Brain–Heart Axis and Biomarkers of Cardiac Damage and Dysfunction after Stroke: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
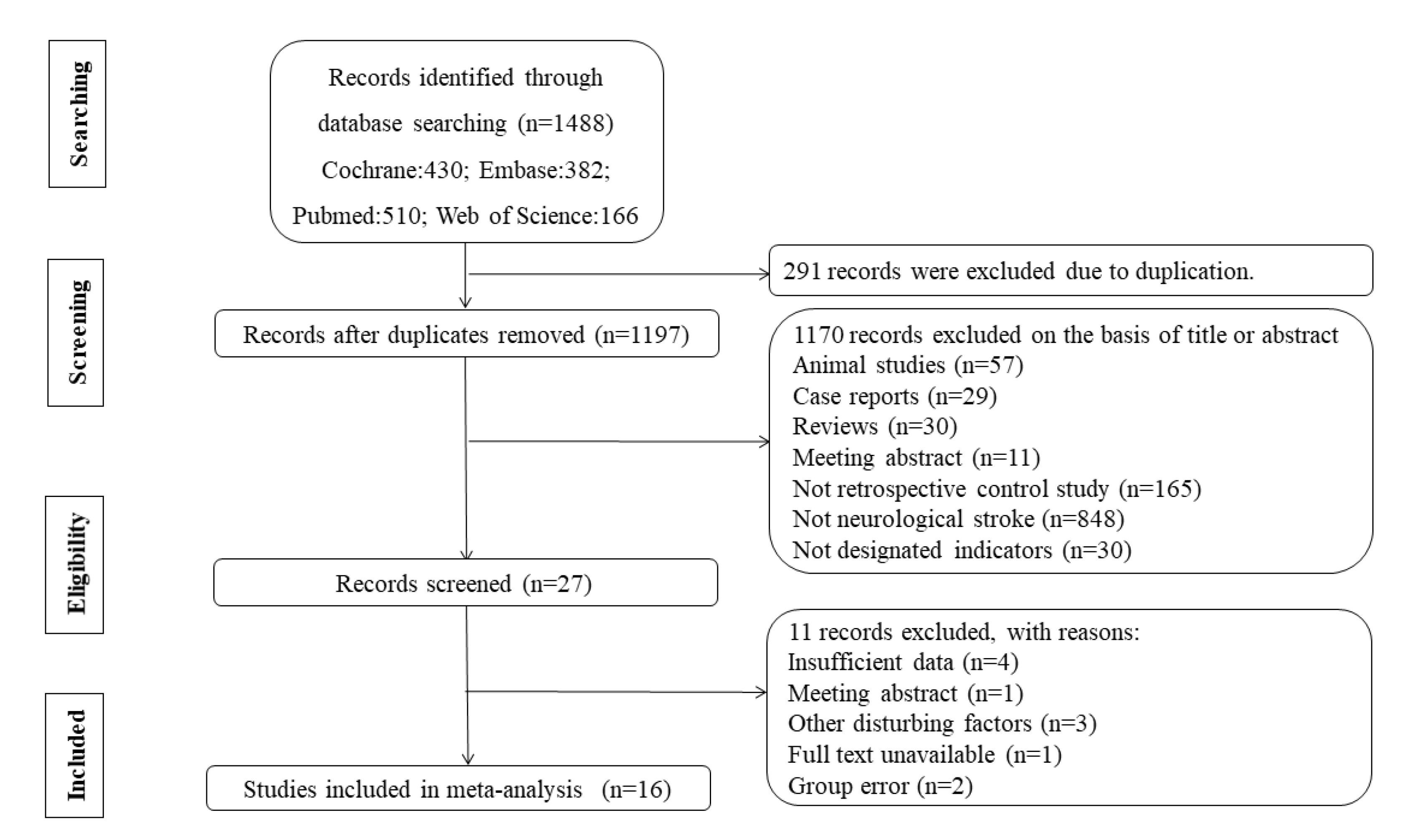
2.1. Study Selection
2.2. Data Extraction and Quality Assessment
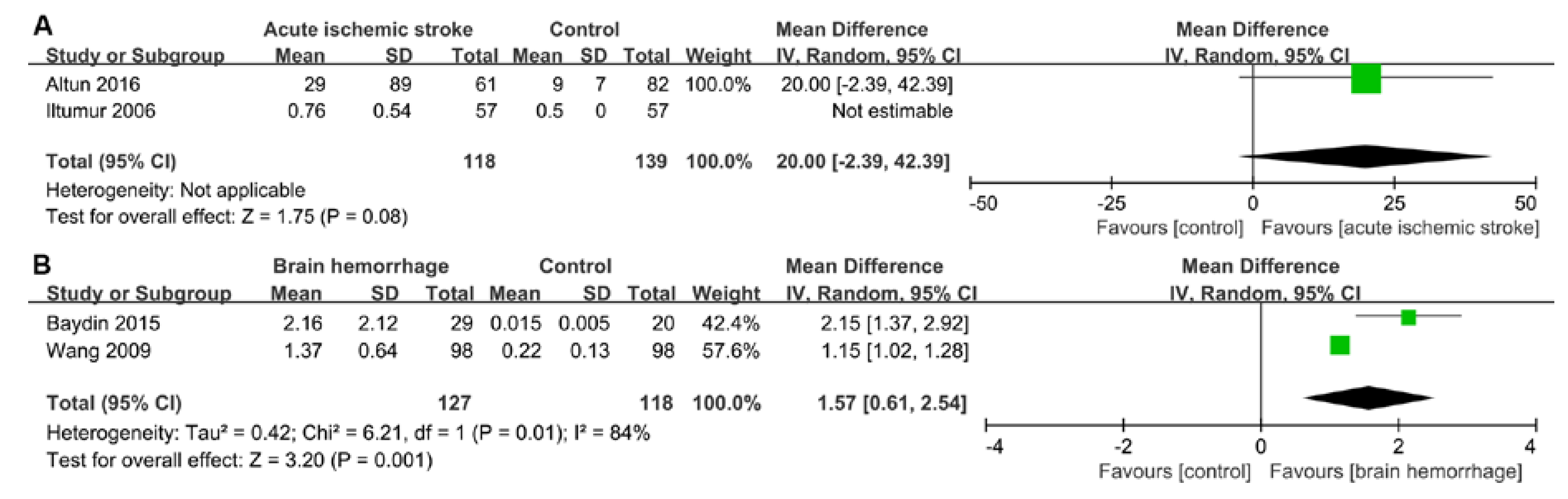
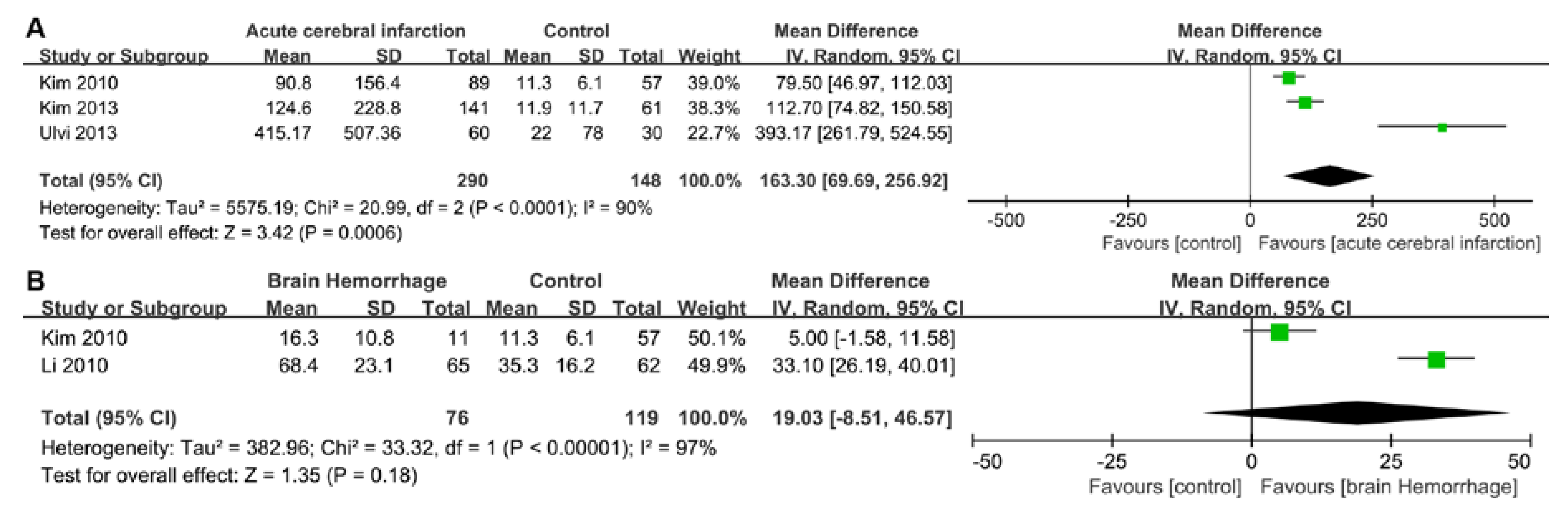
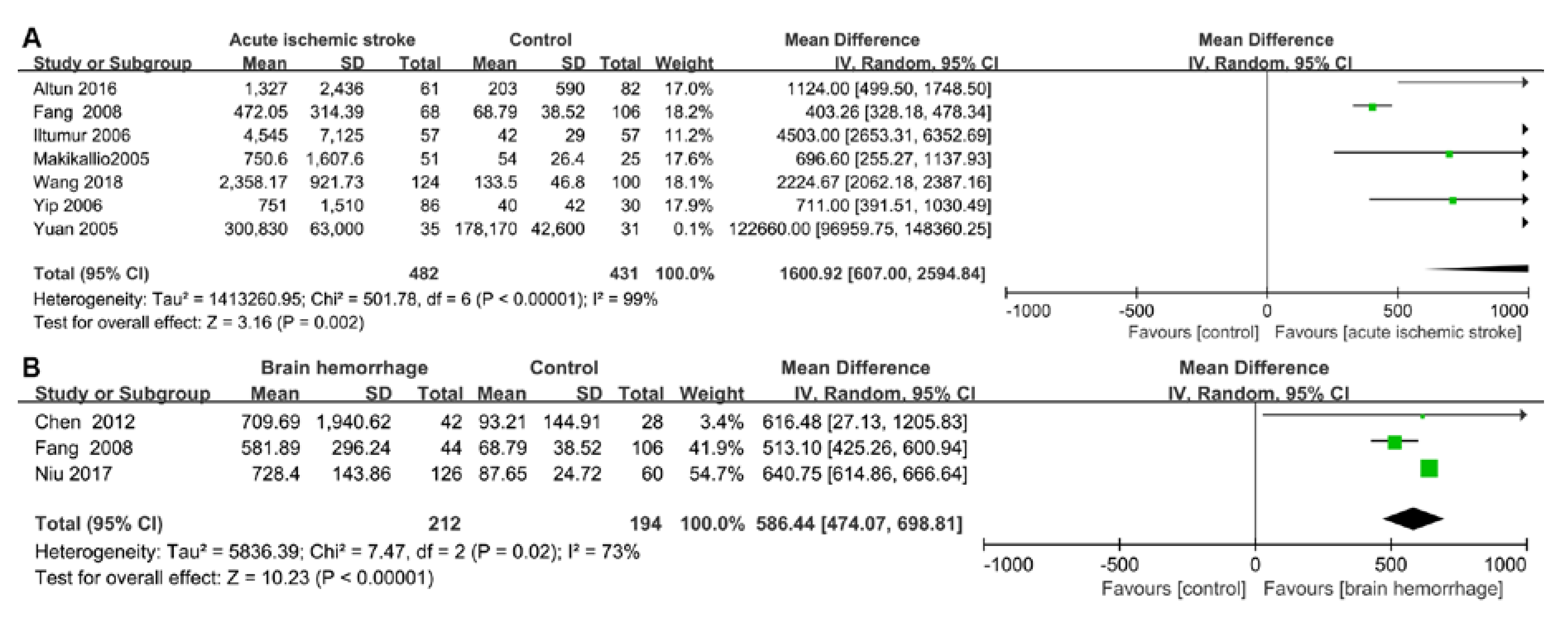
2.3. Pooled Results from Literature-Based Meta-Analysis
3. Discussion
3.1. Cardiac Damage Induced by Ischemic Stroke
3.2. Cardiac Damage induced by a Brain Hemorrhage
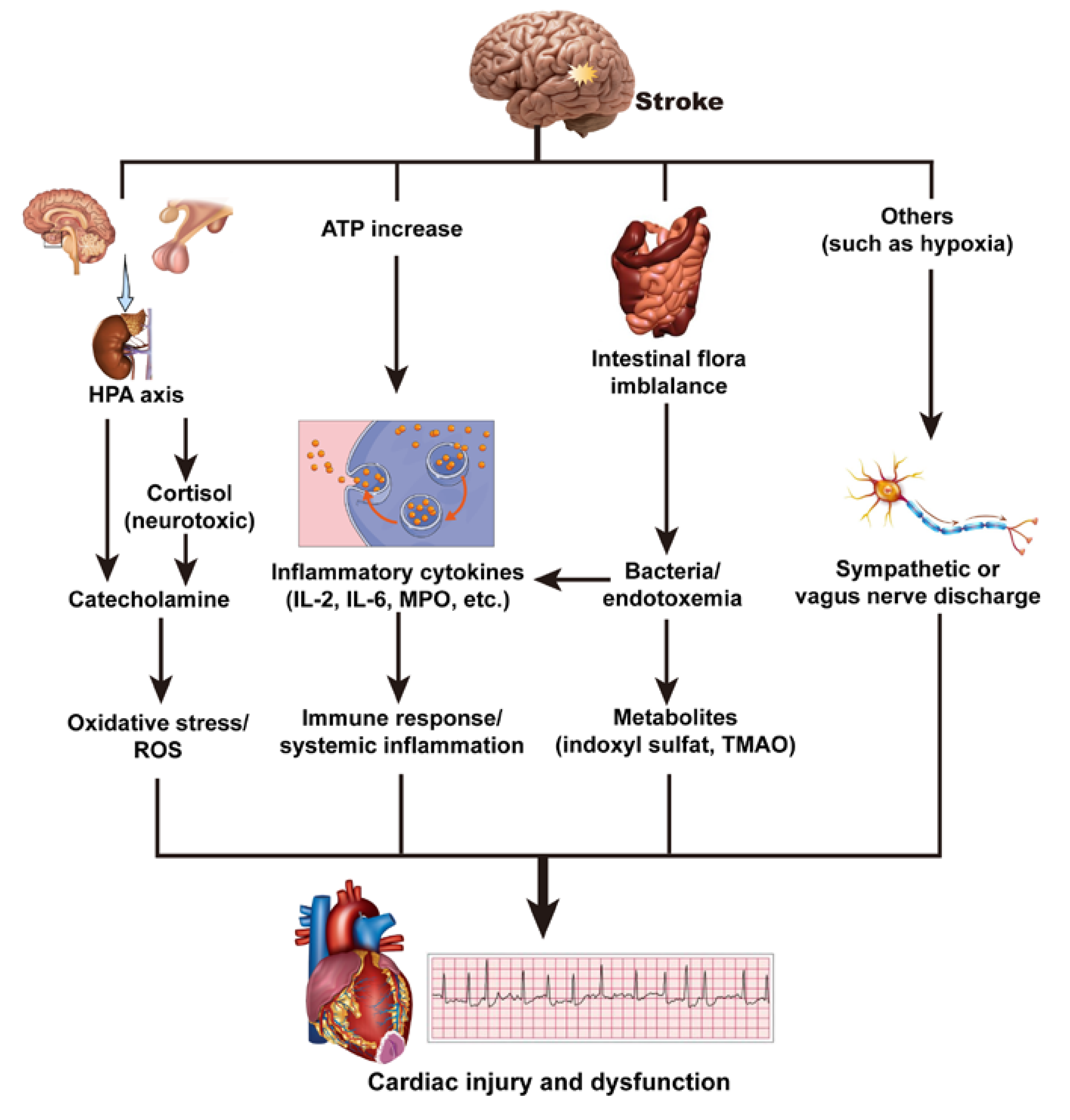
3.3. Mechanism of Brain–Heart Interaction after a Stroke
3.3.1. Hypothalamic–Pituitary–Adrenal Axis and Catecholamine Surge
3.3.2. Immune Response and Systemic Inflammation
3.3.3. Intestinal Flora Imbalance
3.3.4. Others
3.4. Limitations
4. Materials and Methods
4.1. Searching Strategies
4.2. Literature Screening and Selection Criteria
4.3. Data Extraction
4.4. Statistical Analysis
5. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| cTn | Cardiac troponin |
| BNP | Brain natriuretic peptide |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| cTnT | Cardiac troponin T |
| cTnI | Cardiac troponin I |
| cTnC | Cardiac troponin C |
| NOS | Newcastle–Ottawa Quality Assessment Scale |
| SAH | Subarachnoid hemorrhage |
| WMD | Weighted mean difference |
| CI | Confidence interval |
| ECG | Electrocardiogram |
| LVEF | Left ventricular ejection fraction |
| NIHSS | U.S. National Institutes of Health Stroke Scale |
| HPA | Hypothalamic–pituitary–adrenal |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| MPO | Myeloperoxidase |
| ROS | Reactive oxygen species |
| TMAO | Trimethylamine-N-oxide |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| MeSH | Medical subject headings |
References
- Lunardi Baccetto, S.; Lehmann, C. Microcirculatory Changes in Experimental Models of Stroke and CNS-Injury Induced Immunodepression. Int. J. Mol. Sci. 2019, 20, 5184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Ischemic stroke and mitochondria: Mechanisms and targets. Protoplasma 2019, 257, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Baydin, A.; Amanvermez, R.; Tuncel, Ö.K.; Ocak, M.; Meric, M.; Cokluk, C. Ischemia-modified albumin is not better than creatine kinase-MB and cardiac troponin i in predicting a cardiac injury in nontraumatic subarachnoid hemorrhage. Am. J. Emerg. Med. 2015, 33, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.V.; Finlay, C.W.; Jacob, S.; Raina, S.K.; Lee, R.W.; Hinduja, A. Can admission BNP level predict outcome after intravenous thrombolysis in acute ischemic stroke? Neurologist 2019, 24, 6–9. [Google Scholar] [CrossRef]
- Fujiki, Y.; Matano, F.; Mizunari, T.; Murai, Y.; Tateyama, K.; Koketsu, K.; Kubota, A.; Kobayashi, S.; Yokota, H.; Morita, A. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2018, 129, 870–875. [Google Scholar] [CrossRef] [Green Version]
- Tahsili-Fahadan, P.; Geocadin, R.G. Heart-Brain Axis: Effects of Neurologic Injury on Cardiovascular Function. Circ. Res. 2017, 120, 559–572. [Google Scholar] [CrossRef]
- Nagai, M.; Hoshide, S.; Kario, K. The insular cortex and cardiovascular system: A new insight into the brain-heart axis. J. Am. Soc. Hypertens: JASH 2010, 4, 174–182. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Y.; Teng, J. Levels of plasma N-terminal pro-brain natriuretic peptide and D-dimer on the prognosis of patients with acute cerebral infarction. Pak. J. Med. Sci. 2018, 34, 855–858. [Google Scholar] [CrossRef]
