Restriction of Manganese Intake Prevents the Onset of Brain Manganese Overload in Zip14−/− Mice
Abstract
:1. Introduction
2. Results
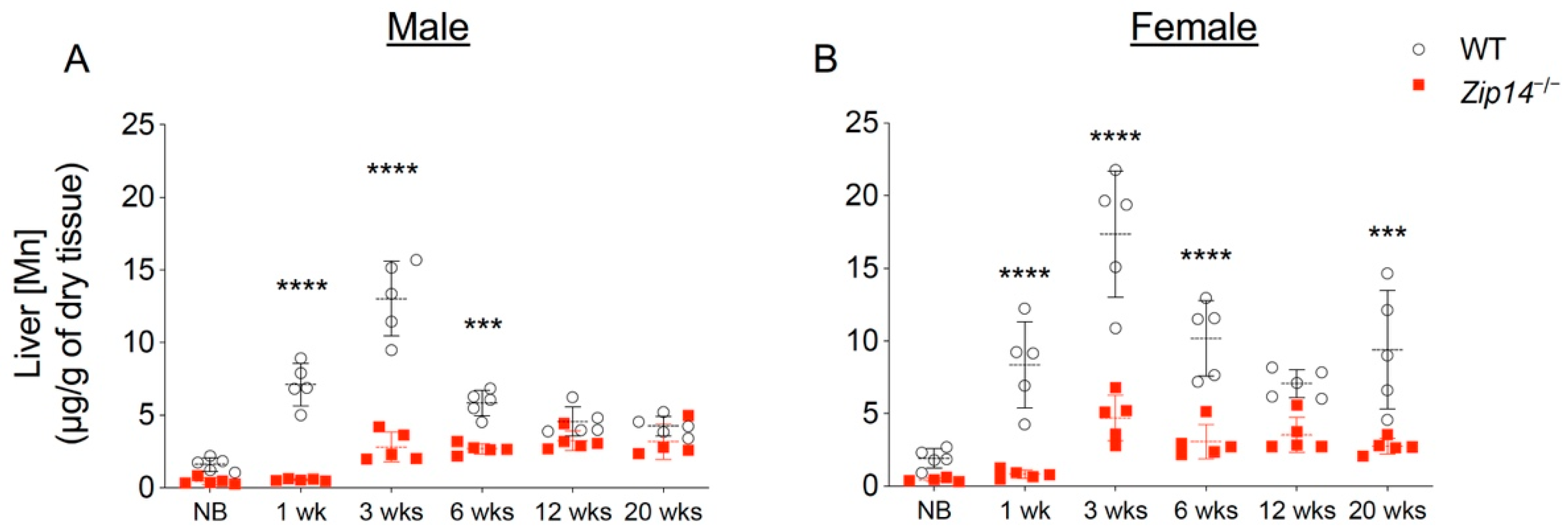
2.1. Zip14−/− Mice Are Born without Manganese Overload
2.2. Metal Chelation Approach Using CaNa2EDTA Cannot Prevent Brain Manganese Loading in Zip14−/− Mice
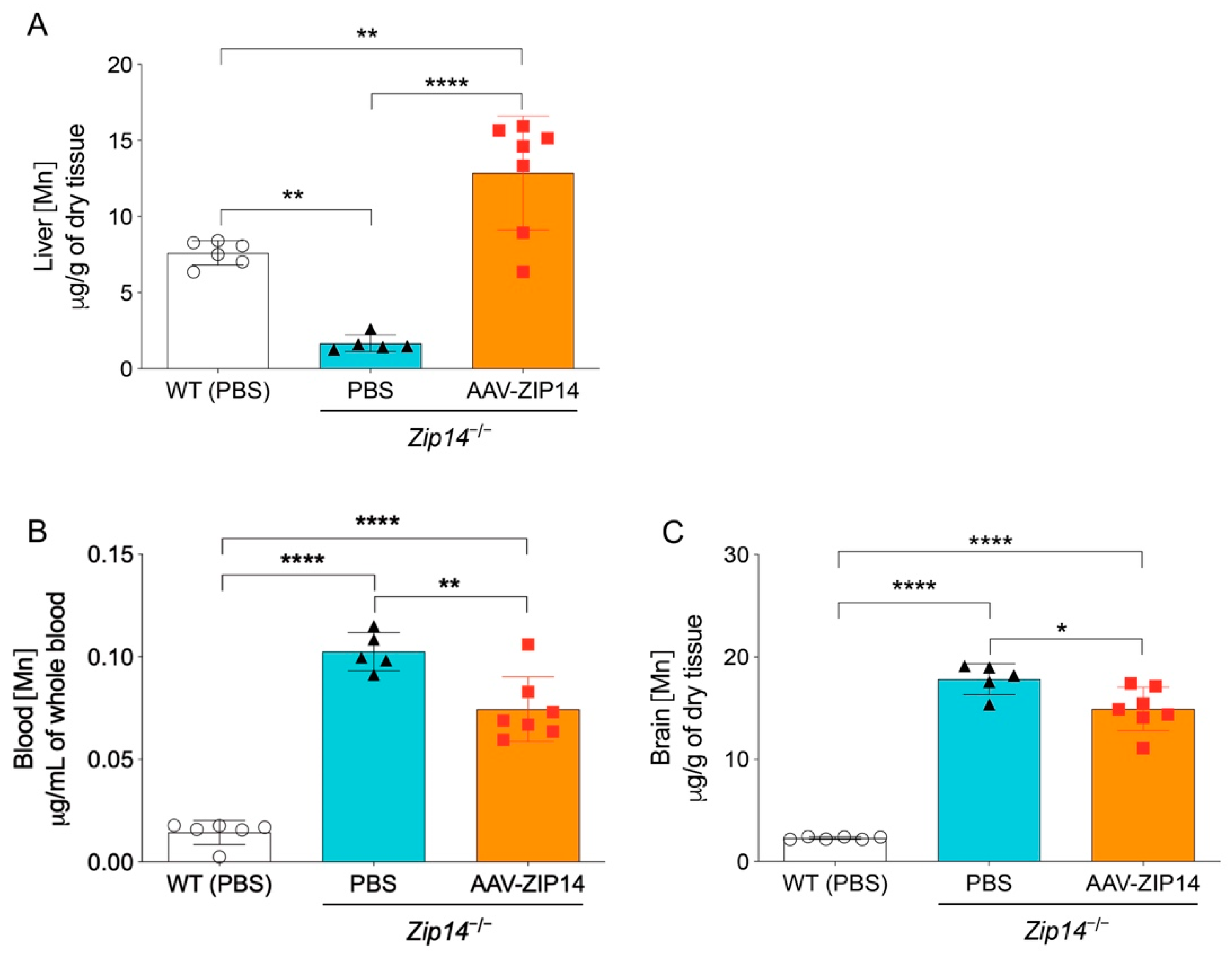
2.3. Hepatic ZIP14 Restoration Increases Liver Manganese, but Does Not Effectively Prevent the Accumulation of Manganese in the Brain
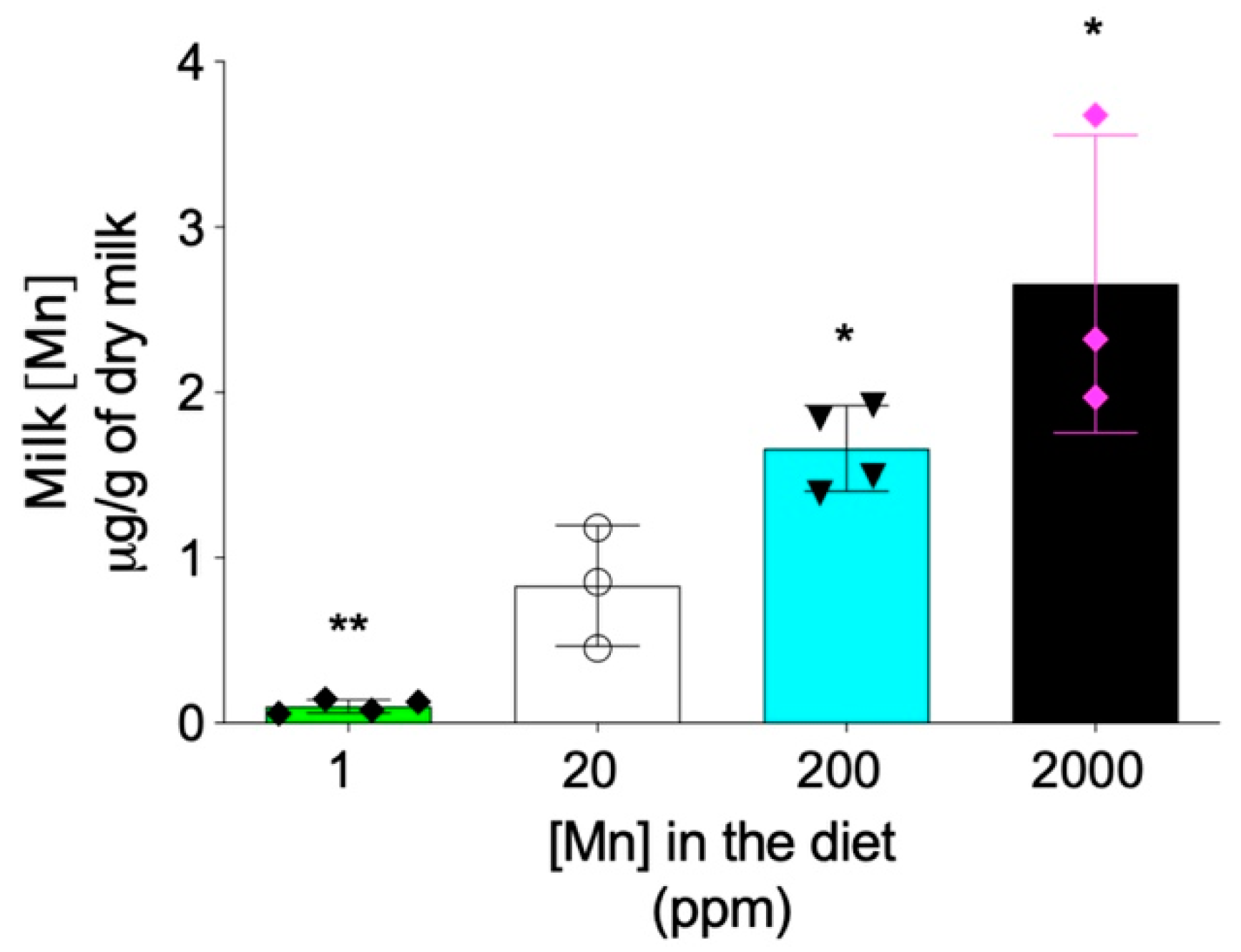
2.4. Maternal Milk Manganese Level Can Be Altered by Dietary Intervention
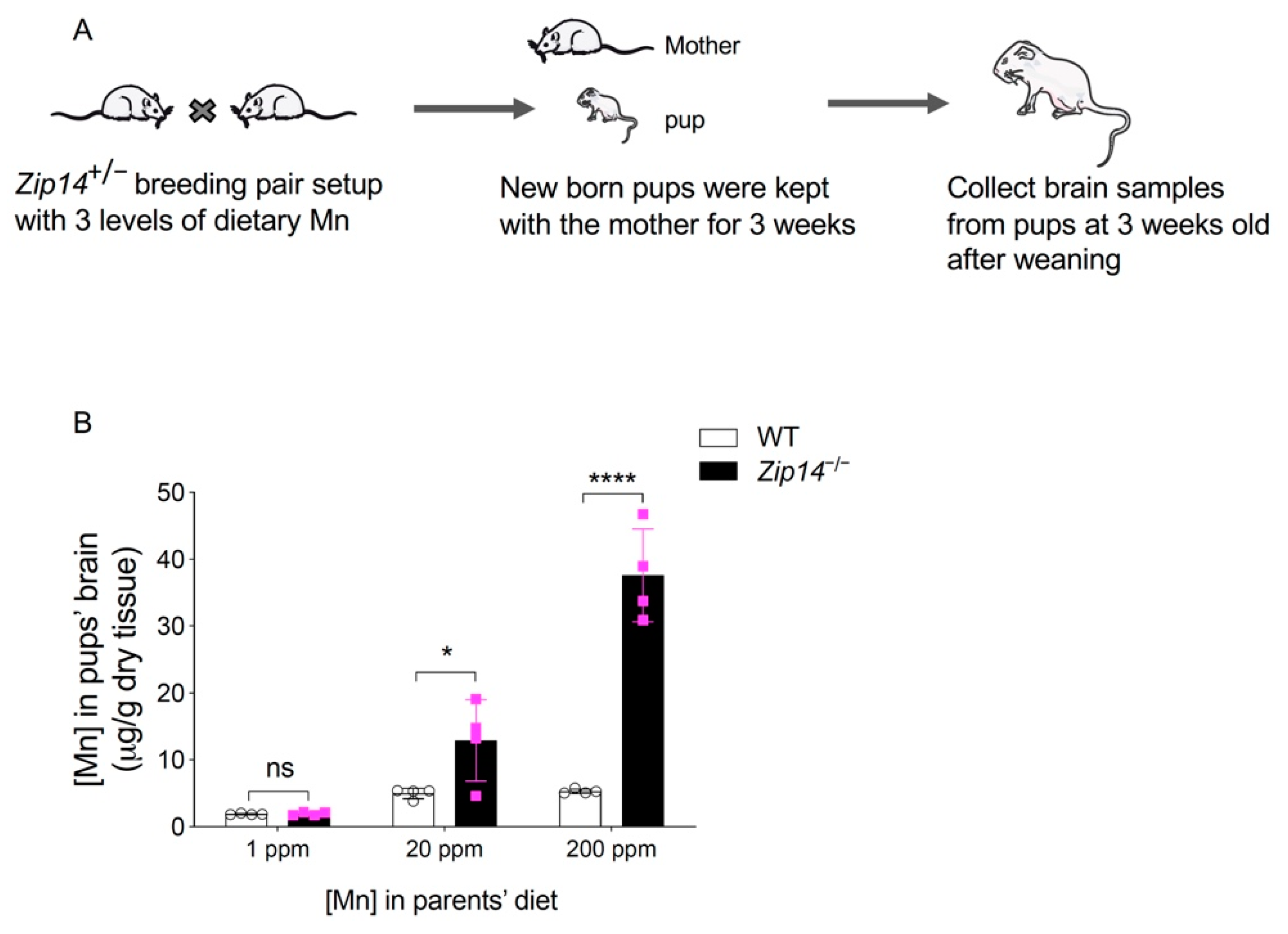
2.5. Early Intervention to Restrict Manganese Intake Prevents the Onset of Brain Manganese Loading in Zip14−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animals, Genotyping and Tissue Collection
4.2. AAV Production and Injection
4.3. Dietary Intervention and Chelator Injection
4.4. Tissue Metal Analysis by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.5. Immunoblotting Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinilla-Tenas, J.J.; Sparkman, B.K.; Shawki, A.; Illing, A.C.; Mitchell, C.J.; Zhao, N.; Liuzzi, J.P.; Cousins, R.J.; Knutson, M.D.; MacKenzie, B. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 2011, 301, C862–C871. [Google Scholar] [CrossRef] [Green Version]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 Gene Encodes ZIP14, A Metal/Bicarbonate Symporter: Similarities to the ZIP8 Transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005, 579, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troche, C.; Aydemir, T.B.; Cousins, R.J. Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E258–E268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winslow, J.W.W.; Limesand, K.H.; Zhao, N. The Functions of ZIP8, ZIP14, and ZnT10 in the Regulation of Systemic Manganese Homeostasis. Int. J. Mol. Sci. 2020, 21, 3304. [Google Scholar] [CrossRef]
- Marti-Sanchez, L.; Ortigoza-Escobar, J.D.; Darling, A.; Villaronga, M.; Baide, H.; Molero-Luis, M.; Batllori, M.; Vanegas, M.I.; Muchart, J.; Aquino, L.; et al. Hypermanganesemia due to mutations in SLC39A14: Further insights into Mn deposition in the central nervous system. Orphanet J. Rare Dis. 2018, 13, 2–8. [Google Scholar] [CrossRef]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef] [Green Version]
- Juneja, M.; Shamim, U.; Joshi, A.; Mathur, A.; Uppili, B.; Sairam, S.; Ambawat, S.; Dixit, R.; Faruq, M. Aradhna A novel mutation in SLC39A14 causing hypermanganesemia associated with infantile onset dystonia. J. Gene Med. 2018, 20, e3012. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Akinyode, A.; Paulus, E.; Weiskirchen, R.; Hojyo, S.; Fukada, T.; Giraldo, G.; Schrier, J.; Garcia, A.; Janus, C.; et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E1769–E1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Y.; Gao, H.; Wang, J.; Qiang, Y.; Imam, M.U.; Li, Y.; Wang, J.; Zhang, R.; Zhang, H.; Yu, Y.; et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 2017, 3, 17025. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Kim, M.-H.; Kim, J.; Colon-Perez, L.M.; Banan, G.; Mareci, T.H.; Febo, M.; Cousins, R.J. Metal Transporter Zip14 (Slc39a14) Deletion in Mice Increases Manganese Deposition and Produces Neurotoxic Signatures and Diminished Motor Activity. J. Neurosci. 2017, 37, 5996–6006. [Google Scholar] [CrossRef] [Green Version]
