Lung Cancer (LC) in HIV Positive Patients: Pathogenic Features and Implications for Treatment
Abstract
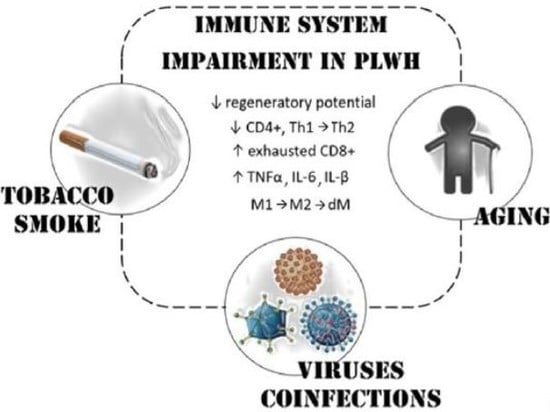
:1. HIV and Cancer Risk
2. Lung Cancer Features in HIV Infected Patients
3. Therapy
3.1. Surgery and Radiotherapy
3.2. Chemotherapy and Target Therapy
3.3. Immunotherapy
4. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global HIV & AIDS Statistics—2019 Fact Sheet|UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 10 October 2019).
- Lee, J.Y.; Dhakal, I.; Casper, C.; Noy, A.; Palefsky, J.M.; Haigentz, M.; Krown, S.E.; Ambinder, R.F.; Mitsuyasu, R.T. Risk of Cancer among Commercially Insured HIV-Infected Adults on Antiretroviral Therapy. J. Cancer Epidemiol. 2016, 2016, 2138259. [Google Scholar] [CrossRef] [Green Version]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Impact of the Expanded AIDS Surveillance Case Definition on AIDS Case Reporting—United States, First Quarter, 1993. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00020374.htm (accessed on 14 July 2019).
- Siegfried, N.; Uthman, O.A.; Rutherford, G.W. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst. Rev. 2010, 17, CD008272. [Google Scholar]
- Rubinstein, P.G.; Aboulafia, D.M.; Zloza, A. Malignancies in HIV/AIDS: From epidemiology to therapeutic challenges. AIDS 2014, 28, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiels, M.S.; Pfeiffer, R.M.; Hall, H.I.; Li, J.; Goedert, J.J.; Morton, L.M.; Hartge, P.; Engels, E.A. Proportions of Kaposi Sarcoma, Selected Non-Hodgkin Lymphomas, and Cervical Cancer in the United States Occurring in Persons With AIDS, 1980-2007. JAMA 2011, 305, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, S.; Engels, E. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS 2017, 25, 1032–1057. [Google Scholar]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, H. Malignancies in HIV-Infected and AIDS patients. Adv. Exp. Med. Biol. 2017, 1018, 167–179. [Google Scholar]
- Shiels, M.; Islam, J.; Rosenberg, P.; Hall, H.; Jacobson, E.; Engels, E. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann. Intern. Med. 2019, 168, 866–873. [Google Scholar] [CrossRef]
- Sigel, K.; Makinson, A.; Thaler, J. Lung cancer in persons with HIV. Curr. Opin. HIV AIDS 2017, 12, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Shiels, M.S.; Cole, S.R.; Kirk, G.D.; Poole, C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 2009, 52, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearz, A.; Vaccher, E.; Talamini, R.; Berretta, M.; Tirelli, U. Comment on ‘Lung cancer in the Swiss HIV Cohort Study: Role of smoking, immunodeficiency and pulmonary infection’. Br. J. Cancer 2012, 106, 1899–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mdodo, R.; Frazier, E.L.; Dube, S.R.; Mattson, C.L.; Sutton, M.Y.; Brooks, J.T.; Skarbinski, J. Cigarette Smoking Prevalence Among Adults With HIV Compared With the General Adult Population in the United States. Ann. Intern. Med. 2015, 162, 335. [Google Scholar] [CrossRef]
- Sigel, K.; Wisnivesky, J.; Gordon, K.; Dubrow, R.; Justice, A.; Brown, S.T.; Goulet, J.; Butt, A.A.; Crystal, S.; Rimland, D.; et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012, 26, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Rossouw, T.M.; Anderson, R.; Feldman, C. Impact of HIV infection and smoking on lung immunity and related disorders. Eur. Respir. J. 2015, 46, 1781–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, E.A.; Brock, M.V.; Chen, J.; Hooker, C.M.; Gillison, M.; Moore, R.D. Elevated Incidence of Lung Cancer Among HIV-Infected Individuals. J. Clin. Oncol. 2006, 24, 1383–1388. [Google Scholar] [CrossRef]
