Functional Diversity Effects of Vegetation on Runoff to Design Herbaceous Hedges for Sediment Retention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Functional Traits Measurements
2.3. Characterisation of the Community Functional Structure
2.4. Hydraulic Measurements
2.5. Data Analysis
3. Results
3.1. Variation of Community-Weighted Trait and the Unit Stream Power
3.2. Variation of Functional Diversity within the Communities
3.3. Variation of Functional Diversity and the Unit Stream Power
4. Discussion
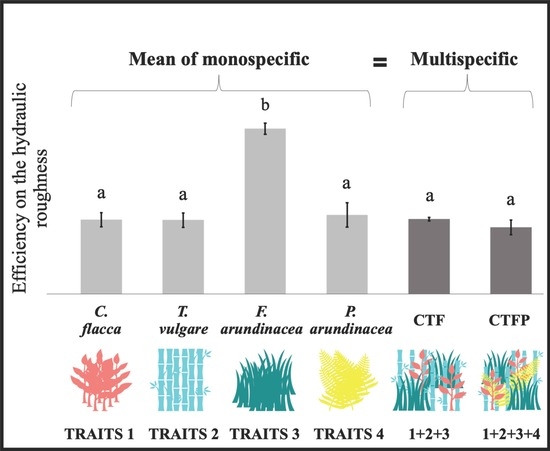
4.1. Non-Additive Effect of the Functional Diversity on Hydraulic Roughness
4.2. Implication to Design Herbaceous Hedges for Sediment Retention
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lavorel, S.; McIntyre, S.; Landsberg, J.; Forbes, T.D.A. Plant functional classifications: From general groups to specific groups based on response to disturbance. Trends Ecol. Evol. 1997, 12, 474–478. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; Chapin, F.S.; Tecco, P.A.; Gurvich, D.E.; Grigulis, K. Functional Diversity—At the Crossroads between Ecosystem Functioning and Environmental Filters. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; pp. 81–91. ISBN 978-3-540-32729-5. [Google Scholar]
- Tilman, D. Functional Diversity. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 109–120. ISBN 978-0-12-226865-6. [Google Scholar]
- Faucon, M.-P.; Houben, D.; Lambers, H. Plant functional traits: Soil and ecosystem services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, P.; Li, G.; Zhou, D. Relationships between functional diversity and ecosystem functioning: A review. Acta Ecol. Sin. 2014, 34, 85–91. [Google Scholar] [CrossRef]
- Cadotte, M.W. Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 2017, 20, 989–996. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quetier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [Green Version]
- Petchey, O.L. Integrating methods that investigate how complementarity influences ecosystem functioning. Oikos 2003, 101, 323–330. [Google Scholar] [CrossRef]
- Loreau, M.; Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, B.; Wang, S.; Zhu, L.; Zhang, L.; Jiao, L.; Wang, C. Reducing soil erosion by improving community functional diversity in semi-arid grasslands. J. Appl. Ecol. 2015, 52, 1063–1072. [Google Scholar] [CrossRef]
- Erktan, A.; Cécillon, L.; Roose, E.; Frascaria-Lacoste, N.; Rey, F. Morphological diversity of plant barriers does not increase sediment retention in eroded marly gullies under ecological restoration. Plant Soil 2013, 370, 653–669. [Google Scholar] [CrossRef]
- Kervroëdan, L.; Armand, R.; Saunier, M.; Faucon, M.-P. Effects of plant traits and their divergence on runoff and sediment retention in herbaceous vegetation. Plant Soil 2019, 441, 511–524. [Google Scholar] [CrossRef]
