Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Screening of Cardiovascular Agents against Schistosomes
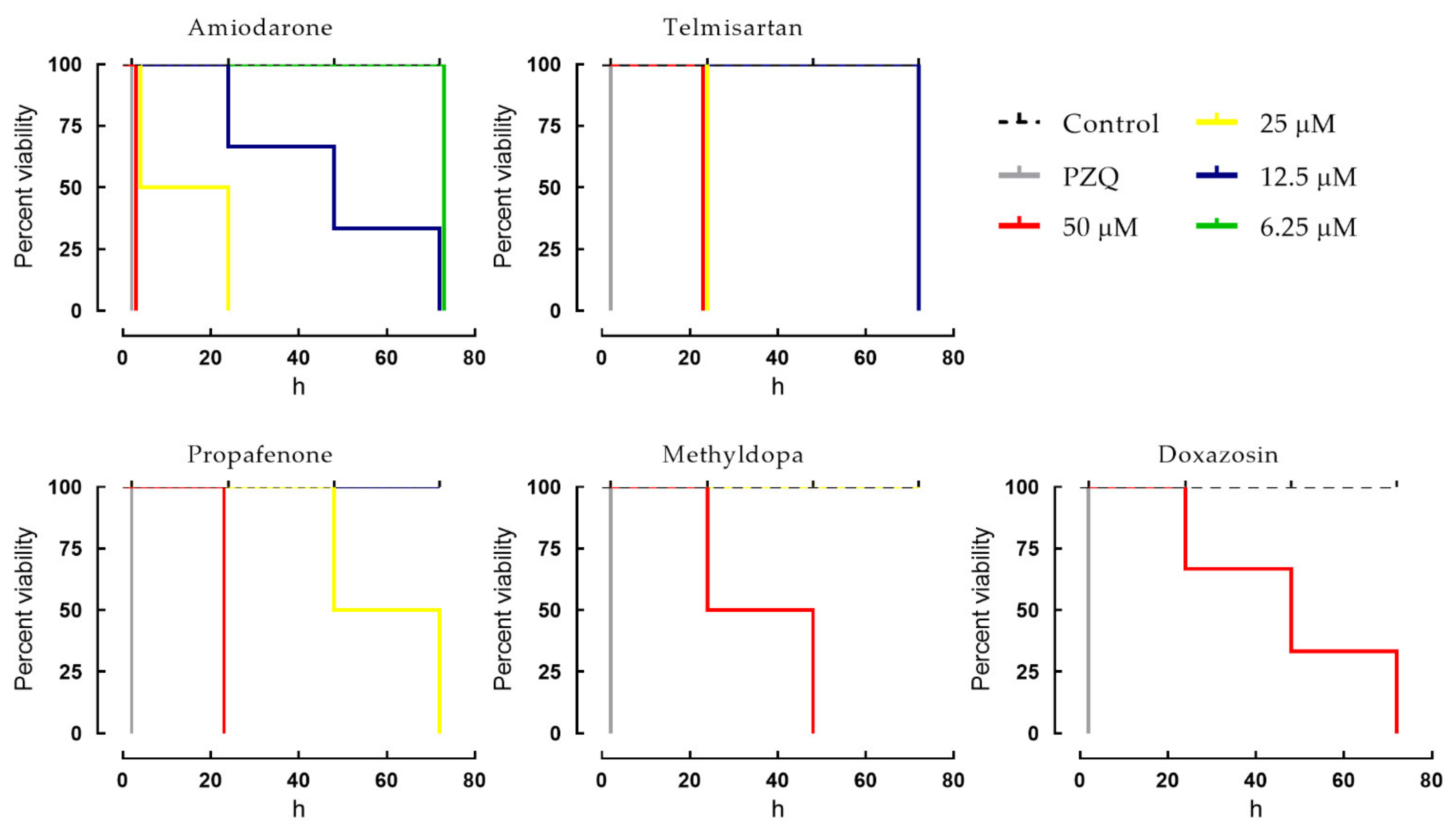
2.2. Dose-Dependent Effects of Cardiovascular Agents against Schistosomes
2.3. Effects of Cardiovascular Agents on the Tegument of Schistosomes
2.4. Antischistosomal Properties of Amiodarone in Mice Harboring Either Chronic or Early S. mansoni Infections