- Scheiber, I.F.; Wu, Y.; Morgan, S.E.; Zhao, N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 2019, 294, 9147–9160. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Thorn, T.L.; Ruggiero, C.H.; Pompilus, M.; Febo, M.; Cousins, R.J. Intestine-specific deletion of metal transporter Zip14 (Slc39a14) causes brain manganese overload and locomotor defects of manganism. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G673–G681. [Google Scholar] [CrossRef]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Aschner, M. Manganese toxicity in the central nervous system: The glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med. 2013, 273, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hutchens, S.; Jursa, T.; Shawlot, W.; Polishchuk, E.V.; Polishchuk, R.S.; Dray, B.K.; Gore, A.C.; Aschner, M.; Smith, D.R.; et al. Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J. Biol. Chem. 2017, 292, 16605–16615. [Google Scholar] [CrossRef] [Green Version]
- Keen, C.L.; Clegg, M.S.; Lönnerdal, B.; Hurley, L.S. Whole-blood manganese as an indicator of body manganese. N. Engl. J. Med. 1983, 308, 1230. [Google Scholar]
- Discalzi, G.; Pira, E.; Hernandez, E.H.; Valentini, C.; Turbiglio, M.; Meliga, F. Occupational Mn parkinsonism: Magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation. NeuroToxicology 2000, 21, 863–866. [Google Scholar]