- Iltumur, K.; Karabulut, A.; Apak, I.; Aluclu, U.; Ariturk, Z.; Toprak, N. Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am. Heart J. 2006, 151, 1115–1122. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.Y.; Park, S.H.; Jang, H.C.; Lim, E.J.; Chang, S.J.; Lee, S.S. Plasma B-type natriuretic peptide level in patients with acute cerebral infarction according to infarction sub-type and infarction volume. Int. J. Med. Sci. 2012, 10, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.; Zhen, X.; Hu, H.; Li, C.; Zhan, W.; Zeng, H. Research of Plasma Concentration of Amino-Terminal Pro-Brain Natriuretic Peptide and Prognosis Ipact in Ptients Suffering from of Cerebrovascular Accident. Chin. J. Arterioscler. 2008, 16, 311–313. [Google Scholar]
- Suleiman, H.M.; Aliyu, I.S.; Abubakar, S.A.; Isa, M.S.; El-Bashir, J.M.; Adamu, R.; Ibrahim, M.Z.; Mohammed, A.; Yusuf, R.; Manu, M.; et al. Cardiac Troponin T and creatine kinase MB fraction levels among patients with acute ischemic stroke in Nigeria. Niger. J. Clin. Pract. 2017, 20, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhao, M.; Xu, C.; Zhang, T.; Jia, Y.; Wang, T.; Zhu, B. Diagnostic Roles of Postmortem cTn I and cTn T in Cardiac Death with Special Regard to Myocardial Infarction: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Teng, F. Clinical significance of plasma levels of N-terminal brain natriuretic peptide and copeptin in patients with acute cerebral haemorrhage. Biomed. Res. -India 2017, 28, 5638–5641. [Google Scholar]
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart disease and stroke statistics--2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, e25–e146. [Google Scholar] [CrossRef] [Green Version]
- Grysiewicz, R.A.; Thomas, K.; Pandey, D.K. Epidemiology of ischemic and hemorrhagic stroke: Incidence, prevalence, mortality, and risk factors. Neurol. Clin. 2008, 26, 871–895. [Google Scholar] [CrossRef]
- Hartling, L.; Milne, A.; Hamm, M.P.; Vandermeer, B.; Ansari, M.; Tsertsvadze, A.; Dryden, D.M. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013, 66, 982–993. [Google Scholar] [CrossRef]
- Altun, I.; Unal, Y.; Basaran, O.; Akin, F.; Emir, G.K.; Kutlu, G.; Biteker, M. Increased Epicardial Fat Thickness Correlates with Aortic Stiffness and N-Terminal Pro-Brain Natriuretic Peptide Levels in Acute Ischemic Stroke Patients. Tex. Heart Inst. J. 2016, 43, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y. Dynamic changes of plasma cardiac troponin-I and its clinical significance in patients with acute cerebral hemorrhage. Acta Univ. Sci. Med. Chongqing 2009, 34, 971–973. [Google Scholar]
- Kim, M.H.; Kang, S.Y.; Kim, M.C.; Lee, W.I. Plasma Biomarkers in the Diagnosis of Acute Ischemic Stroke. Ann. Clin. Lab. Sci. 2010, 40, 336–341. [Google Scholar] [PubMed]
- Ulvi, H.; Bebek, I.; Demir, R.; Özel, L.; Düdükçü, M.; Aygül, R. Brain natriuretic peptide level and microproteinuria in patients with ischemic stroke. Turk. Klin. J. Med. Sci. 2013, 33, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dai, J.; Li, X.; Huang, G.; Huang, H. Relationship between the plasma levels of brain natriuretic peptide and high sensitive C-reactive protein and brain. edema around hematoma in patients with intracerebral. hemorrhage. China J. Mod. Med. 2010, 20, 2012–2015. [Google Scholar]
