Provincial Dietary Intake Study (PDIS): Energy and Macronutrient Intakes of Children in a Representative/Random Sample of 1–<10-Year-Old Children in Two Economically Active and Urbanized Provinces in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Structure of the Sample and the Sampling Procedure
2.3. Selection of Households
2.4. Selection of Children within Households
2.5. Fieldwork Teams
2.6. Measures
2.6.1. Sociodemographic Questionnaire
2.6.2. Hunger Scale Questionnaire
2.6.3. Dietary Intake
2.6.4. Anthropometry of Mothers
2.7. Data Analyses
2.8. Ethics
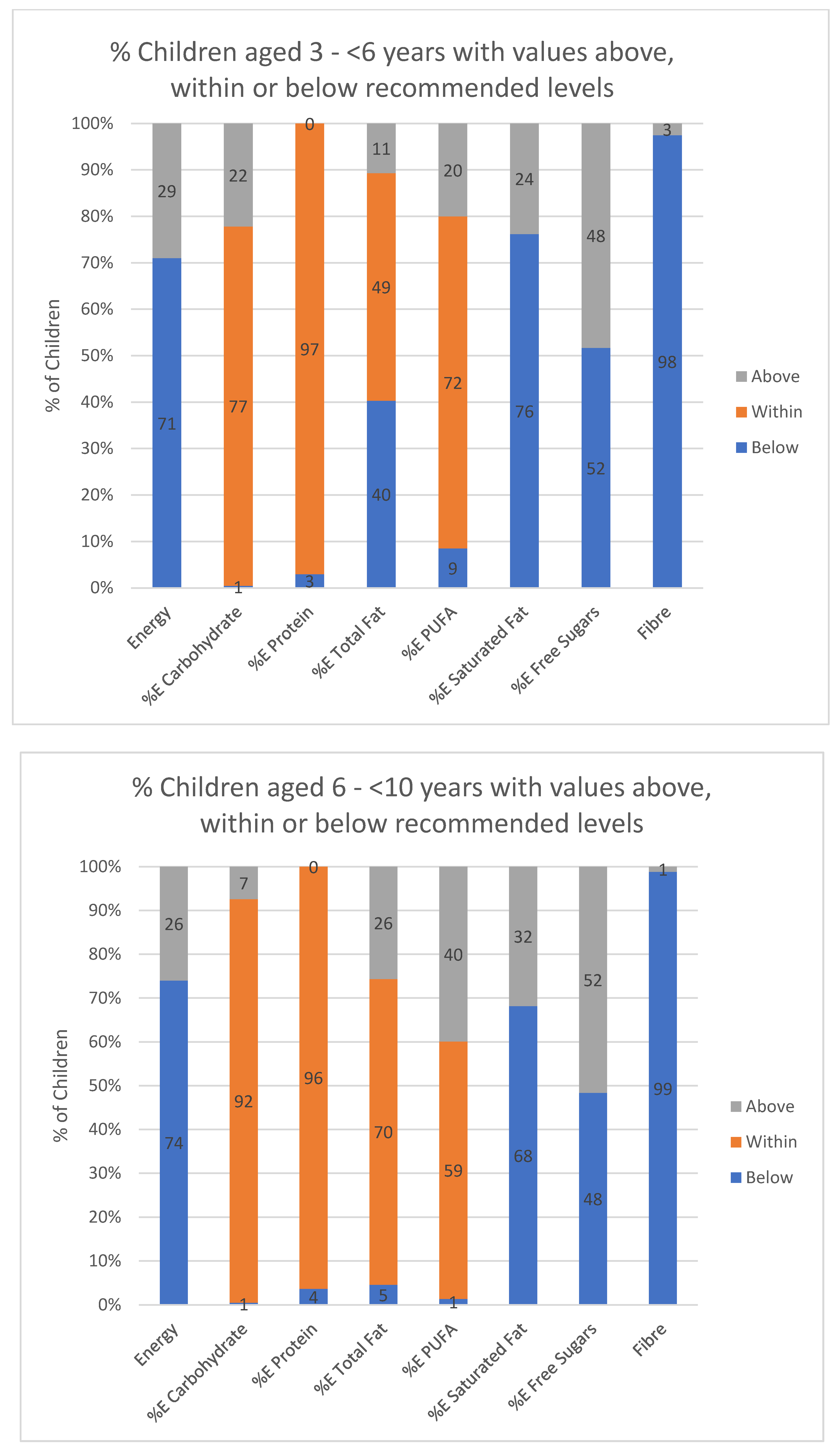
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nasreddine, L.M.; Kassis, A.N.; Ayoub, J.J.; Naja, F.A.; Hwalla, N.C. Nutritional status and dietary intakes of children amid the nutrition transition: The case of the Eastern Mediterranean Region. Nutr. Res. 2018, 57, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The Prevalence of Micronutrient Deficiencies and Inadequacies in the Middle East and Approaches to Interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Winichagoon, P. Transition of maternal and child nutrition in Asia: Implications for public health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Conde, W.L.; Monteiro, C.A. Nutrition transition and double burden of undernutrition and excess of weight in Brazil. Am. J. Clin. Nutr. 2014, 100, 1617S–1622S. [Google Scholar] [CrossRef] [PubMed]
- Tzioumis, E.; Adair, L.S. Childhood dual burden of under- and overnutrition in low- and middle-income countries: A critical review. Food Nutr. Bull. 2014, 35, 230–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016, 12, 12–26. [Google Scholar] [CrossRef]
- Mendez, M.A.; Adair, L.S. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J. Nutr. 1999, 129, 1555–1562. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A. Long-Term Adverse Effects of Early Growth Acceleration or Catch-Up Growth. Ann. Nutr. Metab. 2017, 70, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Senekal, M.; Nel, J.H.; Malczyk, S.; Drummond, L.; Harbron, J.; Steyn, N.P. Provincial Dietary Intake Study (PDIS): Prevalence and Sociodemographic Determinants of the Double Burden of Malnutrition in A Representative Sample of 1 to Under 10-Year-Old Children from Two Urbanized and Economically Active Provinces in South Africa. Int. J. Environ. Res. Public Health 2019, 16, 3334. [Google Scholar] [CrossRef] [Green Version]
