Oxytocin Signaling Acts as a Marker for Environmental Stressors in Zebrafish
Abstract
:1. Introduction
2. Results
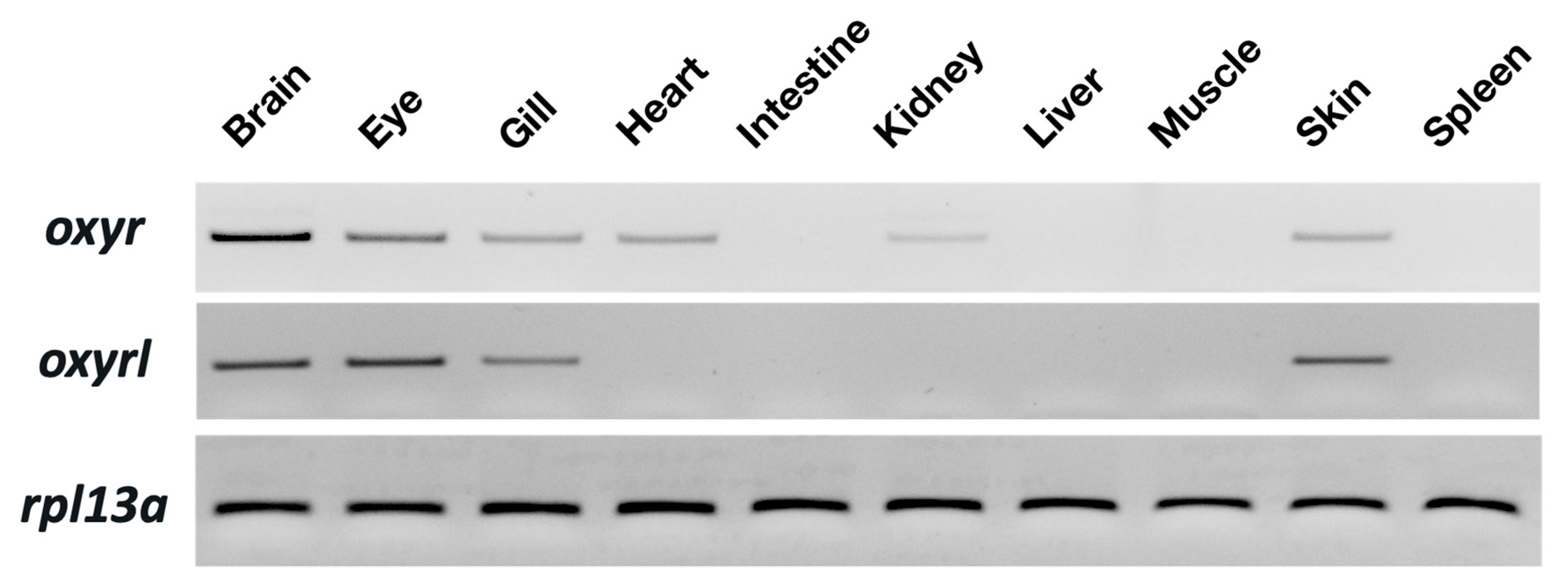
2.1. Oxyr and Oxyrl Were Expressed in the Brains and Gills of Adult Zebrafish
2.2. Environmental Stressors Downregulated Oxyr mRNA Expression in the Gills
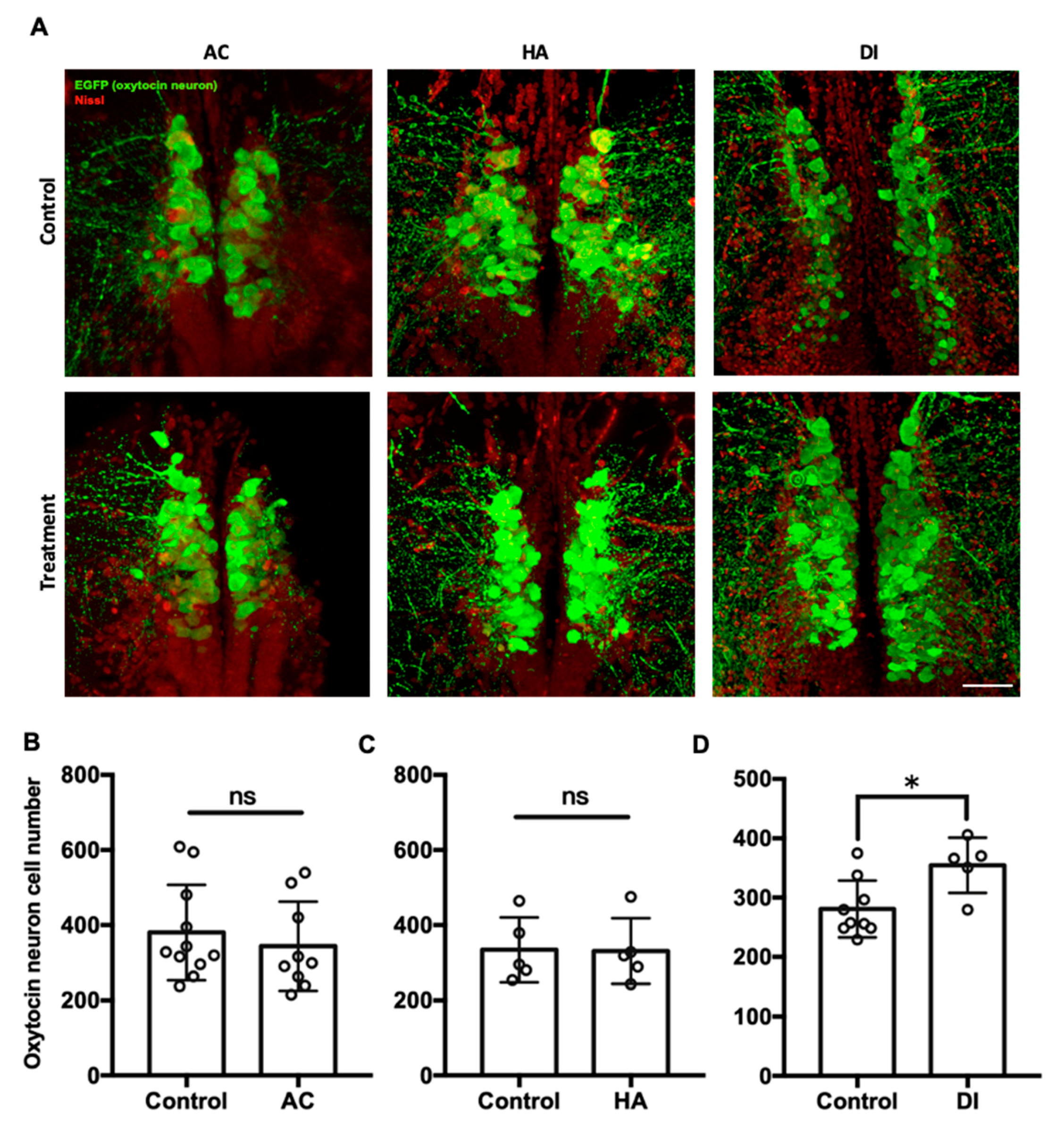
2.3. DI Water Elevated the Number of Oxytocin Neurons in the Brain
2.4. Mapping Central Projections of Oxytocin Neurons in Adult Zebrafish
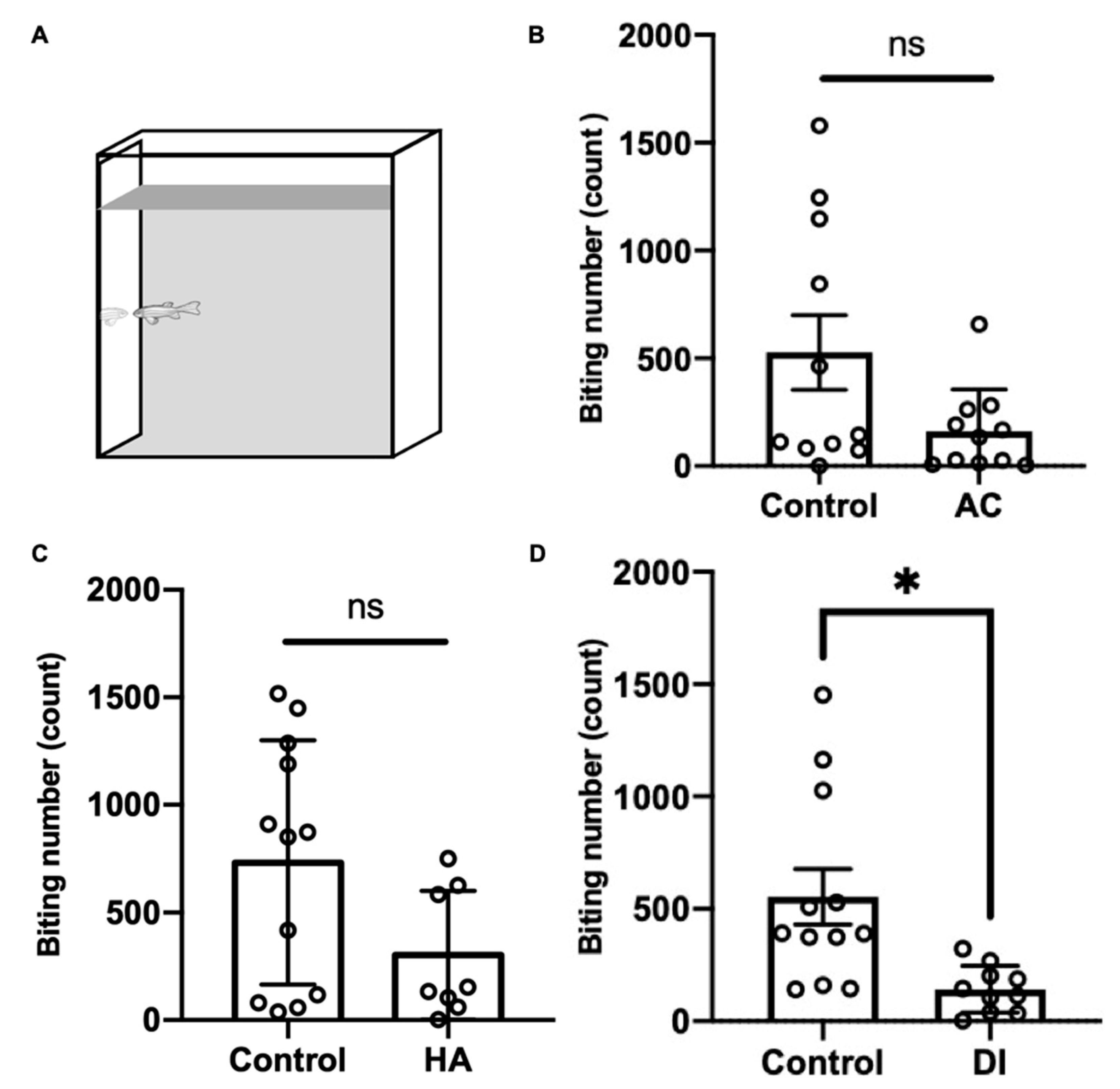
2.5. Effects of Environmental Stressors on Swimming and Aggressive Behaviors
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Environmental Challenges to Adult Zebrafish
4.3. Total RNA Isolation and Complementary DNA Preparation
4.4. mRNA Expression of Oxytocin Receptors
4.5. Real-Time Quantitative Polymerase Chain Reaction
4.6. Brain Fixation and Sectioning
4.7. Immunohistochemistry
4.8. Cell Counting for Oxytocin Neurons
4.9. Novel Tank Diving Test
4.10. Mirror-Biting Test
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | acidic |
| HA | high ammonia |
| DI | double-ionized |
| HPA | hypothalamic–pituitary–adrenal |
| GR | glucocorticoid receptor |
| MO | morpholino |
| AuNP-PVP | gold nanoparticles coated with PVP |
| PPa | the anterior part of the parvocellular preoptic nucleus |
| PPp | the posterior part of the parvocellular preoptic nucleus |
| TPp | the periventricular nucleus of the posterior tuberculum |
| Hc | the caudal zone of the periventricular hypothalamus |
| Vv | the ventral nuclei of the ventral telencephalic area |
| VM | the ventromedial thalamic nuclei |
| A | the anterior thalamic nuclei |
| PGm | the preglomerular nuclei |
| TPp | the periventricular nucleus of the posterior tuberculum |
| Cans | the commissura ansulata |
| IPN | the interpeduncular nucleus |
| TTBc | the crossed tecto-bulbar tract |
| PVN | the paraventricular nucleus of the hypothalamus |
| PFC | the prefrontal cortex |
| Dpf | day-post-fertilization |
| mPFC | the medial prefrontal cortex |
| CB1 receptor | cannabinoid receptor type-1 |
| RE | the reuniens nuclei in the thalamus |
| RH | the rhomboid nuclei in the thalamus |
| CeL/C | the lateral and capsular division of the central amygdala |
| CeM | the central amygdala |
| dHbM | the medial subregion of the dorsal habenula |
| i/vIPN | the intermediate/ventral interpeduncular nucleus |
| dHbL | the lateral subregion of the dorsal habenula |
| d/iIPN | the dorsal/intermediate interpeduncular nucleus |
| DRN | the dorsal raphe nucleus |
References
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Fishelson, Z.; Hochman, I.; Greene, L.E.; Eisenberg, E. Contribution of heat shock proteins to cell protection from complement-mediated lysis. Int. Immunol. 2001, 13, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Fromm, P.O. A review of some physiological and toxicological responses of freshwater fish to acid stress. Env. Biol. Fishes 1980, 5, 79–93. [Google Scholar] [CrossRef]
