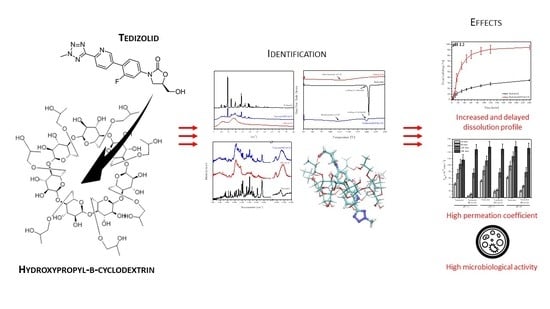
Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. System Preparation
3.2. The Identification of the Systems
3.2.1. Differential Scanning Colorimetry (DSC)
3.2.2. Powder X-ray Diffraction (PXRD)
3.2.3. FT-IR Spectroscopy
3.2.4. Molecular Docking
3.3. Studies of Physicochemical Properties of Systems
3.3.1. Dissolution of Systems
3.3.2. Permeability Studies of Systems
3.3.3. Studies of Bactericidal Activity of Tedizolid in Tedizolid/HP-β-CD Inclusion Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FDA. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/25/infections-and-infectious-diseases (accessed on 6 March 2020).
- Rumondor, A.C.F.; Dhareshwar, S.S.; Kesisoglou, F. Amorphous Solid Dispersions or Prodrugs: Complementary Strategies to Increase Drug Absorption. J. Pharm. Sci. 2016, 105, 2498–2508. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, P.; Causse, M.; Vaquero, M.; Casal, M. In vitro activity of Tedizolid against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2019, 63, e01939-18. [Google Scholar] [CrossRef] [Green Version]
- Michalska, K.; Karpiuk, I.; Król, M.; Tyski, S. Recent development of potent analogues of oxazolidinone antibacterial agents. Bioorg. Med. Chem. 2013, 21, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Love, R.; Adam, H.; Golden, A.; Zelenitsky, S.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Rubinstein, E.; Walkty, A.; et al. Tedizolid: A novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens. Drugs 2015, 75, 253–270. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon PASS. Available online: https://chemicalize.com/app/calculation/tedizolid (accessed on 17 December 2020).
- Im, W.B.; Choi, S.H.; Park, J.-Y.; Choi, S.H.; Finn, J.; Yoon, S.-H. Discovery of torezolid as a novel 5-hydroxymethyl-oxazolidinone antibacterial agent. Eur J. Med. Chem. 2011, 46, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.J.; Barbachyn, M.R. The oxalidinones: Past, present and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef]
- Jornada, D.H.; dos Santos Fernandes, G.F.; Chiba, D.E.; Ferreira de Melo, T.R.; dos Santos, J.L.; Chung, M.C. The prodrug approach: A successful tool for improving drug solubility. Molecules 2016, 21, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Kidney Health. Calcium and Phosphate Balance with Kidney Disease. Available online: https://kidney.org.au/uploads/resources/calcium-and-phosphate-balance-with-kidney-disease-kidney-health-australia-fact-sheet.pdf (accessed on 20 November 2020).