- Steyn, N.P.; McHiza, Z.J. Obesity and the nutrition transition in Sub-Saharan Africa. Ann. N. Y. Acad. Sci. 2014, 1311, 88–101. [Google Scholar] [CrossRef]
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef]
- Bishwajit, G. Nutrition transition in South Asia: The emergence of non-communicable chronic diseases. F1000Res 2015, 4, 8. [Google Scholar] [CrossRef]
- Ochola, S.; Masibo, P.K. Dietary intake of schoolchildren and adolescents in developing countries. Ann. Nutr. Metab. 2014, 64, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Bosu, W.K. An overview of the nutrition transition in West Africa: Implications for non-communicable diseases. Proc. Nutr. Soc. 2015, 74, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Labadarios, D.; Steyn, N.P.; Maunder, E.; MacIntryre, U.; Gericke, G.; Swart, R.; Huskisson, J.; Dannhauser, A.; Vorster, H.H.; Nesmvuni, A.E.; et al. The National Food Consumption Survey (NFCS): South Africa, 1999. Public Health Nutr. 2005, 8, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J.; Steyn, N.P.; Myburgh, N.G.; Nel, J.H. Food items consumed by students attending schools in different socioeconomic areas in Cape Town, South Africa. Nutrition 2006, 22, 252–258. [Google Scholar] [CrossRef] [PubMed]
- MacKeown, J.M.; Pedro, T.M.; Norris, S.A. Energy, macro- and micronutrient intake among a true longitudinal group of South African adolescents at two interceptions (2000 and 2003): The Birth-to-Twenty (Bt20) Study. Public Health Nutr. 2007, 10, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Feeley, A.; Pettifor, J.M.; Norris, S.A. Fast-food consumption among 17-year-olds in the Birth to Twenty cohort. S. Afr. J. Clin. Nutr. 2009, 22, 118–123. [Google Scholar] [CrossRef]
- Harris, T.; Malczyk, S.; Jaffer, N.; Steyn, N. How well are adolescents in the Gouda District of the Western Cape meeting the South African food-based dietary guidelines for fat, sugar and sodium? J. Consumer Sci. 2019, 4. [Google Scholar]
- Statistics South Africa. Mid-Year Population Estimates 2018. Available online: http://www.statssa.gov.za/?p=11341 (accessed on 6 March 2019).