- Serrão, R.; Piñero, C.; Velez, J.; Coutinho, D.; Maltez, F.; Lino, S.; Sarmento, E.; Castro, R.; Tavares, A.P.; Pacheco, P.; et al. Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: The AGING POSITIVE study. Int. J. Infect. Dis. 2019, 79, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Kirk, G.D.; Merlo, C.; O’Driscoll, P.; Mehta, S.H.; Galai, N.; Vlahov, D.; Samet, J.; Engels, E.A. HIV Infection Is Associated with an Increased Risk for Lung Cancer, Independent of Smoking. Clin. Infect. Dis. 2007, 45, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Helleberg, M.; May, M.T.; Ingle, S.M.; Dabis, F.; Reiss, P.; Fätkenheuer, G.; Costagliola, D.; d’Arminio, A.; Cavassini, M.; Smith, C.; et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015, 29, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.A.; Merlo, C.A.; Kirk, G.D. Human Immunodeficiency Virus–Associated Lung Malignancies. Clin. Chest Med. 2013, 34, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Winstone, T.A.; Man, S.F.P.; Hull, M.; Montaner, J.S.; Sin, D.D. Epidemic of Lung Cancer in Patients With HIV Infection. Chest 2013, 143, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althoff, K.N.; McGinnis, K.A.; Wyatt, C.M.; Freiberg, W.M.; Gilbert, C.; Oursler, K.K.; Rimland, D.; Rodriguez-Barradas, R.D.; Park, L.S.; Skanderson, M.; et al. Comparison of Risk and Age at Diagnosis of Myocardial Infarction, End-Stage Renal Disease, and Non-AIDS-Defining Cancer in HIV-Infected Versus Uninfected Adults. Clin. Infect Dis. 2015, 60, 627–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgi, A.; Brodine, S.; Wegner, S.; Milazzo, K.; Wallace, M.R.; Spooner, K.; Blazes, D.L.; Agan, B.K. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer 2005, 104, 1505–1511. [Google Scholar] [CrossRef]
- Powles, T.; Robinson, D.; Stebbing, J.; Shamash, J.; Nelson, M.; Gazzard, B.; Mandelia, S.; Moller, H.; Bower, M. Highly Active Antiretroviral Therapy and the Incidence of Non–AIDS-Defining Cancers in People With HIV Infection. J. Clin. Oncol. 2009, 27, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.D.; Reekie, J.; Mocroft, A.; Reiss, P.; Ledergerber, B.; Gatell, J.; d’Arminio Monforte, A.; Phillips, A.; Lundgren, J.D.; Kirk, O.; et al. Long-term exposure to combination antiretroviral therapy and risk of death from specific causes. AIDS 2012, 26, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Haigentz, M.; Aboulafia, D.M. Lung Cancer in HIV Infection. Clin. Lung Cancer 2012, 13, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, K.; Flores, M.; Raub, W.; Saldana, M. Lung Cancer in Patients with Human Immunodeficiency Virus Infection Compared with Historic Control Subjects. Chest 2019, 102, 1704–1708. [Google Scholar] [CrossRef]
- Tirelli, U.; Spina, M.; Sandri, S.; Serraino, D.; Gobitti, C.; Fasan, M.; Sinicco, A.; Garavelli, P.; Ridolfo, A.L.; Vaccher, E. Lung carcinoma in 36 patients with human immunodeficiency virus infection. Cancer 2000, 88, 563–569. [Google Scholar] [CrossRef]
- Brock, M.V.; Hooker, C.M.; Engels, E.A.; Moore, R.D.; Gillison, M.L.; Alberg, A.J.; Keruly, J.C.; Yang, S.C.; Heitmiller, R.F.; Baylin, S.B.; et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: Implications for patient care. J. Acquir. Immune Defic. Syndr. 2006, 43, 47–55. [Google Scholar] [CrossRef]