- Bassetti, M.; Castaldo, N.; Carnelutti, A.; Peghin, M.; Giacobbe, D.R. Tedizolid phosphate for the treatment of acute bacterial skin and skin-structure infections: An evidence-based review of its place in therapy. Core Evid. 2019, 14, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Dixit, M.; Kini, A.G.; Kulkarni, P.K. Enhancing Solubility and Dissolution of Celecoxib by Spray Drying using Pluronic F 127. Indian J. Pharm. Educ. Res. 2011, 45, 346–352. [Google Scholar]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Scien. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Michalska, K.; Gruba, E.; Cielecka-Piontek, J.; Bednarek, E. Chiral separation of tedizolid using charge single isomer derivatives of cyclodextrins by capillary electrokinetic chromatography. J. Pharm. Biomed. Anal. 2016, 120, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Gruba, E.; Bocian, W.; Cielecka-Piontek, J. Enantioselective recognition of radezolid by cyclodextrin modified capillary electrokinetic chromatography and electronic circular dichroism. J. Pharm. Biomed. Anal. 2017, 139, 98–108. [Google Scholar] [CrossRef]
- Michalska, K.; Pajchel, G.; Tyski, S. Determination of enantiomeric impurity of linezolid by capillary electrophoresis using heptakis-(2,3-diacetyl-6-sulfato)-beta-cyclodextrin. J. Chromatogr. A 2008, 1180, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Bocian, W.; Bednarek, E.; Pałys, B.; Cielecka-Piontek, J. Enantioselective recognition of sutezolid by cyclodextrin modified non-aqueous capillary electrophoresis and explanation of complex formation by means of infrared spectroscopy, NMR and molecular modelling. J. Pharm. Biomed. Anal. 2019, 169, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Cielecka-Piontek, J. Enhanced tedizolid solubility and permeability due to its interactions with hydrophilic biopolymers. Acta Pharm. Hung. 2018, 88, 195. [Google Scholar]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powałowska, D.; Lewandowska, K.; Błaszczak, W.; Gościańska, J.; Pietrzak, R.; Cielecka-Piontek, J. β-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef]
- Paczkowska, M.; Szymanowska-Powałowska, D.; Mizera, M.; Siąkowska, D.; Błaszczak, W.; Piotrowska-Kempisty, H.; Cielecka-Piontek, J. Cyclodextrins as multifunctional excipients: Influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS ONE 2019, 14, e0210694. [Google Scholar] [CrossRef] [Green Version]
- Mizera, M.; Szymanowska, D.; Stasiłowicz, A.; Siąkowska, D.; Lewandowska, K.; Miklaszewski, A.; Plech, T.; Tykarska, E.; Cielecka-Piontek, J. Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity. Biomolecules 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Cyclodextrins Used as Excipients. 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 20 November 2020).
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, E.; Bocian, W.; Michalska, K. Nuclear magnetic resonance spectroscopic study of the inclusion complex of (R)-tedizolid with HDAS-β-CD, β-CD, and γ-cyclodextrin in aqueous solution. J. Pharm. Biomed. Anal. 2019, 169, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Mizera, M.; Lewandowska, K.; Cielecka-Piontek, J. Infrared, Raman and ultraviolet with circular dichroism analysis and theoretical calculations of tedizolid. J. Mol. Struct. 2016, 1115, 136–143. [Google Scholar] [CrossRef]
- Flourence, T.; Malyadri, T.; Thejomoorthy, K. Enhancement of dissolution of Cilostazole by complexation method using Cyclodextrins. World J. Curr. Med. Pharm. Res. 2020, 2, 166–170. [Google Scholar] [CrossRef]
- Gu, W.; Liu, Y. Characterization and stability of beta-acids/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1201, 127159. [Google Scholar] [CrossRef]
- Li, W.; Ran, L.; Liu, F.; Hou, R.; Zhao, W.; Li, Y.; Wang, C.; Dong, J. Preparation and Characterisation of Polyphenol-HP-β-Cyclodextrin Inclusion Complex that Protects Lamb Tripe Protein against Oxidation. Molecules 2019, 24, 4487. [Google Scholar] [CrossRef] [Green Version]
- Rakmai, J.; Cheirsilp, B.; Torrado-Agrasar, A.; Simal-Gandara, J.; Mejuto, J.C. Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: Physiochemical characterization and evaluation of bio-efficacies. CyTA J. Food 2017, 15, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Lo Nostro, P.; Santoni, I.; Bonini, M.; Baglioni, P. Inclusion compound from a semifluorinated alkane and β-cyclodextrin. Langmuir 2013, 19, 2313–2317. [Google Scholar] [CrossRef]
- Hu, S.C.; Lai, Y.C.; Lin, C.-L.; Tzeng, W.-S.; Yen, F.-L. Inclusion complex of saikosaponin-d with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-skin cancer activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef]
- De Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Kawashima Pacheco, S.Y.; Silva, S.S.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; da Silva Ferreira, M.A.; de Almeida, J.C.; et al. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP-β-Cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Sivextro: EPAR- Public Assessment Report. 2015. Available online: https://www.ema.europa.eu/en/documents/assessment-report/sivextro-epar-public-assessment-report_en.pdf (accessed on 17 December 2020).
