecules (
NO/
Ntotal) by reducing the total number of water molecules instead of increasing the number of osmolytes.
To determine if higher ratios of
NO/
Ntotal in the cases of
ISWO = 10 induce State III, we prepare systems with fewer water molecules based on the system of
NO = 125,
ISWO = 10 and
Ntotal = 884 (
NO/
Ntotal = 0.141). In other words, from the final state at 200 ns shown in
Figure 4, we remove some of the water molecules, so that
Ntotal = 850, 800, 750, 700, 650, and 600, which implies that
NO/
Ntotal = 0.147, 0.156, 0.167, 0.179, 0192, and 0.208, respectively. From the 200 ns simulations of the systems, we calculate the occupancy ratio of water in Compartment 1 (
N1/
Ntotal) and the number of water molecules in the CNT (
NCNT). With the cases of
Ntotal =884 in
Figure 4, we summarize the simulation results as a function of
NO/
Ntotal in
Figure 7. The results clearly indicate that at high values of
NO/
Ntotal (>~0.17), the stable states are State III. In other words,
N1/
Ntotal and
NCNT are practically zero. This result may suggest that in the original system of 884 water molecules, if we include more osmolytes, i.e., the ratio of
NO/
Ntotal increases, we will observe State III as a stable state.
Another interesting feature from
Figure 7 is that the number of water molecules in the CNT largely fluctuates in the transition regime between State II and State III, whose
NO/
Ntotal value is approximately 0.15. This fluctuation in
NCNT is a typical behavior observed in the wetting-dewetting transition [
20].
One remaining issue that we have to address is whether the state observed in the case of
NO = 10 and
ISWO = 20 is a global equilibrium state or a metastable state. Here, a metastable state means that the state eventually transitions to another more stable state if we wait. Considering the stable states observed for other values of
NO (see the row of
ISWO = 20 in
Table 1), one would guess that State II is a more stable (equilibrium) state.
To examine whether State II is a more stable state for the case of
NO = 10 and
ISWO = 20, we first prepare five such initial states by removing some osmolytes from the final states (State II) with
NO = 25, 50, 75, 100, and 125 and
ISWO = 20 at 200 ns in
Figure 4, and run MD simulations to determine whether the states characterized as State II are stable. The steady state in terms of
N1 and
NCNT in
Figure 8a indicates that State II is kinetically stable. The relevant configurations for the originally observed state and State II are displayed in
Figure 8b,c, respectively. Then we compare the potential energies between the original state and State II, as displayed in
Figure 8d. From the comparison, we see that the potential energies of State II are lower than the potential energy of the original state observed in
Figure 4. In particular, since the potential energy difference (~700 kJ/mol on average) is significant, we conclude that the stable state in
Figure 4 is a metastable state, and State II is a global equilibrium state. The physical reason for the stability in the metastable state is probably associated with the state being stuck in a local energy minimum, which implies that for further investigations, we may need to examine the spatial arrangements of osmolytes and water molecules in detail.
Finally, if we consider the water transport process as a process to reach an equilibrium state, we can regard the water transport as a transition process from the initial state to State II or State III. In particular, we can call the transition to State III complete transfer of water because all water molecules are transferred from Compartment 1 to Compartment 2 due to the osmolytes.
3.2.3. Stochastic Nature of Water Transport Occurrence
Figure 4 shows that water transport occurs at a very early stage of the simulations when
ISWO is large (20 or 50), while transport does not occur when
ISWO is small (0.1, 1, or 5). However, when
ISWO is intermediate (10), water transport can occur or not depending on the number of osmolytes
NO; in
Figure 4, it occurs only when
NO is greater than 75.
For
ISWO = 10, to better understand the
NO criterion for the occurrence of water transport, we examine more systems between the two systems with
NO = 75 (transport observed) and 50 (no transport) in
Figure 4, which correspond to
NO = 70, 65, 60, 55, and 50. For each system, we perform five independent simulations. The simulation results are shown in
Figure 9.
Interestingly, for the systems of NO = 65, 60, and 55, the occurrence clearly shows a stochastic nature, which means that simulations with a given NO can exhibit water transport or not, and moreover, in the cases where water transport occurs, the waiting time for water transport to occur could vary. For example, when NO = 55, four cases of five display water transport, and their waiting times are 0.2 ns (Case 2), 117.8 ns (Case 3), 153.8 ns (Case 4), and 86.4 ns (Case 5).