- Makikallio, A.M.; Makikallio, T.H.; Korpelainen, J.T.; Vuolteenaho, O.; Tapanainen, J.M.; Ylitalo, K.; Sotaniemi, K.A.; Huikuri, H.V.; Myllyla, V.V. Natriuretic peptides and mortality after stroke. Stroke 2005, 36, 1016–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Zeng, Q.; Wang, J.; Yang, J.; Zhang, Z.-k.; Zhang, Y. Clinical significance of Nt-proBNP1-76 in patients with acute cerebral vascular diseases. J. China Med. Univ. 2005, 34, 49–51. [Google Scholar]
- Yip, H.K.; Sun, C.K.; Chang, L.T.; Chen, M.C.; Liou, C.W. Time course and prognostic value of plasma levels of N-terminal pro-brain natriuretic peptide in patients after ischemic stroke. Circ. J. 2006, 70, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Yang, Q.; Wu, Z. Plasma concentration of N-terminal fragment of pro-brain natriuretic peptide and prognosis in patients with acute cerebral hemorrhage. J. Apoplexy Nerv. Dis. 2012, 29, 35–37. [Google Scholar]
- Yoshimura, S.; Toyoda, K.; Ohara, T.; Nagasawa, H.; Ohtani, N.; Kuwashiro, T.; Naritomi, H.; Minematsu, K. Takotsubo cardiomyopathy in acute ischemic stroke. Ann. Neurol. 2008, 64, 547–554. [Google Scholar] [CrossRef]
- Tay, A.; Tamam, Y.; Yokus, B.; Ustundag, M.; Orak, M. Serum myeloperoxidase levels in predicting the severity of stroke and mortality in acute ischemic stroke patients. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1983–1988. [Google Scholar]
- Mogi, M.; Kawajiri, M.; Tsukuda, K.; Matsumoto, S.; Yamada, T.; Horiuchi, M. Serum levels of renin-angiotensin system components in acute stroke patients. Geriatr. Gerontol. Int. 2014, 14, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Hänggi, J.; Klein, C.; Topka, M.S.; Hiestand, T.; Levinson, R.A.; Jurisic, S.; Lüscher, T.F.; Ghadri, J.-R.; Jäncke, L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019, 40, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.; MacGregor, L.; Lees, K.R.; Diener, H.C.; Hacke, W.; Davis, S. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007, 38, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Rauh, R.; Fischereder, M.; Spengel, F.A. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996, 27, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Lavy, S.; Yaar, I.; Melamed, E.; Stern, S. The effect of acute stroke on cardiac functions as observed in an intensive stroke care unit. Stroke 1974, 5, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doğan, A.; Tunç, E.; Oztürk, M.; Erdemoğlu, A.K. Comparison of electrocardiographic abnormalities in patients with ischemic and hemorrhagic stroke. Anadolu Kardiyol. Derg: AKD = Anatol. J. Cardiol. 2004, 4, 135–140. [Google Scholar]
- Khechinashvili, G.; Asplund, K. Electrocardiographic changes in patients with acute stroke: A systematic review. Cereb. Dis. 2002, 14, 67–76. [Google Scholar] [CrossRef]
- Cushman, M.; Judd, S.E.; Howard, V.J.; Kissela, B.; Gutiérrez, O.M.; Jenny, N.S.; Ahmed, A.; Thacker, E.L.; Zakai, N.A. N-terminal pro-B-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke 2014, 45, 1646–1650. [Google Scholar] [CrossRef] [Green Version]