- Guerrero-Campo, J.; Montserrat-Martí, G. Effects of soil erosion on the floristic composition of plant communities on marl in northeast Spain. J. Veg. Sci. 2000, 11, 329–336. [Google Scholar] [CrossRef]
- Martin, C.; Pohl, M.; Alewell, C.; Körner, C.; Rixen, C. Interrill erosion at disturbed alpine sites: Effects of plant functional diversity and vegetation cover. Basic Appl. Ecol. 2010, 11, 619–626. [Google Scholar] [CrossRef]
- Styczen, M.E.; Morgan, R.P.C. Engineering properties of vegetation. In Slope Stabilization and Erosion Control: A Bioengineering Approach; Morgan, R.P.C., Rickson, R.J., Eds.; E & FN SPON: London, UK, 1995; pp. 4–60. ISBN 978-1-135-83190-5. [Google Scholar]
- Haan, C.T.; Barfield, B.J.; Hayes, J.C. Design Hydrology and Sedimentology for Small Catchments; Academic Press: Cambridge, MA, USA, 1994; ISBN 978-0-08-057164-5. [Google Scholar]
- Cao, L.; Zhang, Y.; Lu, H.; Yuan, J.; Zhu, Y.; Liang, Y. Grass hedge effects on controlling soil loss from concentrated flow: A case study in the red soil region of China. Soil Tillage Res. 2015, 148, 97–105. [Google Scholar] [CrossRef]
- Cantalice, J.R.B.; Silveira, F.P.M.; Singh, V.P.; Silva, Y.J.A.B.; Cavalcante, D.M.; Gomes, C. Interrill erosion and roughness parameters of vegetation in rangelands. CATENA 2017, 148, 111–116. [Google Scholar] [CrossRef]
- Temple, D.M.; Robinson, K.M.; Ahring, R.M.; Davis, A.G. Stability Design of Grass-Lined Open Channels; Agriculture Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1987.
- Akram, S.; Yu, B.; Ghadiri, H.; Rose, C.; Hussein, J. The links between water profile, net deposition and erosion in the design and performance of stiff grass hedges. J. Hydrol. 2014, 510, 472–479. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Bochet, E.; Dutoit, T. Plant functional traits and species ability for sediment retention during concentrated flow erosion. Plant Soil 2012, 353, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Kervroëdan, L.; Armand, R.; Saunier, M.; Ouvry, J.-F.; Faucon, M.-P. Plant functional trait effects on runoff to design herbaceous hedges for soil erosion control. Ecol. Eng. 2018, 118, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Hussein, J.; Yu, B.; Ghadiri, H.; Rose, C. Prediction of surface flow hydrology and sediment retention upslope of a vetiver buffer strip. J. Hydrol. 2007, 338, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Cantalice, J.R.B.; Melo, R.O.; Silva, Y.J.A.B.; Cunha Filho, M.; Araújo, A.M.; Vieira, L.P.; Bezerra, S.A.; Barros, G.; Singh, V.P. Hydraulic roughness due to submerged, emergent and flexible natural vegetation in a semiarid alluvial channel. J. Arid Environ. 2015, 114, 1–7. [Google Scholar] [CrossRef]
