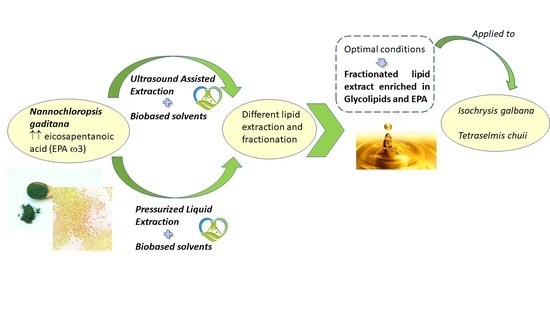
Biobased Solvents for Pressurized Liquid Extraction of Nannochloropsis gaditana Omega-3 Lipids
Abstract
:1. Introduction
2. Results
2.1. Ultrasound Assisted Extraction of Nannochloropsis Gaditana
2.2. Pressurized Liquid Extraction of Nannochloropsis Gaditana
2.3. Analysis of Microalgal Lipids Classes by HPLC-ELSD
2.4. Determination of Fatty Acid Profile of Microalgae Extracts by GC-MS
2.5. Application of Lipid Extraction Method to Other Microalgae
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of Microalgal Biomass
4.2.1. Ultrasound Assisted Extraction
4.2.2. Pressurized Liquid Extraction
4.3. Nannochloropsis Gaditana Extracts Chemical Analysis
4.3.1. HPLC-ELSD Analysis
4.3.2. Fatty Acid Composition by GC-MS
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, I.; Fleurence, J. Microalgae in Health and Disease Prevention; Academic Press: London, UK, 2018. [Google Scholar]
- Sathasivam, R.; Ramalingam, R.; Hashem, A.; Allah, E.F.A. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Virginie, M.; Couzinet-Mossion, A.; Ulmann, L.; Wielgosz-Collin, G. Lipids from Microalgae in Microalgae in Health and Disease Prevention; Levine, I., Fleurence, J., Eds.; Academic Press: London, UK, 2018; pp. 109–131. [Google Scholar]
- Castejón, N.; Señoráns, F.J. Enzymatic modification to produce health-promoting lipids from fish oil, algae and other new omega-3 sources: A review. New Biotechnol. 2020, 57, 45–54. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Jie, X.; Wang, Y.-M.; Xue, C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef]
- Che, H.; Cui, J.; Wen, M.; Xu, J.; Yanagita, T.; Wang, Q.; Xue, C.; Wang, Y. Long Term Effects of Docosahexaenoic Acid Bound Phospholipids and the Combination of Docosahexaenoic Acid Bound Triglyceride and Egg Yolk Phospholipid on Lipid Metabolism in Mice. J. Ocean Univ. China 2018, 17, 392–398. [Google Scholar]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2019, 19, 64–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castejón, N.; Francisco, J.S. Simultaneous extraction and fractionation of omega-3 acylglycerols and glycolipids from wet microalgal biomass of Nannochloropsis gaditana using pressurized liquids. Algal Res. 2019, 37, 74–82. [Google Scholar] [CrossRef]
- Vaz, B.D.S.; Moreira, J.B.; De Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Okolie, C.L.; Taiwo, O.A.; Beth, M.; Chibuike, C.U.; Alberta, N.A.A. Influence of conventional and recent extraction technologies on physicochemical properties of bioactive macromolecules from natural sources: A review. Food Res. Int. 2019, 116, 827–839. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium sp. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from Mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Denis, P.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar]
- De Jesus, S.S.; Ferreira, G.F.; Moreira, L.S.; Maciel, M.R.W.; Filho, R.M. Comparison of several methods for effective lipid extraction from wet microalgae using green solvents. Renew. Energy 2019, 143, 130–141. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Ferreira, G.F.; Fregolente, L.V.; Filho, R.M. Laboratory extraction of microalgal lipids using sugarcane bagasse derived green solvents. Algal Res. 2018, 35, 292–300. [Google Scholar] [CrossRef]