- Statistics South Africa Census 2011 Metadata. Available online: http://www.statssa.gov.za/census/census_2011/census_products/Census_2011_Metadata.pdf (accessed on 30 January 2020).
- ICF International. Demographic and Health Survey Sampling and Household Listing Manual: MEASURE DHS; Inner City Fund (ICF) International: Calverton, MD, USA, 2012. [Google Scholar]
- Steyn, N.P.; Labadarios, D.; Maunder, E.; Nel, J.; Lombard, C.; Directors of the National Food Consumption, S. Secondary anthropometric data analysis of the National Food Consumption Survey in South Africa: The double burden. Nutrition 2005, 21, 4–13. [Google Scholar] [CrossRef]
- Filmer, D.; Pritchett, L. Estimating wealth effects without expenditure data—Or tears: With an application to educational enrollments in the states of India. In The World Bank Development Research Group; World Bank: Washington, DC, USA, 1998. [Google Scholar]
- South African Medical Research Council (MRC). South Africa Demographic and Health Survey: 2016; SA MRC: Pretoria, South Africa, 2017. [Google Scholar]
- Wehler, C.; Scott, R.; Anderson, J. The community childhood hunger identification project: A model of domestic hunger-demonstration. J. Nutr. Educ. 1992, 24, 295–355. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Kipnis, V.; Buckman, D.W.; Carroll, R.J.; Freedman, L.S.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Dodd, K.W. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat. Med. 2010, 29, 2857–2868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrick, K.A.; Rossen, L.M.; Parsons, R.; Dodd, K.W. Estimating usual dietary intake from National Health and Nutrition Examination 5. Survey data using the National Cancer Institute method. National Center for Health Statistics. Vital. Health. Stat. 2018, 2, 1–63. [Google Scholar]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.; Senekal, M. The Dietary Assessment and Education Kit (DAEK) The Chronic Diseases of Lifestyle Unit of the South African Medical Research Council; MRC: Cape Town, South Africa, 2004. [Google Scholar]
- Steyn, N.P.; Senekal, M.; Norris, S.A.; Whati, L.; Mackeown, J.M.; Nel, J.H. How well do adolescents determine portion sizes of foods and beverages? Asia Pac. J. Clin. Nutr. 2006, 15, 35–42. [Google Scholar]
- Neville, M.C.; Allen, J.C.; Archer, P.C.; Casey, C.E.; Seacat, J.; Keller, R.P.; Lutes, V.; Rasbach, J.; Neifert, M. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am. J. Clin. Nutr. 1991, 54, 81–92. [Google Scholar] [CrossRef]
- Lee, R.D.; Nieman, D.C. Nutritional Assessment, 6th ed.; McGraw-Hill: London, UK, 2013. [Google Scholar]
- WHO. Obesity: Preventing and Managaing the Global Epidemic (Report of a WHO Consultation). Available online: https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 6 March 2019).
- Van Graan, A.E.; Chetty, J.M.; Links, M.R. Food Composition Tables for South Africa, 5th ed.; South African Medical Research Council: Cape Town, South Africa, 2017. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Available online: https://www.nap.edu/read/11537/chapter/1#iii (accessed on 15 October 2019).
- WHO. Guideline: Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children. Available online: https://extranet.who.int/dataform/upload/surveys/666752/files/Draft%20WHO%20SFA-TFA%20guidelines_04052018%20Public%20Consultation(1).pdf (accessed on 20 November 2019).
- WHO. Guideline: Sugars Intake for Adults and Children. Available online: https://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed on 20 November 2019).
- Kibblewhite, R.; Nettleton, A.; McLean, R.; Haszard, J.; Fleming, E.; Kruimer, D.; Te Morenga, L. Estimating Free and Added Sugar Intakes in New Zealand. Nutrients 2017, 9, 1292. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the first 1000 days: The origin of childhood obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F. Early nutrition impact on the insulin-like grwoth factor axis and later health consequences. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 285–292. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Strategy for Infant and Young Child Feeding. Available online: https://www.who.int/nutrition/publications/infantfeeding/9241562218/en/ (accessed on 20 November 2019).