- Mochmann, H.-C.; Scheitz, J.F.; Petzold, G.C.; Haeusler, K.G.; Audebert, H.J.; Laufs, U.; Schneider, C.; Landmesser, U.; Werner, N.; Endres, M.; et al. Coronary Angiographic Findings in Acute Ischemic Stroke Patients With Elevated Cardiac Troponin: The Troponin Elevation in Acute Ischemic Stroke (TRELAS) Study. Circulation 2016, 133, 1264–1271. [Google Scholar] [CrossRef]
- Yildiz, Z.; Kocer, A.; Avsar, S.; Cinier, G. Is Troponin really a reliable marker in patients with acute ischemic stroke? Rom. J. Intern. Med. = Rev. Roum. de Med. interne 2018, 56, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Krause, T.; Werner, K.; Fiebach, J.B.; Villringer, K.; Piper, S.K.; Haeusler, K.G.; Endres, M.; Scheitz, J.F.; Nolte, C.H. Stroke in right dorsal anterior insular cortex Is related to myocardial injury. Ann. Neurol. 2017, 81, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Fiorelli, M.; Toni, D.; Sacchetti, M.L.; Lorenzano, S.; Falcou, A.; Ciarla, M.V.; Suppa, M.; Bonanni, L.; Bertazzoni, G.; et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakoulas, G.; Hatzitolios, A.; Karvounis, H.; Koliakos, G.; Charitandi, A.; Dimitroulas, T.; Savopoulos, C.; Tsirogianni, E.; Louridas, G. N-terminal pro-brain natriuretic peptide levels are elevated in patients with acute ischemic stroke. Angiology 2005, 56, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.A.; Pandey, A.S.; Thompson, B.G.; Keep, R.F.; Hua, Y.; Xi, G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 2018, 134, 240–248. [Google Scholar] [CrossRef]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Bobinger, T.; Burkardt, P.; B Huttner, H.; Manaenko, A. Programmed Cell Death after Intracerebral Hemorrhage. Curr. Neuropharmacol. 2018, 16, 1267–1281. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Chopp, M.; Venkat, P.; Zacharek, A.; Chen, Z.; Landschoot-Ward, J.; Yan, T.; Chen, J. Intracerebral Hemorrhage Induces Cardiac Dysfunction in Mice Without Primary Cardiac Disease. Front. Neurol. 2018, 9, 965. [Google Scholar] [CrossRef]
- Putaala, J.; Lehto, M.; Meretoja, A.; Silvennoinen, K.; Curtze, S.; Kääriäinen, J.; Koivunen, R.J.; Kaste, M.; Tatlisumak, T.; Strbian, D. In-hospital cardiac complications after intracerebral hemorrhage. Int. J. Stroke: Off. J. Int. Stroke Soc. 2014, 9, 741–746. [Google Scholar] [CrossRef]
- Campos-Pires, R.; Edge, C.J.; Dickinson, R. Argon: A Noble Foe for Subarachnoid Hemorrhage. Crit. Care Med. 2016, 44, 1456–1457. [Google Scholar] [CrossRef]
- Fernando, S.M.; Perry, J.J. Subarachnoid hemorrhage. CMAJ 2017, 189, E1421. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, Q.; Wu, H.; Krafft, P.R.; Wang, Z.; Zhang, J.H. The harmful effects of subarachnoid hemorrhage on extracerebral organs. BioMed. Res. Int. 2014, 2014, 858496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wybraniec, M.T.; Mizia-Stec, K.; Krzych, Ł. Neurocardiogenic injury in subarachnoid hemorrhage: A wide spectrum of catecholamin-mediated brain-heart interactions. Cardiol. J. 2014, 21, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadian, A.; Mizzi, A.; Banasiak, M.; Downes, K.; Camporesi, E.M.; Thompson Sullebarger, J.; Vasan, R.; Mangar, D.; van Loveren, H.R.; Agazzi, S. Cardiac manifestations of subarachnoid hemorrhage. Heart Lung Vessel 2013, 5, 168–178. [Google Scholar] [PubMed]