For
NO = 70, however, we observe water transport in all five cases, and the waiting times are relatively narrowly distributed (16.2 ns (Case 1), 6.0 ns (Case 2), 14.2 ns (Case 3), 6.2 ns (Case 4), and 16.0 ns (Case 5)). In contrast, for the cases of
NO = 50, in only one case (Case 4) is water transport observed. From this comparison, as
NO decreases, the waiting time increases in that the waiting time is at least 200 ns for the case with no transport. The observation with
NO = 50 gives rise to a question: if we consider a longer simulation time, such as 1000 ns, than the value of 200 ns in
Figure 9, can we observe more cases showing water transport? To address this question, we further examine longer simulations for more cases in which one might expect no water transport from the 200 ns simulation results in
Figure 4.
We perform 1000 ns simulations for
NO = 52, 50, 45, 40, and 25. For each
NO, we perform five independent simulations. We display the simulation results in
Figure 10. The simulation results for
NO = 50 and 45 indicate that up to 200 ns, only two cases of five and only one case exhibit water transport, respectively, but up to 1000 ns, for each
NO, four cases of five show water transport. Therefore, when we increase the simulation time, the probability for water transport increases.
Figure 10 also shows that when
NO decreases, the number of water transport occurrences is reduced: five, four, four, three, and zero occurrences for
NO = 52, 50, 45, 40, and 25, respectively. Therefore, from the above discussion, we see the general trend that water transport is more likely to be observed when we include more osmolytes and wait longer; moreover, the waiting time is reduced when more osmolytes are added. Thus, although we do not observe water transport for
NO = 25 in
Figure 10, its observation may be possible if we wait much longer.
Finally, related to the discussion of kinetically stable states in
Figure 7, since the CNT is occupied by water at all times in
Figure 9 and
Figure 10, the transition due to the water transport in
Figure 9 and
Figure 10 is from the initial state to State II.
Figure 10 also shows that only the system of
NO = 25, which corresponds to
NO/
Ntotal = 0.028 (= 25/884), does not show water transport. Therefore, to make
Figure 7 more accurate, we must adjust the
NO/
Ntotal criterion value to distinguish between States I and II. However, since determining the exact value of
NO/
Ntotal for the boundary requires large-size ensembles and long-time simulations, which is not our major interest, we do not attempt to update
Figure 7 based on the results in
Figure 9 and
Figure 10. Again, the main point of
Figure 7 is that mainly three kinetically stable states are observed.
3.2.4. Characteristics of the Transition due to Water Transport
Here, we further study the details of water transport observed in
Figure 4,
Figure 9 and
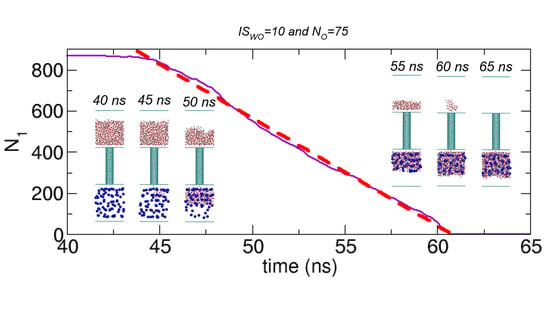
Figure 10. Interestingly, during water transport, the number of water molecules in Compartment 1 appears to linearly decrease with time. To quantify this time-transient behavior, we fit the data to a linear curve. One example is shown in
Figure 11a for the case of
NO = 75 and
ISWO = 10 (also see
Figure 4), where the data of
N1 versus time
t are best fitted with a linear curve using curve fitting in the xmgrace program. The resulting linear curve is
. This fitting is only valid for the time interval corresponding to the water transport. Remarkably, in this case, the correlation coefficient is greater than 0.99. Moreover, we calculate the transition time, which is the time for the transition from the initial state to State II or State III due to water transport. In this case, the transition time is 17.0 ns (= 60.8 ns − 43.8 ns). Additionally, from the linear fitting, we can determine the slope, which gives the transport rate. In this case, the transport rate is 52.3 water molecules/ns.
Since the transition time, linearity of the time-transient behavior, and transport rate can depend on the number of osmolytes
NO, we calculate them as functions of
NO. The results are shown in
Figure 11. Interestingly, according to
Figure 11b, when the number of osmolytes is sufficiently large (
NO > ~60), the transition time is ~ 20 ns almost regardless of the interaction strength
ISWO. Thus, if the osmotic force is sufficiently large beyond a certain value, the transition time reaches a limit, which is ~20 ns in this case. However, more osmolytes can reduce the waiting time for water transport, as we discuss in 3.2.3. When the number of osmolytes is smaller (
NO < ~60), the transition time depends on both
NO and
ISWO. In other words, when the osmotic force decreases by reducing
NO or
ISWO, the transition time increases, or no transition is observed.
