Vaginal Lactobacilli and Vaginal Dysbiosis-Associated Bacteria Differently Affect Cervical Epithelial and Immune Homeostasis and Anti-Viral Defenses
Abstract
:1. Introduction
2. Results
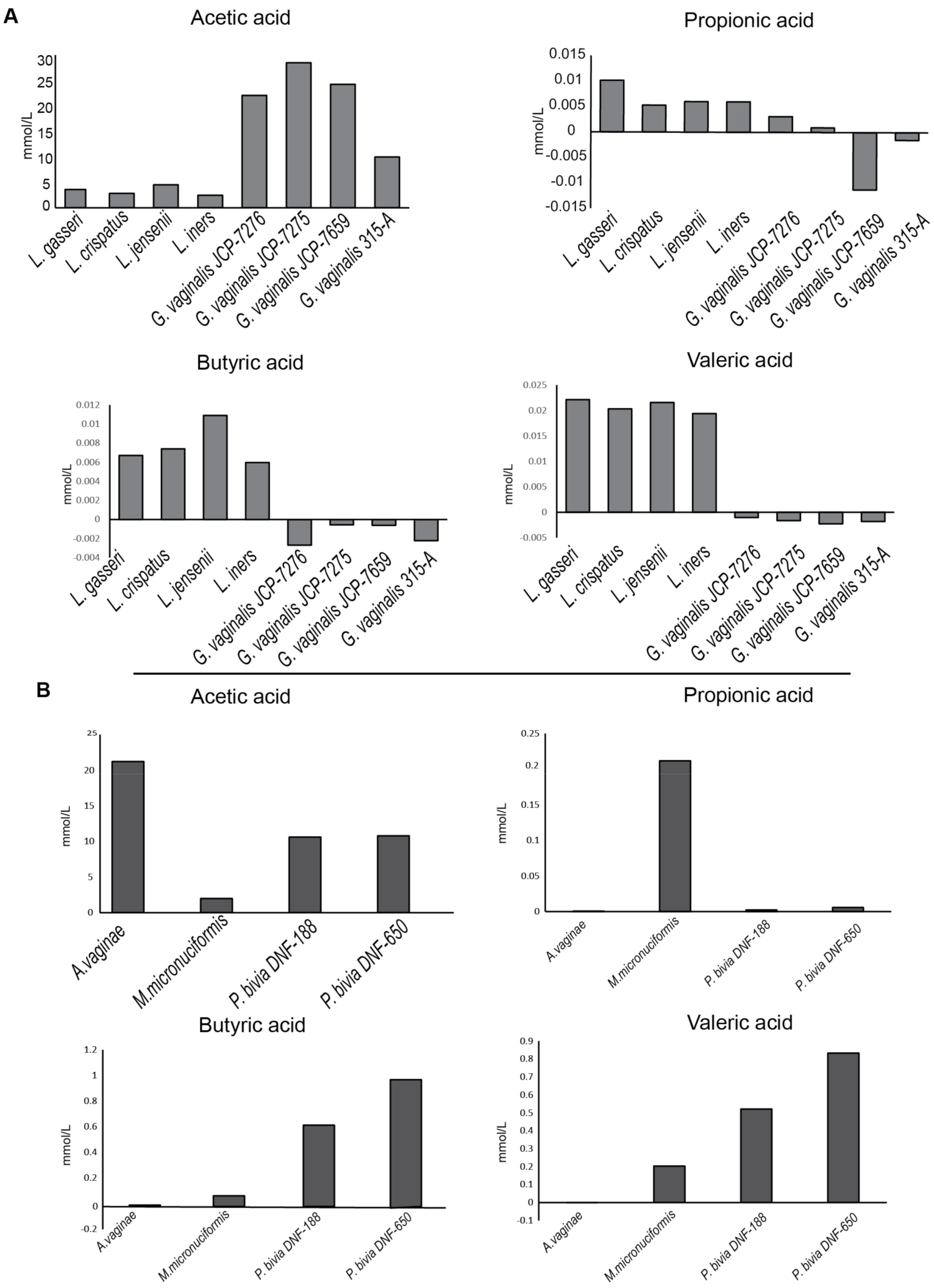
2.1. Bacterial Strains and Analysis of Short Chain Fatty Acids (SCFAs) Production
2.2. Effects of Supernatants and Bacterial Lysates on Viability of Cervical Epithelial Cell Lines
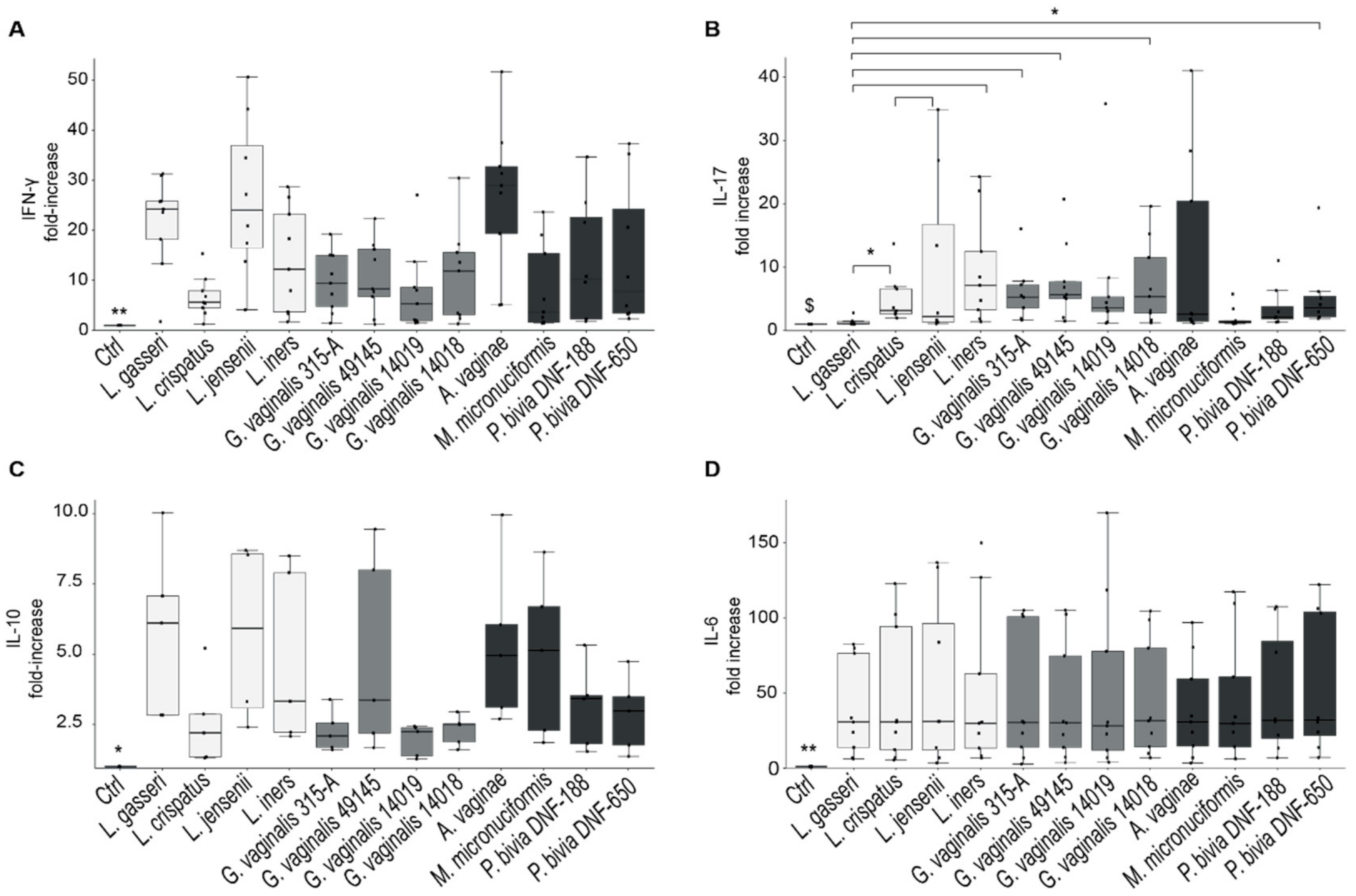
2.3. Cytokine’s Production by CaSki and SiHa Cells Cultured with Vaginal Bacteria
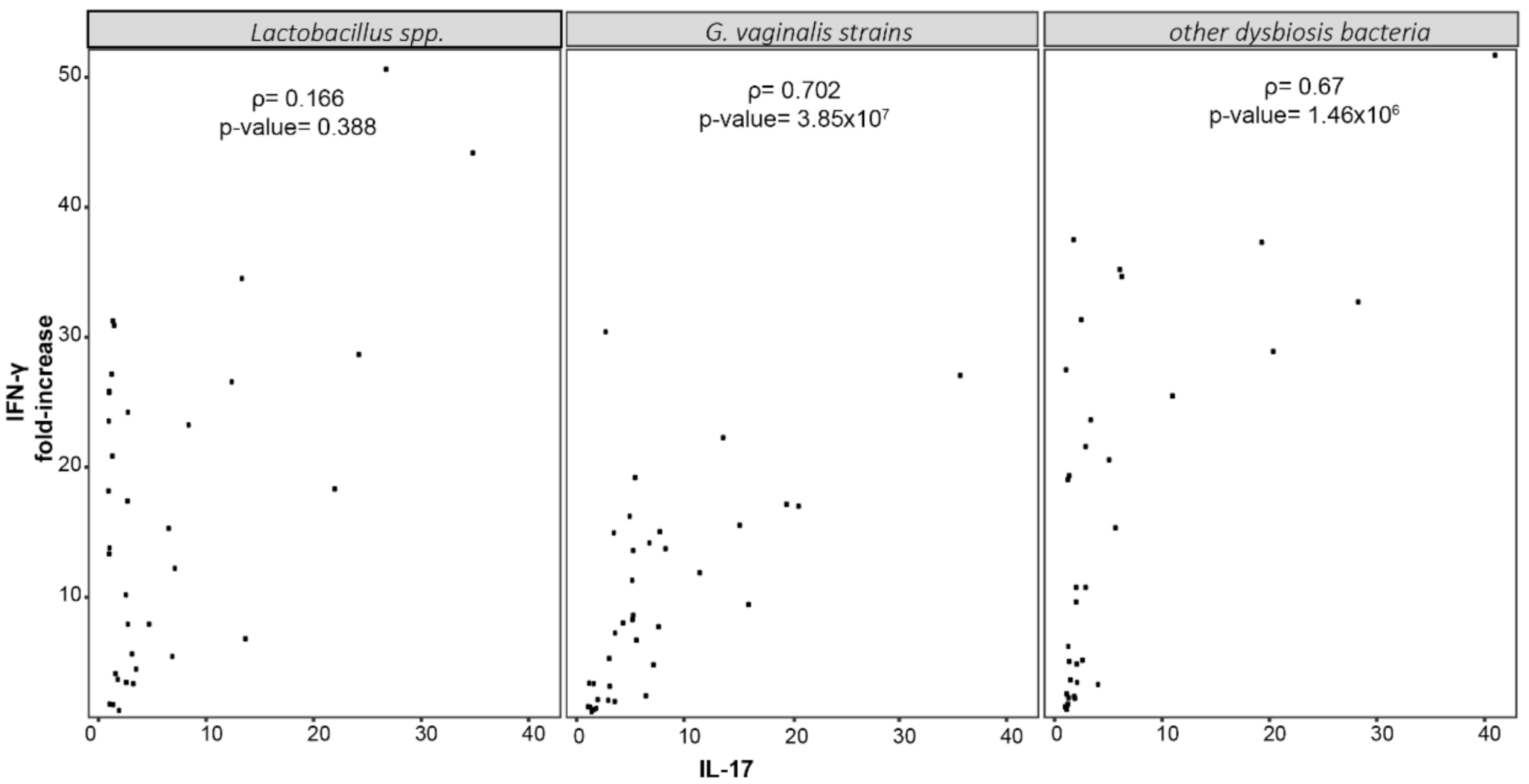
2.4. Cytokine’s Production by PBMCs Stimulated with Dominant Species of Vaginal Microbiota
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Bacterial Cultures
4.3. Epithelial Cell Culture
4.4. PBMCs Isolation and Culture
4.5. Viability Test
4.6. Cytokines’ Evaluation
4.7. SCFAs (Short Chain Fait Acids) Profile of Bacterial Strains
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torcia, M.G. Interplay among Vaginal Microbiome, Immune Response and Sexually Transmitted Viral Infections. Int. J. Mol. Sci. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastromarino, P.; Di Pietro, M.; Schiavoni, G.; Nardis, C.; Gentile, M.; Sessa, R. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int. J. Med. Microbiol. 2014, 304, 654–661. [Google Scholar] [CrossRef]
- Turovskiy, Y.; Noll, K.S.; Chikindas, M.L. The aetiology of bacterial vaginosis. J. Appl. Microbiol. 2011, 110, 1105–1128. [Google Scholar] [CrossRef]
- Pybus, V.; Onderdonk, A.B. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1999, 1, 285–292. [Google Scholar] [CrossRef]
- Peebles, K.; Velloza, J.; Balkus, J.E.; McClelland, R.S.; Barnabas, R.V. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2019, 46, 304–311. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 31 March 2021).