- Hernandez, E.H.; Discalzi, G.; Valentini, C.; Venturi, F.; Chio, A.; Carmellino, C.; Rossi, L.; Sacchetti, A.; Pira, E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. NeuroToxicology 2006, 27, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.P.M.; Andrade, V.; Aschner, M. Neuroprotective and Therapeutic Strategies for Manganese–Induced Neurotoxicity. Clin. Pharmacol. Transl. Med. 2017, 1, 54–62. [Google Scholar]
- Gao, J.; Chen, J.; de Domenico, I.; Koeller, D.M.; Harding, C.O.; Fleming, R.E.; Koeberl, D.D.; Enns, C.A. Hepatocyte-targeted HFE and TFR2 control hepcidin expression in mice. Blood 2010, 115, 3374–3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.-S.; Gao, J.; Koeberl, D.D.; Enns, C.A. The Role of Hepatocyte Hemojuvelin in the Regulation of Bone Morphogenic Protein-6 and Hepcidin Expression in Vivo. J. Biol. Chem. 2010, 285, 16416–16423. [Google Scholar] [CrossRef] [Green Version]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Laboratory Animals, 4th ed.; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Sato, I.; Matsusaka, N.; Kobayashi, H.; Nishimura, Y. Effects of Dietary Manganese Contents on 54Mn Metabolism in Mice. J. Radiat. Res. 1996, 37, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007, 221, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Aschner, M.; Erikson, K.M.; Hernández, E.H.; Tjalkens, R. Manganese and its Role in Parkinson’s Disease: From Transport to Neuropathology. NeuroMolecular Med. 2009, 11, 252–266. [Google Scholar] [CrossRef]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Totten, M.; Zhang, Z.; Bucinca, H.; Erikson, K.; Santamaría, A.; Bowman, A.B.; Aschner, M. Iron and manganese-related CNS toxicity: Mechanisms, diagnosis and treatment. Expert Rev. Neurother. 2019, 19, 243–260. [Google Scholar] [CrossRef]