2.4.1. Effect of Amiodarone on Worm Burden
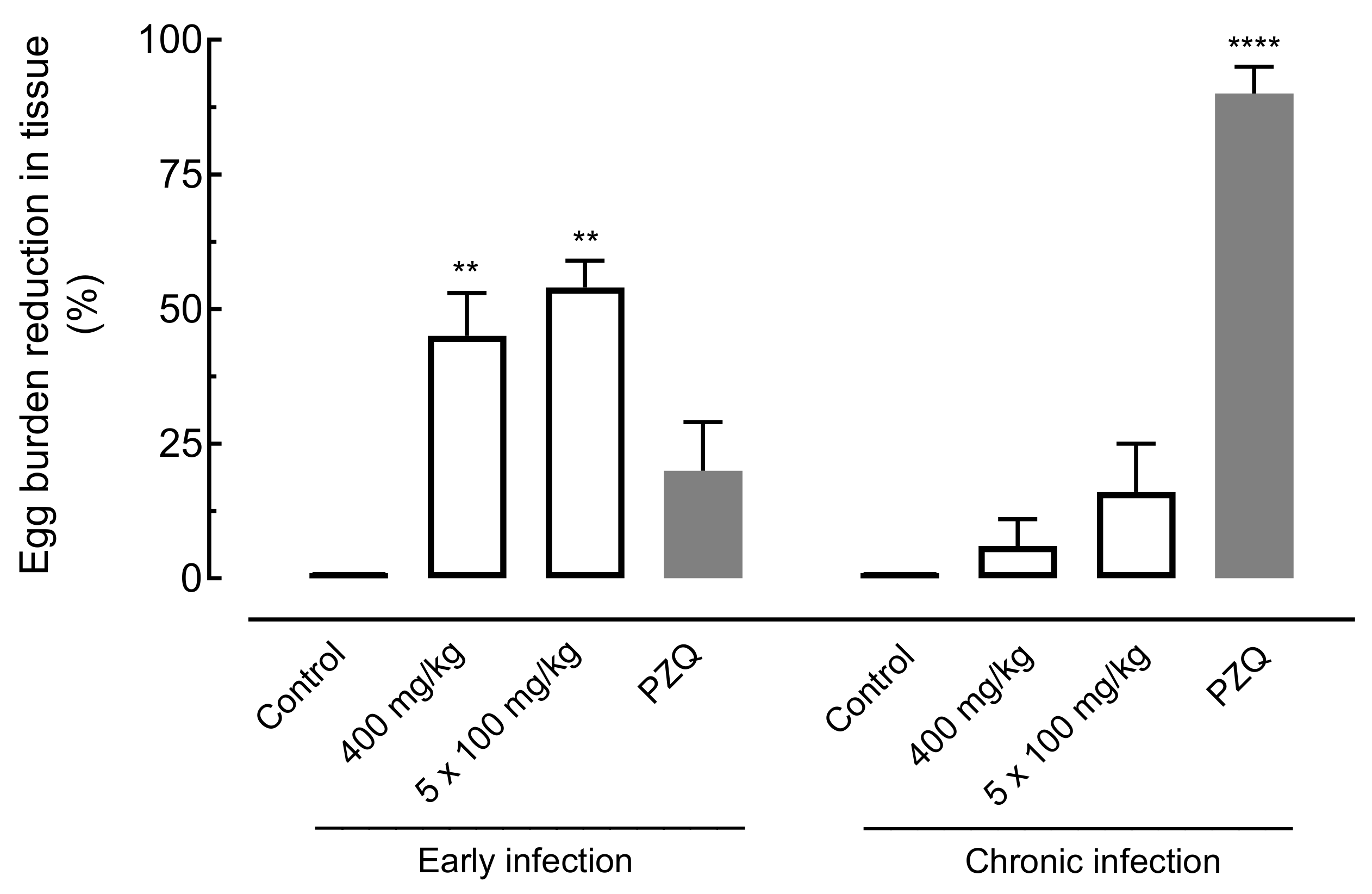
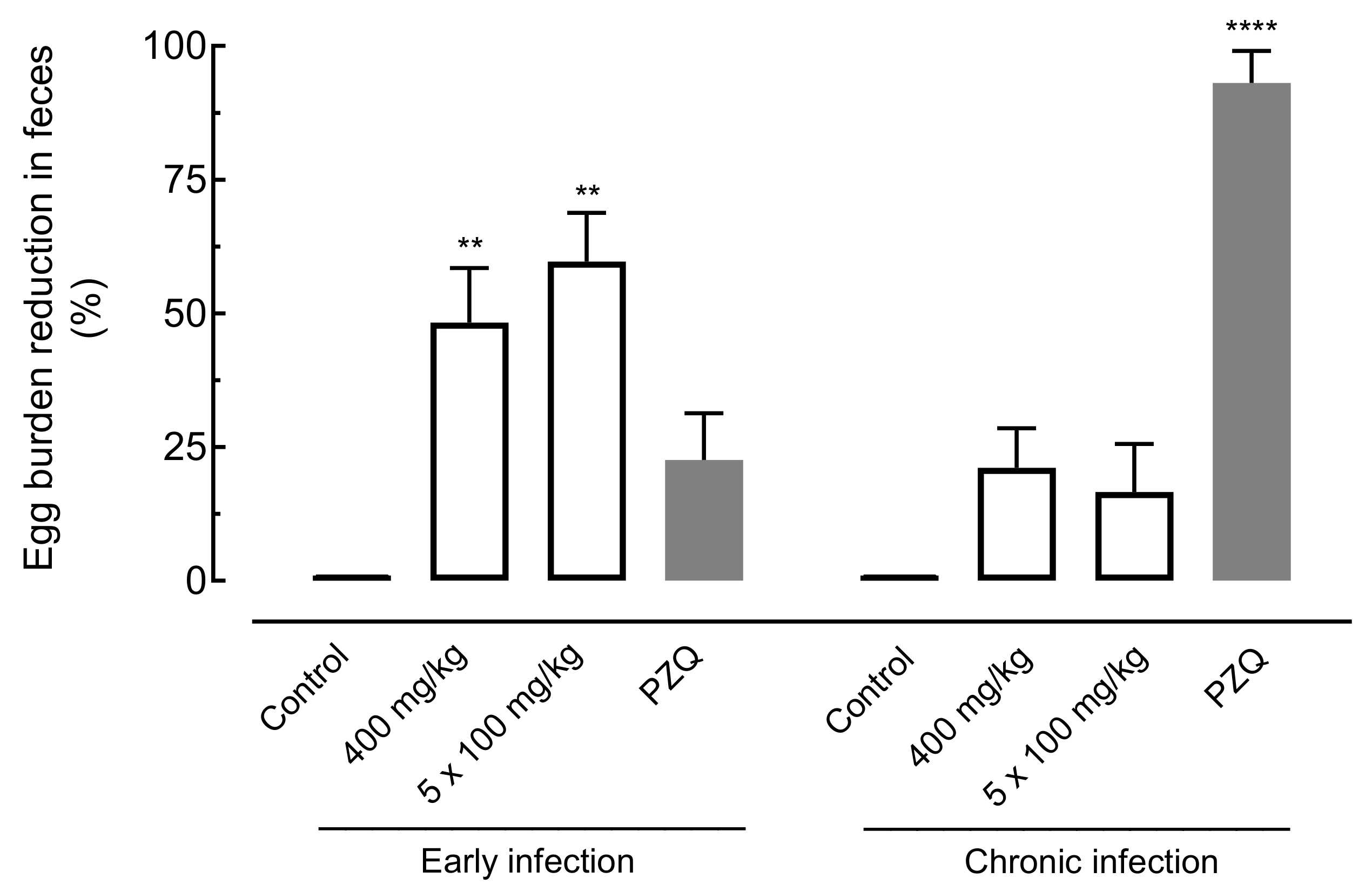
2.4.2. Effect of Amiodarone on Egg Burden
3. Discussion
4. Materials and Methods
4.1. Reagents and Drugs
4.2. Animals and Parasite Maintenance
4.3. Primary Screening
4.4. Secondary Screening
4.5. Scanning Electron Microscopy Analysis
4.6. In Vivo Studies in an Animal Model of Schistosomiasis
4.7. Randomization and Blinding
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McManus, D.P.; Dunne, D.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef]
- World Health Organization. Schistosomiasis. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 16 June 2021).