- Järvelä, J. Flow resistance of flexible and stiff vegetation: A flume study with natural plants. J. Hydrol. 2002, 269, 44–54. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mason, N.W.H.; MacGillivray, K.; Steel, J.B.; Wilson, J.B. An index of functional diversity. J. Veg. Sci. 2003, 14, 571–578. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and dissimilarity coefficients: A unified approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Leps, J.; De Bello, F.; Lavorel, S.; Berman, S. Quantifying and interpreting functional diversity of natural communities: Practical considerations matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Richet, J.-B.; Ouvry, J.-F.; Saunier, M. The role of vegetative barriers such as fascines and dense shrub hedges in catchment management to reduce runoff and erosion effects: Experimental evidence of efficiency, and conditions of use. Ecol. Eng. 2017, 103, 455–469. [Google Scholar] [CrossRef]
- Yang, C.T. Unit stream power and sediment transport. ASCE J. Hydraul. Div. 1972, 98, 1805–1826. [Google Scholar]
- Govers, G. Relationship between discharge, velocity and flow area for rills eroding loose, non-layered materials. Earth Surf. Process. Landf. 1992, 17, 515–528. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Ouin, A.; Burel, F. Influence of herbaceous elements on butterfly diversity in hedgerow agricultural landscapes. Agric. Ecosyst. Environ. 2002, 93, 45–53. [Google Scholar] [CrossRef]




| Discharges | C | T | F | P | CTF | CTFP | ANOVA |
|---|---|---|---|---|---|---|---|
| Q1 = 2 L s−1 m−1 | 0.0035 (±0.0008) a | 0.0035 (±0.0007) a | 0.0015 (±0.0001) b | 0.0034 (±0.0011) a | 0.0033 (±0.0001) a | 0.0039 (±0.0008) a | 5.29 ** |
| Q2 = 4 L s−1 m−1 | 0.0046 (±0.0007) a | 0.0051 (±0.0009) a | 0.0021 (±0.0001) b | 0.0046 (±0.0011) a | 0.0044 (±0.0002) a | 0.0053 (±0.0008) a | 8.8 *** |
| Q3 = 8 L s−1 m−1 | 0.0066 (±0.001) a | 0.0083 (±0.0013) a | 0.0032 (±0.0002) b | 0.0067 (±0.0013) a | 0.0064 (±0.0004) a | 0.0077 (±0.0015) a | 10.92 *** |
| Q4 = 11 L s−1 m−1 | 0.0078 (±0.0011) a | 0.0105 (±0.0015) a | 0.0038 (±0.0001) b | 0.0079 (±0.0016) a | 0.0077 (±0.0006) a | 0.009 (±0.0017) a | 12.64 *** |
| Community-Weighted Traits | C | T | F | P | CTFP | CTF | ANOVA /Kruskal-Wallis |
|---|---|---|---|---|---|---|---|
| CW-LA (mm2) | 1575.8 (±144.6) a | 4168.5 (±1049.5) b | 3130 (±298.4) bc | 3054.4 (±331.1) bc | 3116.2 (±605.6) bc | 2715.9 (±224.8) ac | 9.28 *** |
| CW-LD5 (dm−2) | 96.3 (±15.6) a | 12 (±2.6) b | 193.1 (±73.3) c | 46.6 (±24.5) a | 91.2 (±7.1) abc | 111.2 (±21.8) abc | 18.99 ** |
| CW-LD10 (dm−2) | 148.8 (±30.8) a | 20.8 (±1.5) b | 280.3 (±79.6) c | 74.9 (±43.1) ab | 154.8 (±37.8) a | 171.7 (±31.4) ac | 15.53 *** |
| CW-LD20 (dm−2) | 236.5 (±43.4) a | 38.8 (±2.6) b | 423.9 (±61.4) c | 108.5 (±61.4) bd | 214.8 (±79.7) ad | 212.8 (±40.2) ad | 24.89 *** |
| CW-DLA5 (mm2 dm−2) | 153,175 (±35,395) a | 50,527 (±20,693) b | 599,417 (±210,360) c | 147,223 (±90,166) ab | 229,484 (±25,910) abc | 251,565 (±59,698) abc | 17.96 ** |
| CW-DLA10 (mm2 dm−2) | 236,709 (±61,527) a | 86,420 (±23,545) b | 868,938 (±216,666) c | 237,161 (±156,858) a | 406,719 (±121,334) abc | 389,669 (±77,960) abc | 17.63 ** |