- Hattingh, J. The surface area of mudfish. J. Fish. Biol. 1976, 8, 19–22. [Google Scholar] [CrossRef]
- Iwama, G.K.; Afonso, L.O.; Todgham, A.; Ackerman, P.; Nakano, K. Are hsps suitable for indicating stressed states in fish? J. Exp. Biol. 2004, 207, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmini, E.; Rani, M.U. Evaluation of oxidative stress biomarkers in hepatocytes of grey mullet inhabiting natural and polluted estuaries. Sci. Total Environ. 2009, 407, 4533–4541. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.; Brandon, C.S.; Lee, F.S.; Lomax, M.I.; Dillmann, W.H.; Cunningham, L.L. Hsp70 inhibits aminoglycoside-induced hair cell death and is necessary for the protective effect of heat shock. J. Assoc. Res. Otolaryngol. 2008, 9, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.H.; Chang, I.C.; Chen, C.H.; Lee, T.H.; Hwang, P.P. Phenotypic changes in mitochondrion-rich cells and responses of Na+/K+-ATPase in gills of tilapia exposed to deionized water. Zool. Sci. 2008, 25, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tine, M.; Bonhomme, F.; McKenzie, D.J.; Durand, J.D. Differential expression of the heat shock protein Hsp70 in natural populations of the tilapia, Sarotherodon melanotheron, acclimatised to a range of environmental salinities. BMC Ecol. 2010, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Khotinul, U.; Chuang, H.J.; Chiu, L.; Yang, W.K.; Wang, Y.C.; Wu, W.Y.; Lee, T.H. Potential osmoprotective roles of branchial heat shock proteins towards Na+, K+ -ATPase in milkfish (Chanos chanos) exposed to hypotonic stress. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2020, 248, 110749. [Google Scholar]
- Harper, C.; Wolf, J.C. Morphologic effects of the stress response in fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedemeyer, G.; Barton, B.B.; McLeay, D.J. Stress and acclimation. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 451–489. [Google Scholar]
- Lin, C.H.; Shih, T.H.; Liu, S.T.; Hsu, H.H.; Hwang, P.P. Cortisol regulates acid secretion of H+-ATPase-rich ionocytes in zebrafish (Danio rerio) embryos. Front. Physiol. 2015, 6, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jezova, D.; Skultetyova, I.; Tokarev, D.I.; Bakos, P.; Vigas, M. Vasopressin and oxytocin in stress. Ann. N. Y. Acad. Sci. 1995, 771, 192–203. [Google Scholar] [CrossRef]
- Weninger, S.C.; Dunn, A.; Muglia, L.J.; Dikkes, P.; Miczek, K.A.; Swiergiel, A.H.; Berridge, C.W.; Majzoub, J.A. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc. Natl. Acad. Sci. USA 1999, 96, 8283–8288. [Google Scholar] [CrossRef] [Green Version]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Hu, Y.C.; Chu, K.F.; Hwang, L.Y.; Lee, T.H. Cortisol regulation of Na+, K+ -ATPase β1 subunit transcription via the pre-receptor 11β-hydroxysteroid dehydrogenase 1-like (11β-Hsd1L) in gills of hypothermal freshwater milkfish, Chanos chanos. J. Steroid Biochem. Mol. Biol. 2019, 192, 105381. [Google Scholar] [CrossRef]