- Mduluza, T.; Mutapi, F. Putting the treatment of paediatric schistosomiasis into context. Infect. Dis. Poverty 2017, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vale, N.; Gouveia, M.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; da Costa, J.M.C. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017, 61, e02582. [Google Scholar] [CrossRef] [Green Version]
- Kabuyaya, M.; Chimbari, M.J.; Mukaratirwa, S. Efficacy of praziquantel treatment regimens in pre-school and school aged children infected with schistosomiasis in sub-Saharan Africa: A systematic review. Infect. Dis. Poverty 2018, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Spangenberg, T. Alternatives to praziquantel for the prevention and control of schistosomiasis. ACS Infect. Dis. 2021, 7, 939–942. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, J.; Geary, T.G. FDA-approved antiparasitic drugs in the 21st century: A success for helminthiasis? Trends Parasitol. 2020, 36, 573–575. [Google Scholar] [CrossRef]
- Mafud, A.C.; Ferreira, L.L.G.; Mascarenhas, Y.; Andricopulo, A.D.; de Moraes, J. Discovery of novel antischistosomal agents by molecular modeling approaches. Trends Parasitol. 2016, 32, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Lago, E.M.; Xavier, R.P.; Teixeira, T.R.; Silva, L.M.; Filho, A.A.D.S.; de Moraes, J. Antischistosomal agents: State of art and perspectives. Future Med. Chem. 2018, 10, 89–120. [Google Scholar] [CrossRef] [PubMed]
- Lago, E.M.; Silva, M.P.; Queiroz, T.G.; Mazloum, S.F.; Rodrigues, V.D.C.; Carnaúba, P.U.; Pinto, P.L.; Rocha, J.A.; Ferreira, L.L.; Andricopulo, A.D.; et al. Phenotypic screening of nonsteroidal anti-inflammatory drugs identified mefenamic acid as a drug for the treatment of schistosomiasis. EBioMedicine 2019, 43, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roquini, D.B.; Cogo, R.M.; Mengarda, A.C.; Mazloum, S.F.; Morais, C.S.; Xavier, R.P.; Salvadori, M.C.; Teixeira, F.S.; Ferreira, L.E.; Pinto, P.L.; et al. Promethazine exhibits antiparasitic properties in vitro and reduces worm burden, egg production, hepatomegaly, and splenomegaly in a schistosomiasis animal model. Antimicrob. Agents Chemother. 2019, 63, 01208-19. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.P.; Mengarda, A.C.; Silva, M.P.; Roquini, D.B.; Salvadori, M.C.; Teixeira, F.S.; Pinto, P.L.; Morais, T.R.; Ferreira, L.L.G.; Andricopulo, A.D.; et al. H1-antihistamines as antischistosomal drugs: In vitro and in vivo studies. Parasites Vectors 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.C.; Mengarda, A.C.; Silva, B.C.; Relvas-Lima, T.S.; Rodrigues, V.C.; Salvadori, M.C.; Teixeira, F.S.; Lopes, A.F.; Rando, D.G.; de Moraes, J. New evidence for tamoxifen as an antischistosomal agent: In vitro, in vivo and target fishing studies. Future Med. Chem. 2021, 13, 945–957. [Google Scholar] [CrossRef] [PubMed]
- De Brito, M.G.; Mengarda, A.C.; Oliveira, G.L.; Cirino, M.E.; Silva, T.C.; de Oliveira, R.N.; Allegretti, S.M.; de Moraes, J. Therapeutic effect of diminazene aceturate on parasitic blood fluke Schistosoma mansoni infection. Antimicrob. Agents Chemother. 2020, 64, 01372-20. [Google Scholar] [CrossRef]
- Guerra, R.A.; Silva, M.P.; Silva, T.C.; Salvadori, M.; Teixeira, F.S.; de Oliveira, R.N.; Rocha, J.A.; Pinto, P.L.S.; de Moraes, J. In vitro and in vivo studies of spironolactone as an antischistosomal drug capable of clinical repurposing. Antimicrob. Agents Chemother. 2019, 63, 01722-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, J. Natural products with antischistosomal activity. Future Med. Chem. 2015, 7, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Padalino, G.; Chalmers, I.W.; Brancale, A.; Hoffmann, K.F. Identification of 6-(piperazin-1-yl)-1,3,5-triazine as a chemical scaffold with broad anti-schistosomal activities. Wellcome Open Res. 2020, 5, 169. [Google Scholar] [CrossRef]