| CW-DLA20 (mm2 dm−2) | 376,697 (±93,429) a | 163,234 (±48,516) b | 1,325,651 (±212,015) c | 343,506 (±223,961) ab | 573,873 (±251,557) abc | 474,577 (±79,275) abc | 15.91 ** |
| CW-DSA5 (mm2 dm−2) | 4675.3 (±1258.3) a | 2263.4 (±593.7) a | 19,926 (±7469.9) b | 3880.1 (±2061) a | 6088.6 (±1345) ab | 5579.4 (±1499.8) ab | 15.48 ** |
| CW-DSA10 (mm2 dm−2) | 9226.5 (±2471.1) a | 4412.9 (±1116.5) a | 40,673 (±15,603) b | 7458.3 (±3858.2) a | 12,040 (±2561.4) ab | 11,169 (±2975.2) ab | 15.95 ** |
| CW-DSA20 (mm2 dm−2) | 16,841 (±4587.3) a | 8739.8 (±2140.3) a | 82,565 (±30,896) b | 14,356 (±7515.9) a | 23,190 (±5038.9) ab | 21,497 (±5331.6) ab | 16.09 ** |
| CW-DSDm5 (mm dm−2) | 93.5 (±25.2) a | 45.3 (±11.9) a | 398.5 (±149.4) b | 77.6 (±41.2) a | 121.8 (±26.9) ab | 111.6 (±30) ab | 15.48 ** |
| CW-DSDm10 (mm dm−2) | 92.3 (±24.7) a | 44.1 (±11.2) a | 406.7 (±156) b | 74.6 (±38.6) a | 120.4 (±25.6) ab | 111.7 (±29.8) ab | 15.95 ** |
| CW-DSDm20 (mm dm−2) | 91.5 (±24) a | 43.7 (±10.7) a | 412.8 (±154.5) b | 71.8 (±37.6) a | 119.2 (±25.4) ab | 110.6 (±27.1) ab | 15.95 ** |
| Traits | CTF | CTFP | t/W |
|---|---|---|---|
| LA (mm2) | 0.7454 (±0.0836) | 0.7614 (±0.0735) | −0.24864 ns |
| LD5 (dm−2) | 0.9855 (±0.0009) | 0.9883 (±0.0006) | −4.5579 * |
| LD10 (dm−2) | 0.9879 (±0.0008) | 0.9905 (±0.0007) | −4.1791 * |
| LD20 (dm−2) | 0.9889 (±0.0009) | 0.9917 (±0.001) | −3.6474 * |
| DSA5 (mm2 dm−2) | 0.9967 (±0.0002) | 0.9974 (±0.0002) | −4.9464 * |
| DSA10 (mm2 dm−2) | 0.9972 (±0.0002) | 0.9979 (±0.0001) | −5.5758 ** |
| DSA20 (mm2 dm−2) | 0.9976 (±0.0001) | 0.9982 (±0.0001) | −6.6792 ** |
| DSDm5 (mm dm−2) | 0.9878 (±0.0013) | 0.9903 (±0.0009) | −2.7224 ° |
| DSDm10 (mm dm−2) | 0.9878 (±0.0012) | 0.9902 (±0.0009) | −2.8 ° |
| DSDm20 (mm dm−2) | 0.9878 (±0.001) | 0.9902 (±0.0009) | −3.0036 * |
| DLA5 (mm2 dm−2) | 0.9986 (±0.00003) | 0.999 (±0.00003) | 0 ns |
| DLA10 (mm2 dm−2) | 0.9987 (±0.00002) | 0.9991 (±0.0001) | −11.108 ** |
| DLA20 (mm2 dm−2) | 0.9988 (±0.00003) | 0.9992 (±0.0001) | −8.8503 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kervroëdan, L.; Armand, R.; Saunier, M.; Faucon, M.-P. Functional Diversity Effects of Vegetation on Runoff to Design Herbaceous Hedges for Sediment Retention. Diversity 2020, 12, 131. https://0-doi-org.brum.beds.ac.uk/10.3390/d12040131
Kervroëdan L, Armand R, Saunier M, Faucon M-P. Functional Diversity Effects of Vegetation on Runoff to Design Herbaceous Hedges for Sediment Retention. Diversity. 2020; 12(4):131. https://0-doi-org.brum.beds.ac.uk/10.3390/d12040131
Chicago/Turabian StyleKervroëdan, Léa, Romain Armand, Mathieu Saunier, and Michel-Pierre Faucon. 2020. "Functional Diversity Effects of Vegetation on Runoff to Design Herbaceous Hedges for Sediment Retention" Diversity 12, no. 4: 131. https://0-doi-org.brum.beds.ac.uk/10.3390/d12040131