- Marcus, J.L.; Chao, C.; Leyden, W.A.; Xu, L.; Yu, J.; Horberg, M.A.; Klein, D.; Towner, W.J.; Quesenberry, C.P.; Abrams, D.I.; et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Kiderlen, T.R.; Siehl, J.; Hentrich, M. HIV-associated lung cancer. Oncol. Res. Treat. 2017, 40, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Sigel, C.; Beasley, M.B.; Wisnivesky, J.; Crothers, K.; Bauml, J.; Hysell, K.; Emu, B.; Borsu, L.; Sigel, K. Clinically significant mutations in HIV-infected patients with lung adenocarcinoma. Br. J. Cancer 2017, 117, 1392–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichardo, R.; Go, R.F.; Qu, L.; Hussein, L.; Gupta, S. HIV-associated Non-small-cell Lung Cancer with Rearrangement of the Anaplastic Lymphoma Kinase Gene: A Report of Two Patients. Cureus 2019, 11, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Wang, L.; Cheng, Z.; Pei, Z.; Zhang, Z.; Li, Z.; Zhang, X.; Yan, D.; Xia, Q.; Feng, Y.; et al. Molecular Changes of Lung Malignancy in HIV Infection. Sci. Rep. 2018, 8, 13128. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Christensen, K.L.; Jedlicka, P.; Coletta, R.D.; Barón, A.E.; Harrell, J.C.; Horwitz, K.B.; Billheimer, D.; Heichman, K.A.; Welm, A.L.; et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J. Clin. Investig. 2009, 119, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Karim, B.O.; Rhee, K.-J.; Liu, G.; Yun, K.; Brant, S.R. Prom1 function in development, intestinal inflammation, and intestinal tumorigenesis. Front. Oncol. 2014, 4, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santerre, M.; Chatila, W.; Wang, Y.; Mukerjee, R.; Sawaya, B.E. HIV-1 Nef promotes cell proliferation and microRNA dysregulation in lung cells. Cell Cycle 2019, 18, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Coghill, A.E.; Shiels, M.S.; Suneja, G.; Engels, E.A. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J. Clin. Oncol. 2015, 33, 2376–2383. [Google Scholar] [CrossRef]
- Sigel, K.; Crothers, K.; Dubrow, R.; Krauskopf, K.; Jao, J.; Sigel, C.; Moskowitz, A.; Wisnivesky, J. Prognosis in HIV-infected patients with non-small cell lung cancer. Br. J. Cancer 2013, 109, 1974–1980. [Google Scholar] [CrossRef] [Green Version]
- Shiels, M.S.; Cole, S.R.; Mehta, S.H.; Kirk, G.D. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J. Acquir. Immune Defic. Syndr. 2010, 55, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Di Shen, X. Human immunodeficiency virus infection and mortality risk among lung cancer patients. Medcine 2018, 97, e0361. [Google Scholar] [CrossRef] [PubMed]
- Suneja, G.; Lin, C.C.; Simard, E.P.; Han, X.; Engels, E.A.; Jemal, A. Disparities in cancer treatment among patients infected with the human immunodeficiency virus. Cancer 2016, 122, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Hooker, C.M.; Meguid, R.A.; Hulbert, A.; Taylor, J.T.; Shin, J.; Wrangle, J.; Rodgers, K.; Lee, B.; Laskshmanan, S.; Brown, T.; et al. Human immunodeficiency virus infection as a prognostic factor in surgical patients with non-small cell lung cancer. Ann. Thorac. Surg. 2012, 93, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horberg, M.A.; Hurley, L.B.; Klein, D.B.; Follansbee, S.E.; Quesenberry, C.; Flamm, J.A.; Green, G.M.; Luu, T. Surgical outcomes in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Arch. Surg. 2006, 141, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- Sigel, K.M.; Stone, K.; Wisnivesky, J.P.; Park, L.S.; Kong, C.Y.; Silverberg, M.J.; Brown, S.; Goetz, M.; Rodriguez-Barradas, M.C.; Gibert, C.; et al. Short-term outcomes for lung cancer resection surgery in HIV infection. AIDS 2019, 33, 1353–1360. [Google Scholar] [CrossRef]