- Rodan, L.H.; Hauptman, M.; d’Gama, A.M.; Qualls, A.E.; Cao, S.; Tuschl, K.; Al-Jasmi, F.; Hertecant, J.; Hayflick, S.J.; Wessling-Resnick, M.; et al. Novel founder intronic variant in SLC39A14 in two families causing Manganism and potential treatment strategies. Mol. Genet. Metab. 2018, 124, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zeglam, A.; Abugrara, A.; Kabuka, M. Autosomal-recessive iron deficiency anemia, dystonia and hypermanganesemia caused by new variant mutation of the manganese transporter gene SLC39A14. Acta Neurol. Belg. 2018, 119, 379–384. [Google Scholar] [CrossRef]
- Jones, M.M.; Basinger, M.A.; Gale, G.R.; Atkins, L.M.; Smith, A.B.; Stone, A. Effect of chelate treatments on kidney, bone and brain lead levels of lead-intoxicated mice. Toxicology 1994, 89, 91–100. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wei, G.; Zhao, N. Restriction of Manganese Intake Prevents the Onset of Brain Manganese Overload in Zip14−/− Mice. Int. J. Mol. Sci. 2021, 22, 6773. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136773
Wu Y, Wei G, Zhao N. Restriction of Manganese Intake Prevents the Onset of Brain Manganese Overload in Zip14−/− Mice. International Journal of Molecular Sciences. 2021; 22(13):6773. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136773
Chicago/Turabian StyleWu, Yuze, Guojun Wei, and Ningning Zhao. 2021. "Restriction of Manganese Intake Prevents the Onset of Brain Manganese Overload in Zip14−/− Mice" International Journal of Molecular Sciences 22, no. 13: 6773. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136773