- Hontela, A.; Rasmussen, J.B.; Audet, C.; Chevalier, G. Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and Mercury. Arch. Environ. Contam. Toxicol. 1992, 22, 278–283. [Google Scholar] [CrossRef]
- Teles, M.; Soares, A.M.V.M.; Tort, L.; Guimarães, L.; Oliveira, M. Linking cortisol response with gene expression in fish exposed to gold nanoparticles. Sci. Total Environ. 2017, 584–585, 1004–1011. [Google Scholar] [CrossRef]
- Canli, E.G.; Dogan, A.; Canli, M. Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Ranabir, S.; Reetu, K. Stress and hormones. Indian J. Endocrinol. Metab. 2011, 1, 18–22. [Google Scholar] [CrossRef]
- Dale, H.H. On some physiological actions of ergot. J. Physiol. 1906, 34, 163–206. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [Green Version]
- Sharpey-Schafer, E.A.; Mackenzie, K. The action of animal extracts on milk secretion. Proc. R. Soc. Lond. B Biol. Sci. 1911, 84, 16–22. [Google Scholar]
- Nishioka, T.; Anselmo-Franci, J.A.; Li, P.; Callahan, M.F.; Morris, M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998, 781, 57–61. [Google Scholar] [CrossRef]
- Torner, L.; Plotsky, P.M.; Neumann, I.D.; Jong, T.R. Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology 2017, 77, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Amico, J.A.; Mantella, R.C.; Vollmer, R.R.; Li, X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004, 16, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hoge, E.A.; Pollack, M.H.; Kaufman, R.E.; Zak, P.J.; Simon, N.M. Oxytocin levels in social anxiety disorder. Cns Neurosci. Ther. 2008, 14, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Wei, F.Y.; Matsunaga, T.; Matsunaga, N.; Kaitsuka, T.; Tomizawa, K. Oxytocin protects against stress-induced cell death in murine pancreatic β-cells. Sci. Rep. 2016, 6, 25185. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.; Ott, V.; Rapp, K.; Brede, S.; Piccinini, F.; Cobelli, C.; Lehnert, H.; Hallschmid, M. Oxytocin improves β-cells responsivity and glucose tolerance in healthy men. Diabetes 2017, 66, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, D.D.; Champagne, F.C.; Meaney, M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000, 12, 1145–1148. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Certin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, M.Y.; Hung, J.C.; Wu, L.C.; Hwang, S.P.L.; Hwang, P.P. Isotocin controls ion regulation through regulating ionocytes progenitor differentiation and proliferation. Cell. Mol. Life Sci. 2011, 68, 2797–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warfvinge, K.; Krause, D.; Edvinsson, L. The distribution of oxytocin and the oxytocin receptor in rat brain: Relation to regions active in migraine. J. Headache Pain. 2020, 21, 10. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, H.; Latt, H.M.; Koga, Y.; Nishiki, T.; Matsui, H. Oxytocin and stress: Neural mechanisms, stress-related disorders and therapeutic approaches. Neuroscience 2019, 417, 1–10. [Google Scholar] [CrossRef]