- De Almeida, L.M.S.; de Carvalho, L.S.A.; Gazolla, M.; Pinto, P.L.S.; da Silva, M.P.N.; de Moraes, J.; Filho, A.A.D.S. Flavonoids and sesquiterpene lactones from Artemisia absinthium and Tanacetum parthenium against Schistosoma mansoni worms. Evid. Based Complement. Altern. Med. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.R.; da Silveira, L.S.; Mengarda, A.C.; Júnior, I.J.A.; da Silva, O.O.Z.; Miguel, F.B.; Silva, M.P.; Almeida, A.D.C.; Torres, D.D.S.; Pinto, P.D.F.; et al. Antischistosomal properties of aurone derivatives against juvenile and adult worms of Schistosoma mansoni. Acta Trop. 2021, 213, 105741. [Google Scholar] [CrossRef]
- De Moraes, J.; De Oliveira, R.N.; Costa, J.; Júnior, A.L.G.; de Sousa, D.; Freitas, R.M.; Allegretti, S.; Pinto, P.L.S. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.P.; de Oliveira, R.N.; Mengarda, A.C.A.; Roquini, D.B.; Allegretti, S.M.; Salvadori, M.; Teixeira, F.S.; de Sousa, D.; Pinto, P.L.; Filho, A.A.D.S.; et al. Antiparasitic activity of nerolidol in a mouse model of schistosomiasis. Int. J. Antimicrob. Agents 2017, 50, 467–472. [Google Scholar] [CrossRef]
- Mengarda, A.; Mendonça, P.S.; Morais, C.S.; Cogo, R.M.; Mazloum, S.F.; Salvadori, M.C.; Teixeira, F.S.; Morais, T.R.; Antar, G.M.; Lago, J.H.G.; et al. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop. 2020, 205, 105350. [Google Scholar] [CrossRef]
- Soares, J.B.R.C.; Menezes, D.; Vannier-Santos, M.; Ferreira-Pereira, A.; Almeida, G.T.; Venancio, T.; Verjovski-Almeida, S.; Zishiri, V.K.; Kuter, D.; Hunter, R.; et al. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PLoS Negl. Trop. Dis. 2009, 3, e477. [Google Scholar] [CrossRef] [Green Version]
- Stahl, E.; Ellis, J. Novel allosteric effects of amiodarone at the muscarinic M5 receptor. J. Pharmacol. Exp. Ther. 2010, 334, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, R.S.; Hill, R.J.; Cannon, N.J.; Duff, H.J. Amiodarone: Biochemical evidence for binding to a receptor for class I drugs associated with the rat cardiac sodium channel. Circ. Res. 1989, 65, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, K.; Kimber, M.J.; Day, T.A.; Ribeiro, P. A constitutively active G protein-coupled acetylcholine receptor regulates motility of larval Schistosoma mansoni. Mol. Biochem. Parasitol. 2015, 202, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, R.M. Ion channels and drug transporters as targets for anthelmintics. Curr. Clin. Microbiol. Rep. 2014, 1, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bais, S.; Greenberg, R.M. TRP channels in schistosomes. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, G.; Madigan, R.T.; Joubert, L.-M.; Bozzi, A.; Sayed, N.; Wu, J.C.; Stevens, D.A. A combination of itraconazole and amiodarone is highly effective against Trypanosoma cruzi infection of human stem cell-derived cardiomyocytes. Am. J. Trop. Med. Hyg. 2019, 101, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Martiín, X.; Garciía-Marchan, Y.; Fernandez, A.; Rodriguez, N.; Rojas, H.; Visbal, G.; Benaim, G. Amiodarone destabilizes intracellular Ca2+ homeostasis and biosynthesis of sterols in Leishmania mexicana. Antimicrob. Agents Chemother. 2009, 53, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Franqueza, L.; Valenzuela, C.; Delpón, E.; Longobardo, M.; Caballero, R.; Tamargo, J. Effects of propafenone and 5-hydroxy-propafenone on hKv1. 5 channels. Br. J. Pharmacol. 1998, 125, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latini, R.; Tognoni, G.; Kates, R.E. Clinical pharmacokinetics of amiodarone. Clin. Pharmacokinet. 1984, 9, 136–156. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Santangeli, P.; Di Biase, L.; Burkhardt, J.D.; Bai, R.; Mohanty, P.; Pump, A.; Natale, A. Examining the safety of amiodarone. Expert Opin. Drug Saf. 2012, 11, 191–214. [Google Scholar] [CrossRef]