- Frontera, J.A.; Parra, A.; Shimbo, D.; Fernandez, A.; Schmidt, J.M.; Peter, P.; Claassen, J.; Wartenberg, K.E.; Rincon, F.; Badjatia, N.; et al. Cardiac arrhythmias after subarachnoid hemorrhage: Risk factors and impact on outcome. Cereb. Dis. 2008, 26, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junttila, E.; Vaara, M.; Koskenkari, J.; Ohtonen, P.; Karttunen, A.; Raatikainen, P.; Ala-Kokko, T. Repolarization abnormalities in patients with subarachnoid and intracerebral hemorrhage: Predisposing factors and association with outcome. Anesth. Analg. 2013, 116, 190–197. [Google Scholar] [CrossRef]
- Christensen, H.; Boysen, G.; Christensen, A.F.; Johannesen, H.H. Insular lesions, ECG abnormalities, and outcome in acute stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 269–271. [Google Scholar] [CrossRef] [Green Version]
- Ay, H.; Arsava, E.M.; Saribaş, O. Creatine kinase-MB elevation after stroke is not cardiac in origin: Comparison with troponin T levels. Stroke 2002, 33, 286–289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Z.; Qi, S. Cardiac Troponin Elevation and Outcome after Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2015, 24, 2375–2384. [Google Scholar] [CrossRef]
- Deibert, E.; Barzilai, B.; Braverman, A.C.; Edwards, D.F.; Aiyagari, V.; Dacey, R.; Diringer, M. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J. Neurosurg. 2003, 98, 741–746. [Google Scholar] [CrossRef]
- Horowitz, M.B.; Willet, D.; Keffer, J. The use of cardiac troponin-I (cTnI) to determine the incidence of myocardial ischemia and injury in patients with aneurysmal and presumed aneurysmal subarachnoid hemorrhage. Acta Neurochir. 1998, 140, 87–93. [Google Scholar] [CrossRef]
- Sandhu, R.; Aronow, W.S.; Rajdev, A.; Sukhija, R.; Amin, H.; D’Aquila, K.; Sangha, A. Relation of cardiac troponin I levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Am. J. Cardiol. 2008, 102, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemorrhage: Who dies, and why? Crit. Care (London, England) 2015, 19, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAteer, A.; Hravnak, M.; Chang, Y.; Crago, E.A.; Gallek, M.J.; Yousef, K.M. The Relationships Between BNP and Neurocardiac Injury Severity, Noninvasive Cardiac Output, and Outcomes After Aneurysmal Subarachnoid Hemorrhage. Biol. Res. Nurs. 2017, 19, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meaudre, E.; Jego, C.; Kenane, N.; Montcriol, A.; Boret, H.; Goutorbe, P.; Habib, G.; Palmier, B. B-type natriuretic peptide release and left ventricular filling pressure assessed by echocardiographic study after subarachnoid hemorrhage: A prospective study in non-cardiac patients. Crit. Care (London, England) 2009, 13, R76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokainish, H. B-type natriuretic levels in critically ill patients: Critically misleading? Crit. Care (London, England) 2009, 13, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banki, N.M.; Kopelnik, A.; Dae, M.W.; Miss, J.; Tung, P.; Lawton, M.T.; Drew, B.J.; Foster, E.; Smith, W.; Parmley, W.W.; et al. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation 2005, 112, 3314–3319. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Inamasu, J.; Hirose, Y.; Kato, Y.; Ito, K.; Iwase, M.; Sugimoto, K.; Watanabe, E.; Takahashi, A.; Ozaki, Y. The role of norepinephrine and estradiol in the pathogenesis of cardiac wall motion abnormality associated with subarachnoid hemorrhage. Stroke 2012, 43, 1897–1903. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Inamasu, J.; Kato, Y.; Yamada, Y.; Ganaha, T.; Oheda, M.; Hattori, N.; Watanabe, E.; Ozaki, Y.; Hirose, Y. Association between elevated plasma norepinephrine levels and cardiac wall motion abnormality in poor-grade subarachnoid hemorrhage patients. Neurosurg. Rev. 2013, 36, 259–266. [Google Scholar] [CrossRef]