- Iwama, G.K.; Vijayan, M.M.; Forsyth, R.B.; Ackerman, P.A. Heat shock proteins and physiological stress in fish. Am. Zool. 1999, 39, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.H.; Lee, T.H. Early response of protein quality control in gills is associated with survival of hypertonic shock in Mozambique tilapia. PLoS ONE 2013, 8, e63112. [Google Scholar] [CrossRef] [Green Version]
- Utne-Palm, A.C.; Smith, A. Fish as laboratory animals. In The Welfare of Fish; Kristiansen, T.S., Fernö, A., Pavlids, M.A., van de Vis, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 375–400. [Google Scholar]
- Hatting, J. The influence of carbon dioxide on the blood sugar concentration in the fresh-water fish, Labeo capensis (smith). Comp. Biol. Physiol. A Comp. Physiol. 1976, 53, 235–236. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Liu, Q.; Sun, J.; He, K.; Adam, A.A.; Luo, J.; Li, Z.; Wang, Y.; Yang, S. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Wu, S.M. Role of cortisol in hypoosmoregulation in larvae of the tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 1993, 92, 318–324. [Google Scholar] [CrossRef]
- Landin, J.; Hovey, D.; Xu, B.; Lagman, D.; Zettergren, A.; Larhammar, D.; Kettunen, P.; Westberg, L. Oxytocin receptors regulate social preference in zebrafish. Sci. Rep. 2020, 10, 5435. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Nunes, A.R.; Teles, M.; Anbalagan, S.; Blechman, J.; Levkowitz, G.; Oliveira, R.F. Genetic variation in the social environment affects behavioral phenotypes of oxytocin receoptor mutants in zebrafish. eLife 2020, 9, e56973. [Google Scholar] [CrossRef]
- Outtandy, P.; Russell, C.; Kleta, R.; Bockenhauer, D. Zebrafish as a model for kidney function and disease. Pediatr. Nephrol. 2019, 34, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklósi, A.; Andrew, R.J. The zebrafish as a model for behavioral studies. Zebrafish 2006, 3, 227–234. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, J.; Brun, N.R.; Zhao, Y.; Werdich, A.A. Rapid zebrafish behavioral profiling assay accelerates the identification of environmental neurodevelopmental toxicants. Environ. Sci. Technol. 2021, 55, 1919–1929. [Google Scholar] [CrossRef]
- Taylor, S.E.; Gonzaga, G.C.; Klein, L.C.; Hu, P.; Greendale, G.A.; Seeman, T.E. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006, 68, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Windle, R.J.; Shank, N.; Lightman, S.L.; Ingram, C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety. Endocrinol. 1997, 138, 2829–2834. [Google Scholar] [CrossRef]