- Asakawa, A.; Horio, H.; Yamamichi, T.; Okui, M.; Harada, M. Clinical features of HIV-infected patients with non-small-cell lung cancer after lung resection. Gen. Thorac. Cardiovasc. Surg. 2019, 68, 38–42. [Google Scholar] [CrossRef]
- Alongi, F.; Giaj-Levra, N.; Sciascia, S.; Fozza, A.; Fersino, S.; Fiorentino, A.; Mazzola, R.; Ricchetti, F.; Buglione, M.; Buonfrate, D.; et al. Radiotherapy in patients with HIV: Current issues and review of the literature. Lancet Oncol. 2017, 18, e379–e393. [Google Scholar] [CrossRef]
- Coghill, A.E.; Suneja, G.; Rositch, A.F.; Shiels, M.S.; Engels, E.A. HIV Infection, Cancer Treatment Regimens, and Cancer Outcomes Among Elderly Adults in the United States. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Mounier, N.; Katlama, C.; Costagliola, D.; Chichmanian, R.M.; Spano, J.P. Drug interactions between antineoplastic and antiretroviral therapies: Implications and management for clinical practice. Crit. Rev. Oncol. Hematol. 2009, 72, 10–20. [Google Scholar] [CrossRef]
- Torres, H.A.; Mulanovich, V. Management of HIV infection in patients with cancer receiving chemotherapy. Clin. Infect. Dis. 2014, 59, 106–114. [Google Scholar] [CrossRef]
- Flepisi, B.T.; Bouic, P.; Sissolak, G.; Rosenkranz, B. Drug-drug interactions in HIV positive cancer patients. Biomed. Pharmacother. 2014, 68, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.H.; Venkataramanan, R.; Rudek, M.A. Pharmacotherapy in cancer patients with HIV/AIDS. Clin. Pharmacol. Ther. 2014, 95, 370–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berretta, M.; Caraglia, M.; Martellotta, F.; Zappavigna, S.; Lombardi, A.; Fierro, C.; Atripaldi, L.; Muto, T.; Valente, D.; De Paoli, P.; et al. Drug-drug interactions based on pharmacogenetic profile between highly active antiretroviral therapy and antiblastic chemotherapy in cancer patients with HIV infection. Front. Pharmacol. 2016, 7, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudek, M.A.; Chang, C.Y.; Steadman, K.; Johnson, M.D.; Desai, N.; Deeken, J.F. Combination antiretroviral therapy (cART) component ritonavir significantly alters docetaxel exposure. Cancer Chemother. Pharmacol. 2014, 73, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levêque, D.; Santucci, R.; Pavillet, J.; Herbrecht, R.; Bergerat, J.P. Paralytic ileus possibly associated with interaction between ritonavir/lopinavir and vincristine. Pharm. World Sci. 2009, 31, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Rudek, M.A.; Flexner, C.; Ambinder, R.F. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011, 12, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, T.; Tseng, A.L. Interactions between antiretrovirals and antineoplastic drug therapy. Clin. Pharmacokinet. 2005, 44, 111–145. [Google Scholar] [CrossRef]
- Alfa-Wali, M.; Allen-Mersh, T.; Antoniou, A.; Tait, D.; Newsom-Davis, T.; Gazzard, B.; Nelson, M.; Bower, M. Chemoradiotherapy for anal cancer in HIV patients causes prolonged CD4 cell count suppression. Ann. Oncol. 2012, 23, 141–147. [Google Scholar] [CrossRef]