- Belvederi Murri, M.; Prestia, D.; Mondelli, V.; Pariante, C.; Patti, S.; Olivieri, B.; Arzani, C.; Masotti, M.; Respino, M.; Antonioli, M.; et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology 2016, 63, 327–342. [Google Scholar] [CrossRef]
- Christensen, H.; Boysen, G.; Johannesen, H.H. Serum-cortisol reflects severity and mortality in acute stroke. J. Neurol. Sci. 2004, 217, 175–180. [Google Scholar] [CrossRef]
- Wester, V.L.; van Rossum, E.F.C. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol. 2015, 173, M1–M10. [Google Scholar] [CrossRef] [PubMed]
- Barugh, A.J.; Gray, P.; Shenkin, S.D.; MacLullich, A.M.J.; Mead, G.E. Cortisol levels and the severity and outcomes of acute stroke: A systematic review. J. Neurol. 2014, 261, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Veltkamp, R.; Uhlmann, S.; Marinescu, M.; Sticht, C.; Finke, D.; Gretz, N.; Gröne, H.-J.; Katus, H.A.; Backs, J.; Lehmann, L.H. Experimental ischaemic stroke induces transient cardiac atrophy and dysfunction. J. Cachexia Sarcopenia Muscle 2019, 10, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rokita, A.G.; Anderson, M.E. New therapeutic targets in cardiology: Arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII). Circulation 2012, 126, 2125–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirokova, N.; Kang, C.; Fernandez-Tenorio, M.; Wang, W.; Wang, Q.; Wehrens, X.H.T.; Niggli, E. Oxidative stress and ca(2+) release events in mouse cardiomyocytes. Biophys. J. 2014, 107, 2815–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorn, G.W. Adrenergic pathways and left ventricular remodeling. J. Card. Fail. 2002, 8, S370–S373. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Li, D.-R.; Zhao, D.; Oritani, S.; Kamikodai, Y.; Tsuda, K.; Okazaki, S.; Maeda, H. Postmortem cardiac troponin T levels in the blood and pericardial fluid. Part 1. Analysis with special regard to traumatic causes of death. Leg. Med. (Tokyo) 2006, 8, 86–93. [Google Scholar] [CrossRef]
- Winklewski, P.J.; Radkowski, M.; Demkow, U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J. Neuroinflamm. 2014, 11, 213. [Google Scholar] [CrossRef]
- van der Bilt, I.A.C.; Vendeville, J.-P.; van de Hoef, T.P.; Begieneman, M.P.V.; Lagrand, W.K.; Kros, J.M.; Wilde, A.A.M.; Rinkel, G.J.E.; Niessen, H.W.M. Myocarditis in patients with subarachnoid hemorrhage: A histopathologic study. J. Crit. Care 2016, 32, 196–200. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Parsha, K.N.; Rahbar, M.H.; Lee, M.; Bui, T.T.; Nguyen, C.; Barreto, A.D.; Bambhroliya, A.B.; Sahota, P.; Yang, B.; et al. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, Y.; Tanaka, Y.; Hoya, K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke 2001, 32, 1989–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, R.; Diringer, M.N. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit. Care 2008, 8, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, M.; Meyer, S.; Knors, H.; Klinke, A.; Radunski, U.K.; Rudolph, T.K.; Rudolph, V.; Spin, J.M.; Tsao, P.S.; Costard-Jäckle, A.; et al. Levosimendan displays anti-inflammatory effects and decreases MPO bioavailability in patients with severe heart failure. Sci. Rep. 2015, 5, 9704. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta; Int. J. Clin. Chem. 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Granger, D.N. Leukocyte recruitment and ischemic brain injury. Neuromol. Med. 2010, 12, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science (New York, N.Y.) 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, e002699. [Google Scholar] [CrossRef] [Green Version]
- Crapser, J.; Ritzel, R.; Verma, R.; Venna, V.R.; Liu, F.; Chauhan, A.; Koellhoffer, E.; Patel, A.; Ricker, A.; Maas, K.; et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging 2016, 8, 1049–1063. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Adrie, C.; Parlato, M.; Salmi, L.; Adib-Conquy, M.; Bical, O.; Deleuze, P.; Fitting, C.; Cavaillon, J.M.; Monchi, M. Bacterial translocation and plasma cytokines during transcatheter and open-heart aortic valve implantation. Shock (Augusta, GA) 2015, 43, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, Y.; Tang, W.H. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Wang, C.; Nie, L.; Zhao, X.; Gu, J.; Guan, X.; Wang, S.; Xiao, T.; Xu, X.; He, T.; et al. Klotho Protects Against Indoxyl Sulphate-Induced Myocardial Hypertrophy. J. Am. Soc. Nephrol. 2015, 26, 2434–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Zaroff, J.G.; Rordorf, G.A.; Titus, J.S.; Newell, J.B.; Nowak, N.J.; Torchiana, D.F.; Aretz, H.T.; Picard, M.H.; Macdonald, R.L. Regional myocardial perfusion after experimental subarachnoid hemorrhage. Stroke 2000, 31, 1136–1143. [Google Scholar] [CrossRef]
- Huang, C.-C.; Huang, C.-H.; Kuo, H.-Y.; Chan, C.-M.; Chen, J.-H.; Chen, W.-L. The 12-lead electrocardiogram in patients with subarachnoid hemorrhage: Early risk prognostication. Am. J. Emerg. Med. 2012, 30, 732–736. [Google Scholar] [CrossRef]
- Murthy, S.B.; Shah, S.; Rao, C.P.V.; Bershad, E.M.; Suarez, J.I. Neurogenic Stunned Myocardium Following Acute Subarachnoid Hemorrhage: Pathophysiology and Practical Considerations. J. Intensive Care Med. 2015, 30, 318–325. [Google Scholar] [CrossRef]
- Zerna, C.; Thomalla, G.; Campbell, B.C.V.; Rha, J.-H.; Hill, M.D. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet (London, England) 2018, 392, 1247–1256. [Google Scholar] [CrossRef]
| Author/Year | Marker | Experimental Groups | Control Groups | |||
|---|---|---|---|---|---|---|
| Disease | Age | n | Age | n | ||
| Makikallio et al., 2005 [25] | NT-proBNP | Brain infarction | 67 ± 10 | 51 | — | 25 |
| Yuan et al., 2005 [26] | NT-proBNP | Brain infarction | 56 (50–67) | 35 | 57 (53–69) | 31 |
| Iltumur et al., 2006 [10] | NT-proBNP, cTnI | Acute ischemic stroke | 64.5 ± 11.3 | 57 | 61.3 ± 6.09 | 57 |
| Yip et al., 2006 [27] | NT-proBNP | Acute ischemic stroke | 66.0 ± 11.0 | 86 | 64.6 ± 6.5 | 30 |
| Fang et al., 2008 [12] | NT-proBNP | Brain infarction | — | 68 | 54.36 ± 8.29 | 106 |
| Fang et al., 2008 [12] | NT-proBNP | Brain hemorrhage | — | 44 | 54.36 ± 8.29 | 106 |