- Stanley, M. Immune responses to human papillomavirus. Vaccine 2006, 24, S16–S22. [Google Scholar] [CrossRef]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: Stratification and integration of immune protection against the transmission of sexually transmitted infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.; Stites, D.P.; Moscicki, A.-B. Th1 cytokine patterns in cervical human papillomavirus infection. Clin. Diagn. Lab. Immunol. 1999, 6, 751–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosmann, C.; Mattarollo, S.R.; Bridge, J.A.; Frazer, I.H.; Blumenthal, A. IL-17 Suppresses Immune Effector Functions in Human Papillomavirus-Associated Epithelial Hyperplasia. J. Immunol. 2014, 193, 2248–2257. [Google Scholar] [CrossRef]
- Xue, J.; Wang, Y.; Chen, C.; Zhu, X.; Zhu, H.; Hu, Y. Effects of Th17 cells and IL-17 in the progression of cervical carcinogenesis with high-risk human papillomavirus infection. Cancer Med. 2017, 7, 297–306. [Google Scholar] [CrossRef]
- Jee, B.; Yadav, R.; Pankaj, S.; Shahi, S.K. Immunology of HPV-mediated cervical cancer: Current understanding. Int. Rev. Immunol. 2020, 1–20. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; A Cone, R.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D.; et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doerflinger, S.Y.; Throop, A.L.; Herbst-Kralovetz, M.M. Bacteria in the Vaginal Microbiome Alter the Innate Immune Response and Barrier Properties of the Human Vaginal Epithelia in a Species-Specific Manner. J. Infect. Dis. 2014, 209, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Brotman, R.M.; Klebanoff, M.A.; Nansel, T.R.; Yu, K.F.; Andrews, W.W.; Zhang, J.; Schwebke, J.R. Bacterial Vaginosis Assessed by Gram Stain and Diminished Colonization Resistance to Incident Gonococcal, Chlamydial, and Trichomonal Genital Infection. J. Infect. Dis. 2010, 202, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.L.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. Vaginal Lactobacilli, Microbial Flora, and Risk of Human Immunodeficiency Virus Type 1 and Sexually Transmitted Disease Acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Myer, L.; Denny, L.; Telerant, R.; De Souza, M.; Wright, J.T.C.; Kuhn, L. Bacterial Vaginosis and Susceptibility to HIV Infection in South African Women: A Nested Case-Control Study. J. Infect. Dis. 2005, 192, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; van den Broek, M.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef] [Green Version]
- Borgogna, J.-L.; Shardell, M.D.; Santori, E.; Nelson, T.; Rath, J.; Glover, E.; Ravel, J.; Gravitt, P.; Yeoman, C.; Brotman, R. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: A cross-sectional analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 182–192. [Google Scholar] [CrossRef]
- Anton, L.; Sierra, L.-J.; Devine, A.; Barila, G.; Heiser, L.; Brown, A.G.; Elovitz, M. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health. Front. Microbiol. 2018, 9, 2181. [Google Scholar] [CrossRef]
- Randis, T.M.; Zaklama, J.; LaRocca, T.J.; Los, F.C.O.; Lewis, E.L.; Desai, P.; Rampersaud, R.; Amaral, F.E.; Ratner, A.J. Vaginolysin Drives Epithelial Ultrastructural Responses to Gardnerella vaginalis. Infect. Immun. 2013, 81, 4544–4550. [Google Scholar] [CrossRef] [Green Version]
- López-Moreno, A.; Aguilera, M. Vaginal Probiotics for Reproductive Health and Related Dysbiosis: Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1461. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Desai, P.J.; Gibson, F.; Genco, C.A. Distinct Proinflammatory Host Responses to Neisseria gonorrhoeae Infection in Immortalized Human Cervical and Vaginal Epithelial Cells. Infect. Immun. 2001, 69, 5840–5848. [Google Scholar] [CrossRef] [Green Version]