- Bryant, A.K.; Huynh-Le, M.-P.; Simpson, D.R.; Gupta, S.; Sharabi, A.B.; Murphy, J.D. Association of HIV Status With Outcomes of Anal Squamous Cell Carcinoma in the Era of Highly Active Antiretroviral Therapy. JAMA Oncol. 2018, 4, 120. [Google Scholar] [CrossRef]
- Smith, D.M.; Salters, K.A.; Eyawo, O.; Franco-Villalobos, C.; Jabbari, S.; Wiseman, S.M.; Press, N.; Montaner, J.S.G.; Man, S.F.P.; Hull, M.; et al. Mortality among people living with HIV/AIDS with non-small-cell lung cancer in the modern HAART Era. AIDS Care 2018, 30, 936–942. [Google Scholar] [CrossRef]
- Okuma, Y.; Hosomi, Y.; Imamura, A. Lung cancer patients harboring epidermal growth factor receptor mutation among those infected by human immunodeficiency virus. Onco Targets. Ther. 2015, 8, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, V.C.; Venkataramanan, R.; Parise, R.A.; Christner, S.M.; Gramignoli, R.; Strom, S.C.; Rudek, M.A.; Beumer, J.H. Ritonavir and efavirenz significantly alter the metabolism of erlotinib—An observation in primary cultures of human hepatocytes that is relevant to HIV patients with cancer. Drug Metab. Dispos. 2013, 41, 1843–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-H.; McCann, C.D.; Mota, T.M.; Wang, C.; Lipkin, S.M.; Jones, R.B. Have Cells Harboring the HIV Reservoir Been Immunoedited? Front. Immunol. 2019, 10, 1842. [Google Scholar] [CrossRef] [Green Version]
- Mylvaganam, G.; Yanez, A.G.; Maus, M.; Walker, B.D. Toward T Cell-Mediated Control or Elimination of HIV Reservoirs: Lessons From Cancer Immunology. Front. Immunol. 2019, 10, 2109. [Google Scholar] [CrossRef]
- Kythreotou, A.; Mauri, F.A.; Shiner, R.; Suardi, E.; Dalla Pria, A.; Akarca, A.; Trivedi, P.; Gupta, N.; Marafioti, T.; Newsom-Davis, T.; et al. The influence of HIV status on programmed-death ligands expression in non-small cell lung cancer. Lung Cancer 2018, 115, S4. [Google Scholar] [CrossRef]
- Domblides, C.; Antoine, M.; Hamard, C.; Rabbe, N.; Rodenas, A.; Vieira, T.; Crequit, P.; Cadranel, J.; Lavolé, A.; Wislez, M. Non-small cell lung cancer from HIV-infected patients expressed PD-L1 with marked inflammatory infiltrates. AIDS 2017, 32, 1. [Google Scholar] [CrossRef]
- Scilla, K.A.; Zandberg, D.P.; Bentzen, S.M.; Mainor, C.; Heath, J.; Ioffe, O.B.; Cellini, A.L.; Edelman, M.J.; Riedel, D.J.; Feliciano, J.L. Case-control study of PD-1, PD-L1 and B7-H3 expression in lung cancer patients with and without human immunodeficiency virus (HIV) infection. Lung Cancer 2018, 123, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.R.; Purvis, I.J.; Labak, C.M.; Guda, M.R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017, 6, 66–75. [Google Scholar] [PubMed]
- Okuma, Y.; Hishima, T.; Kashima, J.; Homma, S. High PD-L1 expression indicates poor prognosis of HIV-infected patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2018, 67, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pantazis, N.; Martin, G.E.; Hickling, S.; Hurst, J.; Meyerowitz, J.; Willberg, C.B.; Robinson, N.; Brown, H.; Fisher, M.; et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016, 12, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Pinato, D.; Kythreotou, A.; Mauri, F.; Suardi, E.; Allara, E.; Shiner, R.; Akarca, A.; Trivedi, P. Functional immune characterization of HIV-associated non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1490. [Google Scholar] [CrossRef]
- Cook, M.R.; Kim, C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019, 5, 1049–1054. [Google Scholar] [CrossRef]