- Ong, V.; Flanagan, S.; Fang, E.; Dreskin, H.J.; Locke, J.B.; Bartizal, K.; Prokocimer, P. Absorption, distribution, metabolism, and excretion of the novel antibacterial prodrug tedizolid phosphate. Drug Metab. Dispos. 2014, 42, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Yee, S. In vitro permeability across Caco3 cells (colonic) can predict in vivo (small intestinal) absorption in man–Fact or myth. Pharm Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- FDA. Clinical Pharmacology Review of Tedizolid Phosphate (SIVEXTRO). 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205435Orig1s000ClinPharmR.pdf (accessed on 17 December 2020).
- Flanagan, S.D.; Bien, P.A.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Pharmacokinetics of tedizolid following oral administration: Single and multiple dose, effect of food, and comparison of two solid forms of the prodrug. Pharmacotherapy 2014, 34, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Shah, H.D. Acute bacterial skin and skin structure infections: Current perspective. Indian J. Dermatol. 2011, 56, 510–512. [Google Scholar] [CrossRef]
- Athanassiou, G.; Michaleas, S.; Lada-Chitiroglou, E.; Tsitsa, T.; Antoniadou-Vyza, E. Antimicrobial activity of β-lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003, 55, 291–300. [Google Scholar] [CrossRef]
- Paczkowska, M.; McDonagh, A.; Bialek, K.; Tajberm, L.; Cielecka-Piontek, J. Mechanochemical activation with cyclodextrins followed by compaction as an approach to improve dissolution of rutin. Int. J. Pharm. 2020, 581, 119294. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Gebhardt, J.; Kleist, C.; Jakobtorweihen, S.; Hansen, N. Validation and Comparison of Force Fields for Native Cyclodextrins in Aqueous Solution. J. Phys. Chem. B 2018, 122, 1608–1626. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Commission of the European Communities. Validation of Analytical Procedures Q2(R2). Proceedings of the International Conference of Harmonisation (ICH). 2018. Available online: https://database.ich.org/sites/default/files/Q2R2-Q14_EWG_Concept_Paper.pdf (accessed on 17 December 2020).
- European Pharmacopeia 9.0. 2.9.3. Dissolution Test for Solid Dosage Forms; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2016. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]






| Tedizolid | Tedizolid/HP-β-CD | |
|---|---|---|
| MIC [mg L−1] | ||
| Enterococcus faecalis ATTC 29212 | 0.5 ± 0.1 | 0.25 ± 0.1 ↓ |
| E. faecalis clinical isolates | 1 ± 0.2 | 0.25 ± 0.1 ↓ |
| Enterococcus faecium ATCC 27270 | 0.5 ± 0.1 | 0.25 ± 0.1 ↓ |
| E. faecium clinical isolates | 1 ± 0.2 | 0.5 ± 0.1 ↓ |
| Staphylococcus aureus ATCC 25923 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| S. aureus clinical isolates | 2 ± 0.2 | 2 ± 0.2 |
| Staphylococcus epidermidis ATCC 12228 | 1 ± 0.2 | 1 ± 0.2 |
| S. epidermidis clinical isolates | 2 ± 0.2 | 2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Rosiak, N.; Tykarska, E.; Michalska, K.; Płazińska, A.; Płaziński, W.; Szymanowska, D.; Cielecka-Piontek, J. Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. Int. J. Mol. Sci. 2021, 22, 115. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010115
Paczkowska-Walendowska M, Rosiak N, Tykarska E, Michalska K, Płazińska A, Płaziński W, Szymanowska D, Cielecka-Piontek J. Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. International Journal of Molecular Sciences. 2021; 22(1):115. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010115
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Natalia Rosiak, Ewa Tykarska, Katarzyna Michalska, Anita Płazińska, Wojciech Płaziński, Daria Szymanowska, and Judyta Cielecka-Piontek. 2021. "Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity" International Journal of Molecular Sciences 22, no. 1: 115. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010115