- Señoráns, M.; Castejón, N.; Señoráns, F.J. Advanced extraction of lipids with DHA from Isochrysis galbana with enzymatic pre-treatment combined with pressurized liquids and ultrasound assisted extractions. Molecules 2020, 25, 3310. [Google Scholar] [CrossRef]
- Peña, E.H.; Medina, A.R.; Callejon, M.J.J.; Sanchez, M.D.M.; Cerdán, L.E.; Moreno, P.A.G.; Grima, E.M. Extraction of free fatty acids from wet Nannochloropsis gaditana biomass for biodiesel production. Renew. Energy 2015, 75, 366–373. [Google Scholar] [CrossRef]
- Callejón, M.J.J.; Medina, A.R.; Sánchez, M.D.M.; Cerdán, L.E.; Moreno, P.A.G.; López, E.N.; Peña, E.H.; Grima, E.M. Obtaining highly pure EPA-rich lipids from dry and wet Nannochloropsis gaditana microalgal biomass using ethanol, hexane and acetone. Algal Res. 2020, 45, 101729. [Google Scholar] [CrossRef]
- Sicaire, A.G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative bio-based solvents for extraction of fat and oils: Solubility prediction, global yield, extraction kinetics, chemical composition and cost of manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef]
- Figueiredo, A.R.; Da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; George, B.; Shah, F.; Mishra, S. A euryhaline Nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production. Algal Res. 2015, 8, 161–167. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Mantecón, L.; Moyano, R.; Cameán, A.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chuii (TetraSOD®) in a 90 day feeding study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, J.-Y.; Pan, X.-R.; Zhang, F.; Ma, L.-L.; Wang, H.-J.; Zeng, R.J. Different DHA or EPA production responses to nutrient stress in the marine microalga Tisochrysis lutea and the freshwater microalga Monodus subterraneus. Sci. Total. Environ. 2019, 656, 140–149. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, Z.; Zhong, C.; Guo, Z.; Chen, B. Pressurized liquid extraction with ethanol as a green and efficient technology to lipid extraction of Isochrysis biomass. Bioresour. Technol. 2019, 293, 122049. [Google Scholar] [CrossRef]
- Ohse, S.; Derner, R.B.; Ozório, R.Á.; Corrêa, R.G.; Furlong, E.B.; Cunha, P.C.R. Lipid content and fatty acid profiles in ten species of microalgae. Idesia 2015, 33, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Wang, J.; Yang, G.; Zhu, B.; Pan, K. Biomass and nutrient productivities of Tetraselmis chuii under mixotrophic culture conditions with various C:N ratios. Chin. J. Oceanol. Limnol. 2016, 35, 303–312. [Google Scholar] [CrossRef]
- Simple Interactive Statistical Analysis (SISA). Online Software. Available online: http://www.quantitativeskills.com/sisa/statistics/t-test.htm (accessed on 11 February 2021).










| Fatty Acid | Rt (min) | % Fatty Acids | ||||||
|---|---|---|---|---|---|---|---|---|
| Ethanol | 2-MeTHF | 2-MeTHF:Ethanol (1:3) | Hexane: Ethanol (3:4) | MTBE:Ethanol (1:3) | 2-MeTHF:isobutanol (1:3) | 2-MeTHF:isopropanol (1:3) | ||
| 14:0 | 9.70 | 4.74 ± 0.11 ᵃ | 5.34 ± 0.35 ᵃ | 4.76 ± 0.76 ᵃ | 4.73 ± 0.28 ᵃ | 5.18 ± 0.88 ᵃ | 4.70 ± 0.25 ᵃ | 4.77 ± 0.46 ᵃ |