- Uldrick, T.S.; Gonçalves, P.H.; Abdul-Hay, M.; Claeys, A.J.; Emu, B.; Ernstoff, M.S.; Fling, S.P.; Fong, L.; Kaiser, J.C.; Lacroix, A.M.; et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer—A Phase 1 Study. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Bari, S.; Chan, A.; Jain, S.R.; Hostler, C.J. Outcomes of programmed cell death protein 1 (PD-1) and programmed death-ligand 1(PD-L1) inhibitor therapy in HIV patients with advanced cancer. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Spano, J.-P.; Veyri, M.; Gobert, A.; Guihot, A.; Perré, P.; Kerjouan, M.; Brosseau, S.; Cloarec, N.; Montaudié, H.; Helissey, C.; et al. Immunotherapy for cancer in people living with HIV. AIDS 2019, 33, F13–F19. [Google Scholar] [CrossRef]
- Ostios-Garcia, L.; Faig, J.; Leonardi, G.C.; Adeni, A.E.; Subegdjo, S.J.; Lydon, C.A.; Rangachari, D.; Huberman, M.S.; Sehgal, K.; Shea, M.; et al. Safety and Efficacy of PD-1 Inhibitors Among HIV-Positive Patients With Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- McCullar, B.; Alloway, T.; Martin, M. Durable complete response to nivolumab in a patient with HIV and metastatic non-small cell lung cancer. J. Thorac. Dis. 2017, 9, E540–E542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guihot, A.; Marcelin, A.; Massiani, M.; Samri, A.; Soulie, C.; Autran, B.; Spano, J. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann. Oncol. 2018, 29, 517–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althoff, K.N.; Gebo, K.A.; Moore, R.D.; Boyd, C.M.; Justice, A.C.; Wong, C.; Lucas, G.M.; Klein, M.B.; Kitahata, M.M.; Crane, H.; et al. Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: A collaboration of cohort studies. Lancet HIV 2019, 6, e93–e104. [Google Scholar] [CrossRef]
- Goedert, J.J.; Hosgood, H.D.; Biggar, R.J.; Strickler, H.D.; Rabkin, C.S. Screening for Cancer in Persons Living with HIV Infection. Trends Cancer 2016, 2, 416–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- National Lung Screening Trial Research Team; Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S. Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [CrossRef] [Green Version]
- Makinson, A.; Eymard-Duvernay, S.; Raffi, F.; Abgrall, S.; Bommart, S.; Zucman, D.; Valour, F.; Cheret, A.; Poizot-Martin, I.; Duvivier, C.; et al. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. AIDS 2016, 30, 573–582. [Google Scholar] [CrossRef]
- Shi, W.; Zhou, L.; Peng, X.; Ren, H.; Wang, Q.; Shan, F.; Zhang, Z.; Liu, L.; Shi, Y. HIV-infected patients with opportunistic pulmonary infections misdiagnosed as lung cancers: The clinicoradiologic features and initial application of CT radiomics. J. Thorac. Dis. 2019, 11, 2274–2286. [Google Scholar] [CrossRef]
- Little, R.F. Cancer clinical trials in persons with HIV infection. Curr. Opin. HIV AIDS 2017, 12, 84–88. [Google Scholar] [CrossRef]
- Uldrick, T.; Ison, G.; Rudek, M.; Noy, A.; Schwartz, K.; Bruinooge, S. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology–Friends of Cancer Research HIV Working Group. J. Clin. Oncol. 2017, 35, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Suneja, G.; Boyer, M.; Yehia, B.R.; Shiels, M.S.; Engels, E.A.; Bekelman, J.E.; Long, J.A. Cancer Treatment in Patients With HIV Infection and Non–AIDS-Defining Cancers: A Survey of US Oncologists. J. Oncol. Pract. 2015, 11, e380–e387. [Google Scholar] [CrossRef] [PubMed]


| Lung Cancer Features | Subgroups | HIV + Patients | General Population | References |
|---|---|---|---|---|