In
Figure 11c, we quantitatively analyze the linearity of the time-transient behavior in water transport by calculating the correlation coefficient from the linear curve fitting to the data of
N1 versus time
t. Surprisingly, when
ISWO = 10, the correlation coefficient is very close to 1, which implies a constant flow of water during the transport. However, as
ISWO increases or when
NO is large, the transient behavior slightly deviates from linearity. This may reflect the nonlinear nature of interactions between molecules involved in water transport.
As shown in
Figure 11d, we calculate the transport rate from the slope obtained from the linear fitting shown in
Figure 11a. Noticeably, the three plots for
ISWO = 10, 20, and 50 are similar, and the common features are that when
NO > ~60, the transport rate is almost a constant value (~53 water molecules/ns) with small variations and when
NO < ~60, it decreases as
NO decreases. Note that the plots in
Figure 11b,d are inversely related to each other. The similarity of the transport rates regardless of
ISWO for the region of ~40 <
NO < ~60 is an interesting feature, but to better understand its physical origin, further detailed analysis may be required, which is beyond the scope of this work.
3.2.5. Effect of Interactions between Water Molecules on Transport
To understand how the interaction between water molecules affects the transport through a nanochannel, we compare the transport of water molecules with the transport of charge-removed water molecules in terms of the number of molecules in Compartment 1 as a function of time. The results are displayed in
Figure 12. Here, water and charge-removed water molecules represent strongly interacting and weakly interacting transported molecules, respectively. Charge-removed water molecules are prepared by removing the electric charges of water molecules or setting the electric charges to zero. The transport of charge-removed water molecules was discussed in detail in our previous work [
1].
In
Figure 12, first, we can compare the cases of water with the cases of charge-removed water in terms of equilibrium states. At equilibrium, while the number of molecules in Compartment 1 (
N1) for water is zero,
N1 for charge-removed water is not zero when
ISWO = 10 and 20; only when
ISWO = 50 does
N1 fluctuate near zero. Without electrostatic interactions, the interactions between charge-removed water molecules are weak, and thus, their physical behavior is similar to that of a gas. Therefore, if the interaction between molecules and osmolytes is not sufficiently strong, then the molecules can fill up the space, and as a result, when
ISWO = 10 and 20, some water molecules remain in Compartment 1 (see the inset of
Figure 12). When
ISWO increases,
N1 is reduced, and especially when
ISWO = 50,
N1 is near zero. However, for water, because of the strong interactions, its behavior is liquid-like, and aggregation of water molecules is energetically favorable. Moreover, because of the osmolytes in Compartment 2, being in Compartment 2 is more favorable than being in Compartment 1 for water molecules. Therefore, combining these factors, we understand that water molecules transfer to Compartment 2, and thus,
N1 for water is zero.
The difference in strength of the interaction between transported molecules can also explain the different time-transient behaviors in
Figure 12. As we discuss in
Section 3.2.4, the time-transient behavior of water is well fitted to a linear function of time, although some deviations from linearity occur when the interaction between water molecules and osmolytes is very strong. This linearity implies a constant transport rate of water molecules, which means that this rate does not depend on the concentration of water in Compartment 1 (zeroth-order rate). Physically, this occurs because water molecules tend to aggregate, so the local concentration of water is almost constant regardless of the available space in Compartment 1 (see the inset of
Figure 11a). In other words, near the CNT, water molecules are continuously supplied for transport, compensating for the loss of molecules due to transport. In a sense, water transport through a nanochannel driven by osmosis is similar to pulling a string at a constant speed.
However, as we discussed in our previous work [
1], the time-transient behavior of charge-removed water is well fitted to an exponential function of time, not a linear function, because the transport rate is proportional to the concentration (first-order rate). For the transport to occur, charge-removed water molecules in Compartment 1 should approach the CNT, and the probability of going into the CNT is proportional to the number of molecules in Compartment 1. Thus, as more molecules are transferred to Compartment 2, the concentration of molecules in Compartment 1 is reduced, and as a result, the transport rate decreases with time. Particularly, if we assume that the rate is proportional to the concentration of charge-removed water, then we mathematically show that the transient behavior exactly follows an exponential function of time. Therefore, the comparison in
Figure 12 indicates that the interaction between transported molecules apparently affects the transport kinetics.