- Liberzon, I.; Young, E.A. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 1997, 22, 411–422. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, Y.B.; Park, E.H.; Kim, J.Y.; Ryu, C.; Kimm, H.Y.; Lee, J.; Pahk, K.; Shanyu, C.; Kim, H.; et al. Long-term isolation elicits depression and anxiety-related behaviors by reducing oxytocin-induced GABAergic transmission in central amygdala. Front. Mol. Neurosci. 2018, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, S.M.; Saslow, L.R.; Garcia, N.; John, O.P.; Keltner, D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 21437–21441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, C.; Dannlowski, U.; Bräuer, D.; Steven, S.; Laeger, I.; Wittmann, H.; Kugel, H.; Dobel, C.; Hurlemann, R.; Reif, A.; et al. Oxytocin receptor gene methylation: Converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology 2015, 40, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.; Metz, J.R.; Ampe, B.; Decostere, A.; Filk, G.; Saeger, S.D. Scales tell a story on the stress history of fish. PLoS ONE 2015, 10, e0123411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shagirtha, K.; Priya; Arthi; Arun; Prabu, M. Histopathological and biochemical changes in grass carp gill and muscle tissues due to nickel exposure may be related to the induction of oxidative stress. Eur. J. Biomed. Pharm. 2018, 5, 439–447. [Google Scholar]
- Gesto, M.; Hernández, J.; López-Patiño, M.A.; Soengas, J.L.; Míguez, J.M. Is gill cortisol concentration a good acute stress indicator in fish? A study in rainbow trout and zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 188, 65–69. [Google Scholar] [CrossRef]
- Basu, N.; Kennedy, C.J.; Iwama, G.K. The effects of stress on the association between hsp70 and the glucocorticoid receptor in rainbow trout. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 655–663. [Google Scholar] [CrossRef]
- Stolte, E.H.; Mazon, A.F.; Leon-Koosterziel, K.M.; Jesiak, M.; Bury, N.R.; Sturm, A.; Savelkoul, H.F.J.; Verburg van Kemenade, B.M.L.; Flik, G. Corticosteroid receptors involved in stress regulation in common carp, Cyprinus carpio. J. Endocrinol. 2008, 198, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Saito, D.; Komatsuda, M.; Urano, A. Functional organization of preoptic vasotocin and isotocin neurons in the brain of rainbow trout: Central and neurohypophysial projections of single neurons. Neuroscience 2004, 124, 973–984. [Google Scholar] [CrossRef]
- Duque-Wilckens, N.; Torres, L.Y.; Yokoyama, S.; Minie, V.A.; Tran, A.M.; Petkova, S.P.; Hao, R.; Ramos-Maciel, S.; Rios, R.A.; Jackson, K.; et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA 2020, 117, 26406–26413. [Google Scholar] [CrossRef]
- Steinman, M.Q.; Duque-Wilckens, N.; Greenberg, G.D.; Hao, R.; Campi, K.L.; Laredo, S.A.; Laman-Maharg, A.; Manning, C.E.; Doig, I.E.; Lopez, E.M.; et al. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol. Psychiatry 2016, 80, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Coleman, F.H.; Doheny, K.K.; Travagli, R.A. Stress adaptation upregulates oxytocin within hypothalamo-vagal neurocircuits. Neuroscience 2018, 390, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Babygirijia, R.; Bulbul, M.; Cerjak, D.; Ludwig, K.; Takahashi, T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G946–G953. [Google Scholar] [CrossRef] [Green Version]