| 16:0 | 11.77 | 16.64 ± 1.21 ᵃ | 17.11 ± 0.66 ᵃ | 16.83 ± 0.93 ᵃ | 14.47 ± 0.77 ᵃ | 18.19 ± 0.50 ᵃ | 17.13 ± 1.09 ᵃ | 17.65 ± 0.57 ᵃ |
| 16:1 | 12.50 | 23.07 ± 0.74 ᵃ | 25.13 ± 1.01 ᵃ | 24.57 ± 0.48 ᵃ | 23.44± 0.62 ᵃ | 25.74 ± 1.36 ᵃ | 24.96 ± 0.67 ᵃ | 25.31 ± 0.18 ᵃ |
| 18:0 | 14.59 | 0.49 ± 0.23 ᵃ | 0.40 ± 0.10 ᵃ | 0.61 ± 0.55 ᵃ | 0.97± 0.15 ᵃ | 0.76 ± 0.20 ᵃ | 0.46± 0.43 ᵃ | 0.39 ± 0.13 ᵃ |
| 18:1 | 15.59 | 4.17 ± 0.12 ᵃ | 4.54 ± 0.50 ᵃ | 4.47 ± 0.58 ᵃ | 4.42± 0.59 ᵃ | 4.38 ± 0.55 ᵃ | 4.34 ± 0.82 ᵃ | 4.49 ± 0.28 ᵃ |
| 18:2 | 17.12 | 3.37 ± 1.01 ᵃ | 4.14 ± 0.34 ᵃ | 3.74 ± 0.26 ᵃ | 3.75± 0.28 ᵃ | 3.57 ± 0.64 ᵃ | 3.69 ± 0.72 ᵃ | 3.29 ± 1.09 ᵃ |
| 20:3 | 21.90 | 0.51 ± 0.52 ᵃ | 0.60 ± 0.12 ᵃ | 0.79 ± 0.33 ᵃ | 1.19 ± 0.40 ᵃ | 0.41 ± 0.16 ᵃ | 0.62 ± 0.41 ᵃ | 0.65 ± 0.10 ᵃ |
| 20:4 | 22.69 | 7.92 ± 0.48 ᵃ | 7.98 ± 0.58 ᵃ | 8.60± 0.85 ᵃ | 8.46 ± 0.94 ᵃ | 8.26 ± 0.88 ᵃ | 7.77± 0.85 ᵃ | 8.01 ± 1.05 ᵃ |
| 20:5 | 24.61 | 37.38 ± 0.66 ᵃᵇ | 34.93 ± 0.69 ᵇ | 36.25 ± 0.42 ᵃᵇ | 38.54 ± 0.93 ᵃ | 33.91 ± 0.55 ᵇ | 36.33 ± 0.68 ᵃᵇ | 35.42 ± 1.33 ᵃᵇ |
| SFA | 21.87 ᵃ | 22.85 ᵇ | 22.20 ᵃᵇ | 20.17 ᶜ | 24.13 ͩ | 22.29 ᵃᵇ | 22.81 ᵇ | |
| MUFA | 27.24 ᵃ | 29.67 ᵇ | 29.04 ᵇ | 27.86 ᵃ | 30.12 ᵇ | 29.30 ᵇ | 29.80 ᵇ | |
| PUFA | 49.18 ᵃ | 47.65 ᵇ | 48.74 ᵃ | 51.94 ᵃ | 46.15 ᵇ | 48.41 ᵃ | 47.37 ᵇ | |
| n-3 | 37.89 ᵃ | 35.54 ᵇ | 37.04 ᵃ | 39.73 ᶜ | 34.32 ᵇ | 36.95 ᵃᵇ | 36.07 ᵇ | |
| n-6 | 11.29 ᵃ | 12.12 ᵃ | 12.34 ᵃ | 12.21 ᵃ | 11.83 ᵃ | 11.46 ᵃ | 11.30 ᵃ | |
| n-6/n-3 | 0.30 ᵃ | 0.34 ᵃ | 0.33 ᵃ | 0.31 ᵃ | 0.34 ᵃ | 0.31 ᵃ | 0.31 ᵃ | |
| Fatty Acid | % Fatty Acids | ||
|---|---|---|---|
| N. gaditana | I. galbana | T. chuii | |
| 14:0 | 4.74 ± 0.11 ᵃ | 22.86 ± 0.59 ᵇ | - |
| 16:0 | 16.64 ± 1.21 ᵃ | 15.11 ± 0.87 ᵃ | 22.02 ± 0.55 ᵃ |
| 16:1 | 23.07 ± 0.74 ᵃ | 18.2 ± 0.33 ᵃ | 1.45 ± 0.57 ᵇ |
| 17:0 | - | 2.85 ± 0.15 ᵃ | - |
| 18:0 | 0.49 ± 0.23 ᵃ | 1.60 ± 0.28 ᵇ | - |
| 18:1 | 4.17 ± 0.12 ᵃ | 9.93 ± 0.46 ᵇ | 11.12 ± 0.22 ᵇ |
| 18:2 | 3.37 ± 1.01 ᵃ | 9.33 ± 0.13 ᵇ | - |
| 18:3 | - | 12.84 ± 0.40 ᵃ | 24.01 ± 0.72 ᵇ |
| 20:0 | - | - | 20.39 ± 0.86 ᵃ |
| 20:3 | 0.51 ± 0.52 ᵃ | - | 7.39 ± 0.28 ᵇ |
| 20:4 | 7.92 ± 0.48 ᵃ | - | - |
| 20:5 | 37.38 ± 0.66 ᵃ | - | 5.13 ± 0.74 ᵇ |
| 22:0 | - | - | 3.56 ± 0.33 ᵃ |
| 22:6 | - | 7.27 ± 0.28 ᵃ | - |
| SFA | 21.87 ᵃ | 42.42 ᵇ | 42.41 ᵇ |
| MUFA | 27.24 ᵃ | 28.13 ᵇ | 12.57 ᶜ |
| PUFA | 49.18 ᵃ | 29.44 ᵇ | 40.09 ᶜ |
| n-3 | 37.89 ᵃ | 20.11 ᵇ | 29.14 ᶜ |
| n-6 | 11.29 ᵃ | 9.33 ᵇ | 7.39 ᶜ |
| n-6/n-3 | 0.30 ᵃ | 0.46 ᵃ | 0.25 ᵃ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Llamero, C.; Señoráns, F.J. Biobased Solvents for Pressurized Liquid Extraction of Nannochloropsis gaditana Omega-3 Lipids. Mar. Drugs 2021, 19, 107. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020107
Blanco-Llamero C, Señoráns FJ. Biobased Solvents for Pressurized Liquid Extraction of Nannochloropsis gaditana Omega-3 Lipids. Marine Drugs. 2021; 19(2):107. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020107
Chicago/Turabian StyleBlanco-Llamero, Cristina, and F. Javier Señoráns. 2021. "Biobased Solvents for Pressurized Liquid Extraction of Nannochloropsis gaditana Omega-3 Lipids" Marine Drugs 19, no. 2: 107. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020107