| Standardized incidence ratio (SIR) | - | 2.6 (2.1–3.1) | - | Shiels MS et al. J Acquir Immune Defic Syndr 2009 |
| Median age at cancer diagnosis | - | 44–52 years | 70 years | Shiels MS, et al. Ann Intern Med 2010 |
| Gender | male | 86% | 57.8% | Spano JP et al. Med Oncol 2004 |
| Race | white | no current available data | ||
| black | ||||
| Stage at diagnosis | stage III or IV | 77–100% | 75% | Kiderlen TR et al. Oncol Res Treat 2017 |
| Histological type | adenocarcinoma | 49% | 36–50% | Kiderlen TR et al. Oncol Res Treat 2017 |
| squamous cell carcinoma | 20% | 27–30% | ||
| large cell carcinoma | 3% | 4–18% | ||
| small cell carcinoma | 15% | 3–9% | ||
| Survival rate | 2-year | 10% | 31% | Biggar RJ et al. J Acquir Immune Defic Syndr 2005 |
| 5-year | 10% | 19% | Marcus JL et al. Cancer Epidemiol Biomarkers Prev 2015 | |
| Trial NCT (Acronimus) | Drug Tested | Trial Title | Endpoint and Results** | Status |
|---|---|---|---|---|
| 01296113 (CHIVA) | Carboplatin plus pemetrexed | Chemotherapy for LC in HIV+ pts with advanced non-squamous NSCLC | DCR after 4 cycles of carboplatin plus pemetrexed | Completed No results posted |
| 00276588 | Gemcitabine plus carboplatin followed by paclitaxel | Gemcitabine and carboplatin followed by paclitaxel in pts with PS = 2,3 or other significant co-morbidity (HIV or s/p organ transplantation) in advanced NSCLC | Sequential ChT is well tolerated and active. The survival is comparable to that of other regimens utilized in PS = 2 pts with superior tolerability. The prognosis for these pts is very poor even with treatment | Completed Results posted |
| 02134886 | Erlotinib | Erlotinib Hydrochloride in treating NSCLC that is metastatic or cannot be removed by surgery in pts with HIV | Safety, tolerability and MTD of erlotinib in combination with ART | Terminated (Poor enrolment) |
| 01822522 | Cabozantinib | Cabozantinib S-Malate in treating pts with advanced solid tumors and HIV | Safety, tolerability and MTD of cabozantinib | Recruiting |
| 03304093 (CHIVA2) | Nivolumab | Immunotherapy by nivolumab for HIV+ pts with advanced NSCLC | DCR | Recruiting |
| 03767465 (PembroHIV) | ICI | Treatment with ICIs of HIV-infected subjects with cancer (advanced melanoma or other cancers in which the use of ICIs is clinically indicated) | Changes in HIV-viral load and immune-phenotype of cellular populations | Completed No results posted |
| 02408861 | Ipilimumab plus nivolumab | Nivolumab and ipilimumab in treating pts with HIV associated relapsed or refractory classical HL or solid tumors (comprising LC) that are metastatic or cannot be removed by surgery | MTD of nivolumab, ORR, immune function, change in immune status/HIV viral load | Recruiting |
| 00791336 | Nelfinavir with RT and ChT | Study to evaluate using nelfinavir with chemoradiation for NSCLC | PCR | Terminated (Poor enrolment) |
| 01249443 | Paclitaxel plus carboplatin | Paclitaxel and carboplatin in treating pts with metastatic or recurrent solid tumors (comprising NSCLC) and HIV | - Safety, tolerability of vorinostat in combination with ChT. - MTD of the combination | Terminated (Inadequate accrual rate) No results posted |
| 01567722 | - | Collecting and studying tissue samples from pts with HIV-Associated Malignancies (diffuse large B-cell lymphomas, LC, anal cancer and cervical cancer) | - To obtain high-quality tissue from pts with HIV-1 malignancy - To study clinical, genetic, and immunologic parameters with prognostic significance and/or involved in the initiation/progression of HIV-1 malignancies, including complete NGS of HIV-associated cancers | Recruiting |