- De Moraes, J. Antischistosomal natural compounds: Present challenges for new drug screens. In Current Topics in Tropical Medicine; Rodriguez-Morales, A.J., Ed.; InTech: London, UK, 2012; pp. 333–358. [Google Scholar]
- Sessa, D.P.; Mengarda, A.C.; Simplicio, P.E.; Antar, G.M.; Lago, J.H.G.; De Moraes, J. 15β-Senecioyl-oxy-ent-kaur-16-en-19-oic acid, a diterpene isolated from Baccharis lateralis, as promising oral compound for the treatment of schistosomiasis. J. Nat. Prod. 2020, 83, 3744–3750. [Google Scholar] [CrossRef]
- Dias, M.M.; Zuza, O.; Riani, L.R.; Faria-Pinto, P.; Pinto, P.L.S.; Silva, M.P.; de Moraes, J.; Ataíde, A.C.Z.; Silva, F.D.O.; Cecílio, A.B.; et al. In vitro schistosomicidal and antiviral activities of Arctium lappa L. (Asteraceae) against Schistosoma mansoni and Herpes simplex virus-1. Biomed. Pharmacother. 2017, 94, 489–498. [Google Scholar] [CrossRef]
- Mafud, A.C.; Silva, M.P.; Nunes, G.B.; de Oliveira, M.A.; Batista, L.F.; Rubio, T.; Mengarda, A.C.A.; Lago, E.M.; Xavier, R.P.; Gutierrez, S.J.; et al. Antiparasitic, structural, pharmacokinetic, and toxicological properties of riparin derivatives. Toxicol. Vitr. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.A.; De Oliveira, R.N.; De Almeida, R.L.; Mafud, A.C.; Sarkis, A.L.V.; Ganassin, R.; Da Silva, M.P.; Roquini, D.B.; Veras, L.M.; Sawada, T.C.H.; et al. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. PLoS ONE 2018, 13, e0196667. [Google Scholar] [CrossRef] [PubMed]
- De Brito, M.R.; Peláez, W.J.; Faillace, M.S.; Militão, G.C.; Almeida, J.R.; Argüello, G.A.; Szakonyi, Z.; Fülöp, F.; Salvadori, M.; Teixeira, F.S.; et al. Cyclohexene-fused 1,3-oxazines with selective antibacterial and antiparasitic action and low cytotoxic effects. Toxicol. Vitr. 2017, 44, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Filho, G.C.L.; Bueno-Silva, B.; de Moraes, J. Brazilian red propolis exhibits antiparasitic properties in vitro and reduces worm burden and egg production in a mouse model harboring either early or chronic Schistosoma mansoni infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef]
- Lombardo, F.C.; Pasche, V.; Panic, G.; Endriss, Y.; Keiser, J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 2019, 14, 461–481. [Google Scholar] [CrossRef]
- Amorim, C.R.; Pavani, T.; Lopes, A.F.; Duque, M.; Mengarda, A.C.; Silva, M.P.; de Moraes, J.; Rando, D.G. Schiff bases of 4-phenyl-2-aminothiazoles as hits to new antischistosomals: Synthesis, in vitro, in vivo and in silico studies. Eur. J. Pharm. Sci. 2020, 150, 105371. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, J.; Oliveira, C.A.; Faria, J.; Cunha, A.S. New approach to the screening of drugs in experimental Schistosomiasis mansoni in mice. Am. J. Trop. Med. Hyg. 1962, 11, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Mengarda, A.C.; Silva, M.P.; Cirino, M.E.; Morais, T.R.; Conserva, G.A.A.; Lago, J.H.G.; de Moraes, J. Licarin A, a neolignan isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), exhibited moderate preclinical efficacy against Schistosoma mansoni infection. Phytother. Res. 2021. [Google Scholar] [CrossRef]