- Insel, T.R.; Young, L.; Wang, Z. Central oxytocin and reproductive behaviours. Rev. Reprod. 1997, 2, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Neumann, I.D. The oxytocin receptor: From intracellular signaling to behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Liao, P.Y.; Chiu, Y.M.; Yu, J.H.; Chen, S.K. Mapping central projection of oxytocin neurons in unmated mice using Cre and alkaline phosphatase reporter. Front. Neuroanat. 2020, 14, 559402. [Google Scholar] [CrossRef]
- Wircer, E.; Blechman, J.; Borodovsky, N.; Tsoory, M.; Nunes, A.R.; Oliveira, R.F.; Levkowitz, G. Homeodomain protein Otp affects developmental neuropeptide switching in oxytocin neurons associated with a long-term effect on social behavior. eLife 2017, 6, e22170. [Google Scholar] [CrossRef]
- Wee, C.L.; Nikitchenko, M.; Wang, W.C.; Luks-Morgan, S.J.; Song, E.; Gagnon, J.A.; Randlett, O.; Bianco, I.H.; Lacoste, A.M.B.; Glushenkova, E.; et al. Zebrafish oxytocin neurons drive nocifensive behavior via brainstem premotor targets. Nat. Neurosci. 2019, 22, 1477–1492. [Google Scholar] [CrossRef]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Takayanagi, Y.; Inoue, K.; Kimura, T.; Young, L.J.; Onaka, T.; Nishimori, K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009, 29, 2259–2271. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nakajima, M.; Ibañez-Tallon, I.; Heintz, N. A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors. Cell 2016, 167, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Labuschagne, I.; Phan, K.L.; Wood, A.; Angstadt, M.; Chua, P.; Heinrichs, M.; Stout, J.C.; Nathan, P.J. Oxytocin attenuates amygdala reactivity to fear in generalized social disorder. Neuropsychopharmacology 2010, 35, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Mairesse, J.; Gatta, E.; Reynaert, M.L.; Marrocco, J.; Morley-Fletcher, S.; Soichot, M.; Deruyter, L.; Camp, G.V.; Bouwalerh, H.; Fagioli, F.; et al. Activation of presynaptic oxytocin receptors enhances glutamaterelease in the ventral hippocampus of prenatally restraint stressed rats. Psychoneuroendocrinology 2015, 62, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Von Trotha, J.W.; Vernier, P.; Bally-Cuif, L. Emotions and motivated behavior converge on an amygdala-like structure in the zebrafish. Eur. J. Neurosci. 2014, 40, 3302–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas Luz, W.; Santos-Silva, M.; Cardoso, P.B.; Assad, N.; da Silva Moraes, E.R.; Grisólia, A.B.A.; Braga, D.V.; Leão, L.K.R.; de Moraes, S.A.S.; Passos, A.C.; et al. Putative Activation of the CB1 Cannabinoid Receptors Prevents Anxiety-Like Behavior, Oxidative Stress, and GABA Decrease in the Brain of Zebrafish Submitted to Acute Restraint Stress. Front. Behav. Neurosci. 2021, 14, 598812. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, T.; Saito, K.; Kawano, H.; Kawamura, K.; Shinozuka, K.; Watanabe, S. Pharmacological effects on mirror approaching behavior and neurochemical aspects of the telencephalon in the fish, medaka (Oryzias latipes). Soc. Neurosci. 2009, 4, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Yoshida, H.; Takaishi, M.; Nakamura, R.; Yoshimi, A.; Whitford, T.J.; Hirayasu, Y. Thalamic shape and volume abnormalities in female patients with panic disorder. PLoS ONE 2018, 13, e0208152. [Google Scholar] [CrossRef]
- Linley, S.B.; Athanason, A.C.; Rojas, A.K.P.; Vertes, R.P. Role of the reuniens and rhomboid thalamic nuclei in anxiety-like avoidance behavior in the rat. Hippocampus 2021, 31, 756–769. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Lebowitz, E.R.; Zhang, F.; Hu, Y.; Liu, Z.; Yang, H.; Wu, J.; Wang, Y.; Silverman, W.K.; et al. Abnormal asymmetry of thalamic volume moderates stress from parents and anxiety symptoms in children and adolescents with social anxiety disorder. Neuropharmacology 2020, 180, 108301. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.K.S.; Chow, T.S.C. The effects of environmental resource and security on aggressive behavior. Aggress. Behav. 2017, 43, 304–314. [Google Scholar] [CrossRef]
- DeVries, A.C.; Young, W.S.; Nelson, R.J. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J. Neuroendocrinol. 1997, 9, 363–368. [Google Scholar] [CrossRef]
- Campbell, A. Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biol. Psychol. 2008, 77, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debiec, J. Peptides of love and fear: Vasopressin and oxytocin modulate the integration of information in the amygdala. Bioassays 2005, 27, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Ne’eman, R.; Perach-barzilay, N.; Fischer-Shofty, M.; Atias, A.; Shamay-Tsoory, S.G. Intranasal administration of oxytocin increases human aggressive behavior. Horm. Behav. 2016, 80, 125–131. [Google Scholar] [CrossRef] [PubMed]
- DeWall, C.N.; Gillath, O.; Pressman, S.D.; Black, L.L.; Bartz, J.A.; Moskovitz, J.; Stetler, D.A. When the love hormone leads to violence: Oxytocin increases intimate partner violence inclinations among high trait aggressive people. Soc. Psychol. Personal. Sci. 2014, 5, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Chou, M.Y.; Amo, R.; Kinoshita, M.; Cherng, B.W.; Shimazaki, H.; Agetsuma, M.; Shiraki, T.; Aoki, T.; Takahoko, M.; Yamazaki, M.; et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science 2016, 352, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Nakajo, H.; Chou, M.Y.; Kinoshita, M.; Appelbaum, L.; Shimazaki, H.; Tsuboi, T.; Okamoto, H. Hunger potentiates the habenular winner pathway for social conflict by orexin-promoted biased alternative splicing of the AMPA receptor gene. Cell Rep. 2020, 31, 107790. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimamoto, A.; Boyson, C.O.; DeBold, J.F.; Miczek, K.A. GABA(B) receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J. Neurosci. 2010, 30, 11771–11780. [Google Scholar] [CrossRef]