| Wang, 2009 [21] | cTnI | Acute cerebral hemorrhage | 62.5 (45–80) | 98 | 63.6 (43–78) | 98 |
| Li et al., 2010 [24] | BNP | Cerebral hemorrhage | 57 ± 10 | 65 | 56 ± 8 | 62 |
| Kim et al., 2010 [22] | BNP | Acute ischemic stroke | 66.6 ± 11.8 | 89 | 43.8 ± 12.0 | 57 |
| Kim et al., 2010 [22] | BNP | Cerebral hemorrhage | 56.4 ± 14.5 | 11 | 43.8 ± 12.0 | 57 |
| Chen et al., 2012 [28] | NT-proBNP | Acute intracerebral hemorrhage | 67 ± 13 | 42 | 57 ± 17 | 28 |
| Kim et al., 2012 [11] | BNP | Acute cerebral infarction | 67.6 ± 11.6 | 141 | 65.5 ± 7.3 | 61 |
| Ulvi et al., 2013 [23] | BNP | Acute cerebral ischemia | 68.5 ± 14.2 | 60 | 60.2 ± 12.4 | 30 |
| Baydin, et al., 2015 [4] | cTnI | Nontraumatic SAH | 58.8 ± 13.0 | 29 | 47.3 ± 8.6 | 20 |
| Altun et al., 2016 [20] | NT-proBNP, cTnI | Acute ischemic stroke | 71.4 ± 11 | 61 | 68.6 ± 8 | 82 |
| Niu et al., 2017 [16] | NT-proBNP | Acute intracerebral hemorrhage | 63.80 ± 8.46 | 126 | 63.52 ± 8.60 | 60 |
| Suleiman et al., 2017 [13] | cTnT | Ischemic stroke | 59 ± 14.08 | 100 | 59 ± 13.91 | 100 |
| Wang et al., 2018 [9] | NT-proBNP | Acute cerebral infarction | 67.4 ± 7.2 | 124 | 68.2 ± 8.1 | 100 |
| Study | Quality Indicators from the Newcastle–Ottawa Scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Outcome Assessment 1 | Exposure | Scores | ||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | ||
| Makikallio et al., 2005 [25] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Yuan et al., 2005 [26] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Iltumur et al., 2006 [10] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Yip et al., 2006 [27] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Fang et al., 2008 [12] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Wang, 2009 [21] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Li et al., 2010 [24] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Kim et al., 2010 [22] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Chen et al., 2012 [28] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Kim et al., 2012 [11] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Ulvi et al., 2013 [23] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Baydin et al., 2015 [4] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Altun et al., 2016 [20] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Niu et al., 2017 [16] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Suleiman et al., 2017 [13] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Wang et al., 2018 [9] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Zheng, A.; He, T.; Cao, Z. Brain–Heart Axis and Biomarkers of Cardiac Damage and Dysfunction after Stroke: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 2347. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072347
Xu C, Zheng A, He T, Cao Z. Brain–Heart Axis and Biomarkers of Cardiac Damage and Dysfunction after Stroke: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2020; 21(7):2347. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072347
Chicago/Turabian StyleXu, Chengyang, Ang Zheng, Tianyi He, and Zhipeng Cao. 2020. "Brain–Heart Axis and Biomarkers of Cardiac Damage and Dysfunction after Stroke: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 21, no. 7: 2347. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072347