| 01748136 (NA_00036809) | - | Screening for LC in the HIV pts | Differences in stage distribution of HIV-seropositive pts at LC diagnosis between those who are screened by spiral CT and historic controls | Completed No results posted |
| 01207986 (EP48 HIV CHEST) | - | Early LC diagnosis in HIV infected population with an important smoking history with low dose CT: a pilot study | Prevalence of LC detected by low-dose CT scan | Completed No results posted |
| 00491335 | - | HIV infection and tobacco use among injection drug users in Baltimore, Maryland: a pilot study of biomarkers | - To characterize smoking habits and compare tobacco use among HIV-infected and uninfected drug users - To compare serum nicotine levels and spirometry results, as a marker of tobacco use and a marker of damage to lung function, respectively | Completed No results posted |
| 01447589 (NelfLung) | Nelfinavir plus radical radiotherapy | Radical lung radiotherapy plus nelfinavir | MTD of nelfinavir | Withdrawn (poor enrolment ) |
| 00589056 | Nelfinavir with RT and ChT | Nelfinavir, RT, cisplatin and etoposide in treating (HIV-uninfected) pts with stage III NSCLC that cannot be removed by surgery | Nelfinavir administered with concurrent ChT-RT is associated with acceptable toxic effects and a promising ORR, local failure, PFS and OS in unresectable NSCLC | Completed |
| 03367754 | Pembrolizumab | A single dose of pembrolizumab in HIV-infected people | Safety of pembrolizumab in PLWH who have a low CD4+ T cell count despite taking medicines that control HIV replication | Recruiting |
| 02595866 | Pembrolizumab | Pembrolizumab in pts with HIV and relapsed/refractory or disseminated malignant neoplasm (comprising NSCLC) | - Frequency of observed AEs - Incidence of immune-related AEs of clinical interest - Incidence of cART-related AEs | Recruiting |
| 03858491 (POP-NSCLC) | Osimertinib | Pharmacokinetic Boosting of Osimertinib in pts with EGFR-mutated NSCLC | Evaluate if systemic exposure of osimertinib is increased when it is co-administered with anti-HIV drug cobicistat | Not yet recruiting |
| 03706625 (IDeATIon) | - | Integrated discovery of new immuno-molecular actionable biomarkers for tumors with immune-suppressed environment | Analyse tumor biomarkers on frozen biopsy for three types of cancer (non-HL, LC and glioma) | Not yet recruiting |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frega, S.; Ferro, A.; Bonanno, L.; Guarneri, V.; Conte, P.; Pasello, G. Lung Cancer (LC) in HIV Positive Patients: Pathogenic Features and Implications for Treatment. Int. J. Mol. Sci. 2020, 21, 1601. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051601
Frega S, Ferro A, Bonanno L, Guarneri V, Conte P, Pasello G. Lung Cancer (LC) in HIV Positive Patients: Pathogenic Features and Implications for Treatment. International Journal of Molecular Sciences. 2020; 21(5):1601. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051601
Chicago/Turabian StyleFrega, Stefano, Alessandra Ferro, Laura Bonanno, Valentina Guarneri, PierFranco Conte, and Giulia Pasello. 2020. "Lung Cancer (LC) in HIV Positive Patients: Pathogenic Features and Implications for Treatment" International Journal of Molecular Sciences 21, no. 5: 1601. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051601