- Takahashi, A.; Lee, R.X.; Iwasato, T.; Itohara, S.; Arima, H.; Bettler, B.; Miczek, K.A.; Koide, T. Glutamate input in the dorsal raphe nucleus as a determinant of escalated aggression in male mice. J. Neurosci. 2015, 35, 6452–6463. [Google Scholar] [CrossRef] [Green Version]
- Balázsfi, D.; Zelena, D.; Demeter, K.; Miskolczi, C.; Varga, Z.K.; Nagyváradi, A.; Nyíri, G.; Cserép, C.; Baranyi, M.; Sperlágh, B.; et al. Differential roles of the two raphe nuclei in amiable social behavior and aggression-An optogenetic study. Front. Behav. Neurosci. 2018, 12, 163. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.W.; Horng, J.L.; Tong, S.K.; Cherng, B.W.; Liao, B.K.; Lin, L.Y.; Chou, M.Y. Exposure to silver impairs learning and social behaviors in adult zebrafish. J. Hazard. Mater. 2021, 403, 124031. [Google Scholar] [CrossRef]
- Gutnick, A.; Blechmen, J.; Kaslin, J.; Herwig, L.; Belting, H.G.; Affolter, M.; Bonkowsky, J.L.; Levkowitz, G. The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Dev. Cell 2011, 21, 642–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Li, C.; Zeng, Q.; Agrawal, I.; Zhu, X.; Gong, Z. Genome-wide identification of suitable zebrafish Danio rerio reference genes for normalization of gene expression data by RT-qPCR. J. Fish. Biol. 2016, 88, 2095–2110. [Google Scholar] [CrossRef]
- Walker, S.J.; Worst, T.J.; Vrana, K.E. Semiquantitative real-time PCR for analysis of mRNA levels. In Drugs of Abuse; Methods in Molecular Medicine; Wang, J.Q., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 220–221. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Abercrombei, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]




| Genes | Primer Sequence (5′–3′) | Amplicon Size | EF | Ensemble ID |
|---|---|---|---|---|
| oxyr | F: TTCAGCATCCCGCAGGTTTA | 123 bp | 95.4% | ENSDART00000176856.2 |
| R: GCACTGGTCCCTCTTCGTCTT | ||||
| oxyrl | F: ACGCCCTTCTTCTTCGTTCAG | 147 bp | 98.4% | ENSDART00000064853.4 |
| R: TATTTCTCCAGTGCCTCTTACAGC | ||||
| rpl13a | F: CCTCGGTCGTCTTTCCGCTATTG | 247 bp | 95.2% | ENSDART00000180298.1 |
| R: CAGCCTGACCCCTCTTGGTTTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, H.-J.; Chang, C.-Y.; Ho, H.-P.; Chou, M.-Y. Oxytocin Signaling Acts as a Marker for Environmental Stressors in Zebrafish. Int. J. Mol. Sci. 2021, 22, 7459. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147459
Chuang H-J, Chang C-Y, Ho H-P, Chou M-Y. Oxytocin Signaling Acts as a Marker for Environmental Stressors in Zebrafish. International Journal of Molecular Sciences. 2021; 22(14):7459. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147459
Chicago/Turabian StyleChuang, Hsin-Ju, Chun-Yung Chang, Huai-Ping Ho, and Ming-Yi Chou. 2021. "Oxytocin Signaling Acts as a Marker for Environmental Stressors in Zebrafish" International Journal of Molecular Sciences 22, no. 14: 7459. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22147459