- De Moraes, J.; Dario, B.S.; Couto, R.A.; Pinto, P.L.S.; Ferreira, A.M.D.C. Antischistosomal activity of oxindolimine-metal complexes. Antimicrob. Agents Chemother. 2015, 59, 6648–6652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rando, D.G.G.; Da Costa, M.O.L.; Pavani, T.; Oliveira, T.; Dos Santos, P.F.; Amorim, C.R.; Pinto, P.L.; De Brito, M.G.; Silva, M.P.N.; Roquini, D.B.; et al. Vanillin-related N-acylhydrazones: Synthesis, antischistosomal properties and target fishing studies. Curr. Top. Med. Chem. 2019, 19, 1241–1251. [Google Scholar] [CrossRef]



| Drug | Class | EC50 (µM) | EC90 (µM) | |
|---|---|---|---|---|
| 1 | Atenolol | Beta-Adrenoceptor Antagonist | >50 | >50 |
| 2 | Bisoprolol | >50 | >50 | |
| 3 | Carvedilol | >50 | >50 | |
| 4 | Esmolol | >50 | >50 | |
| 5 | Metoprolol | >50 | >50 | |
| 6 | Nebivolol | >50 | >50 | |
| 7 | Propranolol | >50 | >50 | |
| 8 | Propafenone | 25.7 ± 4.2 | 40.9 ± 3.5 | |
| 9 | Amlodipine | Calcium Channel Blocker | >50 | >50 |
| 10 | Diltiazem | >50 | >50 | |
| 11 | Felodipine | >50 | >50 | |
| 12 | Isradipine | >50 | >50 | |
| 13 | Nifedipine | >50 | >50 | |
| 14 | Nitrendipine | >50 | >50 | |
| 15 | Verapamil | >50 | >50 | |
| 16 | Amiodarone | Potassium Channel Blocker | 8.2 ± 0.8 | 11.4 ± 1.2 |
| 17 | Quinidine | >50 | >50 | |
| 18 | Benazepril | Angiotensin-Converting Enzyme | >50 | >50 |
| 19 | Captopril | (ACE) Inhibitor | >50 | >50 |
| 20 | Enalapril | >50 | >50 | |
| 21 | Fosinopril | >50 | >50 | |
| 22 | Lisinopril | >50 | >50 | |
| 23 | Perindopril | >50 | >50 | |
| 24 | Quinacril | >50 | >50 | |
| 25 | Ramipril | >50 | >50 | |
| 26 | Losartan | Angiotensin II Receptor Antagonist | >50 | >50 |
| 27 | Olmesartan | >50 | >50 | |
| 28 | Telmisartan | 11.5 ± 1.6 | 20.6 ± 3.7 | |
| 29 | Valsartan | >50 | >50 | |
| 30 | Doxazosin | Alpha-Adrenoceptor Antagonist | 37.8 ± 3.3 | 49.4 ± 2.8 |
| 31 | Prazosin | >50 | >50 | |
| 32 | Clonidine | Alpha2-Adrenoceptor Agonist | >50 | >50 |
| 33 | Guanabenz | >50 | >50 | |
| 34 | Methyldopa | 30.1 ± 3.8 | 45.6 ± 4.3 | |
| 35 | Hydralazine | Vasodilator | >50 | >50 |
| 36 | Isosorbide mononitrate | >50 | >50 | |
| 37 | Nitroglycerin | >50 | >50 | |
| 38 | Nitroprusside | >50 | >50 | |
| 39 | Papaverine | >50 | >50 | |
| 40 | Propatilnitrate | >50 | >50 | |
| 41 | Dobutamine | Beta1-Adrenergic Receptor Agonist | >50 | >50 |
| 42 | Etilefrine | Alpha1-Adrenoceptor Agonist | >50 | >50 |
| 43 | Digoxin | Cardiac Glycoside; Na+/K+-ATPase Inhibitor | >50 | >50 |
| 44 | Sildenafil | Phosphodiesterase Type 5 (PDE5) Inhibitor | >50 | >50 |
| 45 | Warfarin | Anticoagulant | >50 | >50 |
| 46 | Clopidogrel | Antiplatelet Aggregation | >50 | >50 |
| 47 | Praziquantel | 0.98 ± 0.3 | 1.4 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, R.; Mengarda, A.C.; Cajas, R.A.; Salvadori, M.C.; Teixeira, F.S.; Arcanjo, D.D.R.; Siyadatpanah, A.; Pereira, M.d.L.; Wilairatana, P.; Moraes, J.d. Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni. Pharmaceuticals 2021, 14, 686. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070686
Porto R, Mengarda AC, Cajas RA, Salvadori MC, Teixeira FS, Arcanjo DDR, Siyadatpanah A, Pereira MdL, Wilairatana P, Moraes Jd. Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni. Pharmaceuticals. 2021; 14(7):686. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070686
Chicago/Turabian StylePorto, Raquel, Ana C. Mengarda, Rayssa A. Cajas, Maria C. Salvadori, Fernanda S. Teixeira, Daniel D. R. Arcanjo, Abolghasem Siyadatpanah, Maria de Lourdes Pereira, Polrat Wilairatana, and Josué de Moraes. 2021. "Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni" Pharmaceuticals 14, no. 7: 686. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070686