- Lawrence, R.A. Increasing breastfeeding duration: Changing the paradigm. Breastfeeding Med. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Rifas-Shiman, S.L.; Taveras, E.M.; Oken, E.; Gillman, M.W. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011, 127, e544–e551. [Google Scholar] [CrossRef]
- Chaponda, A.; Goon, D.T.; Hoque, M.E. Infant feeding practices among HIV-positive mothers at Tembisa hospital, South Africa. Afr. J. Prim. Health Care Fam. Med. 2017, 9, e1–e6. [Google Scholar] [CrossRef]
- Kassier, S.; Veldman, F. Cry, the beloved bottle: Infant-feeding knowledge and the practices of mothers and caregivers in an urban township outside Bloemfontein, Free State province. S. Afr. J. Clin. Nutr. 2013, 26, 17–22. [Google Scholar] [CrossRef]
- Van Der Merwe, S.; Du Plessis, L.; Jooste, H.; Nel, D. Comparison of infant-feeding practices in two health subdistricts with different baby-friendly status in Mpumalanga province. S. Afr. J. Clin. 2015, 28, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Smuts, M.; Wolmarans, P. The importance of the quality of type of fat in the diet: A food-based dietary guideline for South Africa. S. Afr. J. Clin. Nutr. 2013, 26, S87–S97. [Google Scholar]
- UNICEF, FFI. Monitoring of flour fortification: The case of South Africa. UNICEF: New York, NY, USA, 2014. Available online: http://www.ffinetwork.org/monitor/Documents/SouthAfricaCS.pdf (accessed on 5 January 2020).
- Labadarios, D. (Ed.) The National Food Consumption Survey (NFCS): Children Aged 1–9 Years; Department of Health: Pretoria, South Africa, 1999. [Google Scholar]
- Temple, N.; Steyn, N.P. Community Nutrition Textbook for South Africa: A Rights-based Approach. Cape Town: Chronic Diseases of Lifestyle Unit; UNISA Press: Pretoria, South Africa, 2015. [Google Scholar]
- The Scientific Advisory Committee on Nutrition Recommendations on Carbohydrates, Including Sugars and Fibre, Published 17 July 2015. Public Health England. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 8 January 2020).
- Shroff, M.R.; Perng, W.; Baylin, A.; Mora-Plazas, M.; Marin, C.; Villamor, E. Adherence to a snacking dietary pattern and soda intake are related to the development of adiposity: A prospective study in school-age children. Public Health Nutr. 2014, 17, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Bucher Della Torre, S.; Keller, A.; Laure Depeyre, J.; Kruseman, M. Sugar-Sweetened Beverages and Obesity Risk in Children and Adolescents: A Systematic Analysis on How Methodological Quality May Influence Conclusions. J. Acad. Nutr. Diet. 2016, 116, 638–659. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Orushka, A. Sugar Sweeteened Beverages (SSB) Tax in South Africa: An Analysis of the Tax Design. Available online: http://researchspace.ukzn.ac.za/handle/10413/16470 (accessed on 20 November 2019).
- Bourne, L. South African paediatric food-based dietary guidelines. Matern. Child Nutr. 2007, 3, 227–229. [Google Scholar] [CrossRef]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.; Reddy, P.; Parker, W.; Hoosain, E.; Naidoo, P.; et al. South African National Health and Nutrition Examination Survey (SANHANES-1); HSRC Press: Cape Town, South Africa, 2014. [Google Scholar]
- South African Department of Health. Government Gazette No.34029: Regulations Relating to Trans Fats in Foods. Available online: https://extranet.who.int/ncdccs/Data/ZAF_B17_Regulationtransfatfoodstuffs.pdf (accessed on 10 December 2019).