- Spear, G.T.; St John, E.; Reza, M.R. Bacterial vaginosis and human immunodeficiency virus infection. AIDS Res. Ther. 2007, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Reyes, K.; Pedraza-Brindis, E.J.; Hernández-Flores, G.; Bravo-Cuellar, A.; López-López, B.A.; Rosas-González, V.C.; Ortiz-Lazareno, P.C. The supernatant of cervical carcinoma cells lines induces a decrease in phosphorylation of STAT-1 and NF-κB transcription factors associated with changes in profiles of cytokines and growth factors in macrophages derived from U937 cells. Innate Immun. 2019, 25, 344–355. [Google Scholar] [CrossRef]
- Tayyeb, J.Z.; Popeijus, H.E.; Mensink, R.P.; Konings, M.C.J.M.; Mokhtar, F.B.A.; Plat, J. Short-Chain Fatty Acids (Except Hexanoic Acid) Lower NF-kB Transactivation, Which Rescues Inflammation-Induced Decreased Apolipoprotein A-I Transcription in HepG2 Cells. Int. J. Mol. Sci. 2020, 21, 5088. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, Z.E.; Laniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef] [Green Version]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Kumari, S.; Bhor, V.M. Association of cervicovaginal dysbiosis mediated HPV infection with cervical intraepithelial neoplasia. Microb. Pathog. 2021, 152, 104780. [Google Scholar] [CrossRef]
- Seresini, S.; Origoni, M.; Caputo, L.; Lillo, F.; Longhi, R.; Vantini, S.; Paganoni, A.M.; Protti, M.P. CD4+ T cells against human papillomavirus-18 E7 in patients with high-grade cervical lesions associate with the absence of the virus in the cervix. Immunology 2010, 131, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Serraino, D.; Carrieri, P.; Pradier, C.; Bidoli, E.; Dorrucci, M.; Ghetti, E.; Schiesari, A.; Zucconi, R.; Pezzotti, P.; Dellamonica, P.; et al. Risk of invasive cervical cancer among women with, or at risk for, HIV infection. Int. J. Cancer 1999, 82, 334–337. [Google Scholar] [CrossRef]
- Caselli, E.; D’Accolti, M.; Santi, E.; Soffritti, I.; Conzadori, S.; Mazzacane, S.; Greco, P.; Contini, C.; Bonaccorsi, G. Vaginal Microbiota and Cytokine Microenvironment in HPV Clearance/Persistence in Women Surgically Treated for Cervical Intraepithelial Neoplasia: An Observational Prospective Study. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Macintyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Herzum, A.; Ciccarese, G.; Dezzana, M.; Casazza, S.; Pastorino, A.; Bandelloni, R.; Parodi, A. Ureaplasma parvum as a possible enhancer agent of HPV-induced cervical intraepithelial neoplasia: Preliminary results. J. Med. Virol. 2016, 88, 2023–2024. [Google Scholar] [CrossRef] [PubMed]
- Curty, G.; De Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2019, 21, 222. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Norenhag, J.; Hu, Y.; Brusselaers, N.; Fransson, E.; Ährlund-Richter, A.; Guðnadóttir, U.; Angelidou, P.; Zha, Y.; Hamsten, M.; et al. Vaginal microbiota and human papillomavirus infection among young Swedish women. NPJ Biofilms Microbiomes 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Van Teijlingen, N.H.; Helgers, L.C.; Willems, E.M.Z.; van Hamme, J.L.; Ribeiro, C.M.; Strijbis, K.; Geijtenbeek, T.B. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J. Reprod. Immunol. 2020, 138, 103085. [Google Scholar] [CrossRef]
- Eslami, S.; Hadjati, J.; Motevaseli, E.; Mirzaei, R.; Bonab, S.F.; Ansaripour, B.; Khoramizadeh, M.R. Lactobacillus crispatus strain SJ-3C-US induces human dendritic cells (DCs) maturation and confers an anti-inflammatory phenotype to DCs. APMIS 2016, 124, 697–710. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.-B.; Kyrgiou, M. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Paaso, A.; Koskimaa, H.; Welters, M.J.P.; Kero, K.; Rautava, J.; Syrjänen, K.; Van Der Burg, S.H.; Syrjänen, S. Interferon-γ and IL-5 associated cell-mediated immune responses to HPV16 E2 and E6 distinguish between persistent oral HPV16 infections and noninfected mucosa. Clin. Exp. Dent. Res. 2021. [Google Scholar] [CrossRef]
- Ondondo, R.; Bukusi, E.; Ng’Ang’A, Z.; Kiptoo, M.; Mpoke, S. Cellular immune responses against natural human papillomavirus infections among men in Kisumu, Kenya. Clin. Immunol. 2020, 212, 108211. [Google Scholar] [CrossRef]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Paccosi, S.; Musilli, C.; Caporale, R.; Gelli, A.M.G.; Guasti, D.; Clemente, A.M.; Torcia, M.G.; Filippelli, A.; Romagnoli, P.; Parenti, A. Stimulatory Interactions between Human Coronary Smooth Muscle Cells and Dendritic Cells. PLoS ONE 2014, 9, e99652. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, C.; Lulli, M.; di Pilato, V.; Schiavone, N.; Russo, E.; Nannini, G.; Baldi, S.; Borrelli, R.; Bartolucci, G.; Menicatti, M.; et al. Differential Responses of Colorectal Cancer Cell Lines to Enterococcus faecalis’ Strains Isolated from Healthy Donors and Colorectal Cancer Patients. J. Clin. Med. 2019, 8, 388. [Google Scholar] [CrossRef] [Green Version]


| CST | Family and Genus | Specie | Strain’s Name |
|---|---|---|---|
| I | Lactobacillaceae, Lactobacillus | L. crispatus | JV-V01 |
| II | Lactobacillaceae, Lactobacillus | L. gasseri | SV-16A |
| III | Lactobacillaceae, Lactobacillus | L. iners | UPII-60-B |
| V | Lactobacillaceae, Lactobacillus | L. jenseni | JV-V16 |
| IV | Bifidobacteriaceae, Gardnerella | G. vaginalis | 315-A |
| IV | Bifidobacteriaceae, Gardnerella | G. vaginalis | 49145/JCP-7276 |
| IV | Bifidobacteriaceae, Gardnerella | G. vaginalis | 14019/JCP-7659 |
| IV | Bifidobacteriaceae, Gardnerella | G. vaginalis | 14018/JCP-7275 |
| IV | Atopobiaceae, Atopobium | A. vaginalis | DSM-15829 |
| IV | Prevotellaceae, Prevotella | P. bivia | DNF-00188 |
| IV | Prevotellaceae, Prevotella | P. bivia | DNF-00650 |
| IV | Veillonellaceae, Megasphaera | M. micronuciformis | DNF-00954 |
| SCFAs | Quan. Ion | Qual. Ion | ISTD |
|---|---|---|---|
| Acetic acid | 60 | - | [2H3] Acetic |
| Propionic acid | 74 | 73 | [2H3] Propionic |
| Butyric acid | 60 | 73 | [2H3] Propionic |
| Valeric acid | 60 | 73 | [2H9] iso-Valeric |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolò, S.; Tanturli, M.; Mattiuz, G.; Antonelli, A.; Baccani, I.; Bonaiuto, C.; Baldi, S.; Nannini, G.; Menicatti, M.; Bartolucci, G.; et al. Vaginal Lactobacilli and Vaginal Dysbiosis-Associated Bacteria Differently Affect Cervical Epithelial and Immune Homeostasis and Anti-Viral Defenses. Int. J. Mol. Sci. 2021, 22, 6487. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126487
Nicolò S, Tanturli M, Mattiuz G, Antonelli A, Baccani I, Bonaiuto C, Baldi S, Nannini G, Menicatti M, Bartolucci G, et al. Vaginal Lactobacilli and Vaginal Dysbiosis-Associated Bacteria Differently Affect Cervical Epithelial and Immune Homeostasis and Anti-Viral Defenses. International Journal of Molecular Sciences. 2021; 22(12):6487. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126487
Chicago/Turabian StyleNicolò, Sabrina, Michele Tanturli, Giorgio Mattiuz, Alberto Antonelli, Ilaria Baccani, Chiara Bonaiuto, Simone Baldi, Giulia Nannini, Marta Menicatti, Gianluca Bartolucci, and et al. 2021. "Vaginal Lactobacilli and Vaginal Dysbiosis-Associated Bacteria Differently Affect Cervical Epithelial and Immune Homeostasis and Anti-Viral Defenses" International Journal of Molecular Sciences 22, no. 12: 6487. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22126487