- Te Morenga, L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.J.; MacGregor, G.A. Harmful effects of salt in determining blood pressure: Meta-analysis of controlled trials. Hypertension 2006, 48, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Mchiza, Z.; Puoane, T.; Steyn, N.P. Food sold by street-food vendors in Cape Town and surrounding areas: A focus on food and nutrition knowledge as well as practices related to food preparation of street-food vendors. J. Hunger Environ. Nutr. 2018. [Google Scholar] [CrossRef]
- Pries, A.M.; Filteau, S.; Ferguson, E.L. Snack food and beverage consumption and young child nutrition in low- and middle-income countries: A systematic review. Matern. Child. Nutr. 2019, 15, e12729. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]



| Variables | Recommended Values | Reference |
|---|---|---|
| Energy intake kJ | Estimated energy requirement (EER) for healthy moderately active children: (kJ/day) M (1–2 yrs): 4393 kJ; F (1–2 yrs): 4166 kJ; M (3 yrs): 6213 kJ; F (3 yrs): 5837 kJ; M (4 yrs): 6552 kJ; F (4 yrs): 6171 kJ; M (5 yrs): 6937 kJ; F (5 yrs): 6514 kJ; M (6 yrs): 7289 kJ; F (6 yrs): 6870 kJ; M (7 yrs): 7699 kJ; F (7 yrs): 7192 kJ; M (8 yrs): 8079 kJ; F (8 yrs): 7573 kJ; M (9 yrs): 8548 kJ; F (9 yrs): 7908 kJ | [37] |
| Carbohydrate | Acceptable macronutrient distribution range (AMDR) 45–65% | [37] |
| Total fat | AMDR: %E from fat in 1–3-year-olds 30–40%, 4–9 -year-olds 25–35% | [38] |
| Saturated fat | <10% of energy | [38] |
| Free sugars | <10% of energy | [39] |
| Fibre | Adequate intake (AI) AI: 1–3-year-olds −13 g, 4–8-year-olds 25 g, boys 9 years 31 g, girls 9 years 29 g | [37] |
| Gauteng N = 733 % (95% CI) | Western Cape N = 593 % (95% CI) | Rao–Scott Chi Square p Values | All N = 1326 % (95% CI) | |
|---|---|---|---|---|
| Primary caregiver | ||||
| Mother | 70.1 (65.6–74.6) | 71.0 (64.7–77.2) | 0.0448 * | 70.4 (66.8–74.0) |
| Father | 6.6 (3.4–9.7) | 1.8 (0.2–3.3) | 5.0 (2.8–7.1) | |
| Grandparent | 16.7 (12.9–20.4) | 21.0 (15.5–26.4) | 18.1 (15.0–21.2) | |
| Other (e.g., sibling, aunt) | 6.7 (4.0–9.5) | 6.3 (2.1–10.4) | 6.6 (4.3–8.8) | |
| Age in Years | ||||
| 1–<3-years | 26.3 (22.1–30.6) | 25.3 (19.4–31.2) | 0.9234 | 26.0 (22.6–29.4) |
| 3–<6-years | 35.4 (31.0–39.8) | 35.1 (30.7–39.5) | 35.3 (32.1–38.5) | |
| 6–<10-years | 38.3 (34.1–42.4) | 39.6 (33.1–46.1) | 38.7 (35.2–42.2) | |
| Gender | ||||
| Male | 50.2 (45.5–54.9) | 47.5 (43.1–51.9) | 0.3907 | 49.3 (45.9–52.7) |
| Female | 49.8 (45.1–54.5) | 52.5 (48.1–56.9) | 50.7 (47.3–54.1) | |
| Head of Household | ||||
| Father | 40.2 (33.8–46.6) | 38.8 (34.6–43.0) | 0.1320 | 39.7 (35.3–44.1) |
| Mother | 16.8 (13.8–19.9) | 10.8 (7.0–14.5) | 14.8 (12.5–17.2) | |
| Grandmother | 21.9 (15.5–28.3) | 28.3 (21.8–34.9) | 24.0 (19.3–28.8) | |
| Grandfather | 11.7 (8.3–15.1) | 14.0 (10.0–18.0) | 12.5 (9.9–15.0) | |
| Other (e.g., aunt, uncle) | 9.4 (5.7–13.1) | 8.1 (4.9–11.4) | 9.0 (6.3–11.7) | |
| Marital Status of Mother | ||||
| Unmarried | 41.1 (34.9–47.2) | 34.8 (28.4–41.1) | 0.0002 ** | 39.0 (34.4–43.5) |
| Married | 24.9 (20.5–29.4) | 41.3 (33.3–49.2) | 30.4 (26.4–34.3) | |
| Divorced/widowed | 4.8 (2.5–7.0) | 2.4 (0.7–4.2) | 4.0 (2.4–5.6) | |
| Living together | 27.8 (22.0–33.6) | 20.8 (15.9–25.7) | 25.5 (21.4–29.6) | |
| Other | 1.4 (0.2–2.6) | 0.8 (0.0–1.8) | 1.2 (0.3–2.1) | |
| Mother’s Highest Education | ||||
| Not completing Gr. 12 | 51.2 (44.9–57.4) | 57.7 (47.1–68.3) | 0.1833 | 53.3 (47.9–58.7) |
| Completion of Gr. 12 | 33.9 (28.4–39.4) | 24.7 (17.6–31.8) | 30.8 (26.5–35.2) | |
| Qualification after Gr.12 | 12.2 (8.7–15.7) | 15.6 (7.6–23.6) | 13.3 (9.9–16.8) | |
| Do not know | 2.8 (1.4–4.1) | 2.0 (0.5–3.5) | 2.5 (1.5–3.5) | |
| Father’s Highest Education | ||||
| Not completing Gr. 12 | 26.9 (22.0–31.7) | 33.8 (29.0–38.5) | 0.3232 | 29.1 (25.6–32.7) |
| Completion of Gr. 12 | 32.6 (26.9–38.3) | 30.4 (25.2–35.6) | 31.9 (27.8–36.0) | |
| Qualification after Gr.12 | 13.1 (9.4–16.9) | 10.7 (5.7–15.7) | 12.3 (9.4–15.3) | |
| Do not know | 27.4 (22.4–32.4) | 25.2 (19.7–30.6) | 26.7 (22.9–30.4) | |
| Mother’s Employment Status | ||||
| Yes | 22.4 (17.8–26.9) | 38.4 (31.0–45.9) | <0.0001 *** | 27.7 (23.9–31.5) |
| No | 74.6 (69.6–79.6) | 60.2 (53.0–67.5) | 69.8 (65.8–73.9) | |
| Do not know/not applicable | 3.0 (1.3–4.7) | 1.3 (0.3–2.4) | 2.5 (1.3–3.6) | |
| Father’s Employment Status (%) | ||||
| Yes | 64.8 (60.6–69.1) | 65.3 (59.7–70.9) | 0.9532 | 65.0 (61.6–68.4) |
| No | 21.4 (17.5–25.3) | 20.5 (15.1–25.9) | 21.1 (18.0–24.2) | |
| Do not know/not applicable | 13.8 (11.1–16.4) | 14.1 (10.2–18.1) | 13.9 (11.7–16.1) | |
| Wealth Index Quintiles | ||||
| One | 21.1 (14.6–27.6) | 17.7 (10.7–24.7) | 0.2633 | 20.0 (15.1–24.8) |
| Two | 17.8 (12.0–23.6) | 24.3 (20.0–28.6) | 20.0 (15.9–24.0) | |
| Three | 21.3 (17.0–25.7) | 17.0 (12.6–21.4) | 19.9 (16.7–23.1) | |
| Four | 21.5 (16.7–26.3) | 17.5 (12.4–22.6) | 20.2 (16.6–23.7) | |
| Five | 18.3 (11.6–25.0) | 23.5 (14.5–32.5) | 20.0 (14.7–25.3) | |
| Ethnicity | ||||
| Black African | 97.8 (96.0–99.6) | 27.6 (12.9–42.3) | <0.0001 | 74.5 (69.5–79.4) |
| Mixed ancestry | 2.2 (0.3–4.0) | 68.0 (53.7–82.4) | 24.1 (19.2–28.9) | |
| Other | 0.0 (0.0–0.1) | 4.4 (0.6–8.2) | 1.5 (0.3–2.7) | |
| Type of Residence | ||||
| Rural | 2.4 (0.7–4.1) | 6.6 (1.6–11.5) | 0.1938 | 3.8 (1.9–5.7) |
| Urban formal | 88.9 (82.3–95.4) | 86.8 (79.1–94.5) | 88.2 (83.2–93.2) | |
| Urban informal | 8.7 (2.7–14.7) | 6.6 (1.7–11.5) | 8.0 (3.7–12.3) | |
| Mother’s BMI [39] | ||||
| Underweight/normal BMI ≤ 18.5 & 18.5–24.9 kgm2 | 33.3 (28.0–38.5) | 29.1 (23.6–34.5) | 0.0023 ** | 32.0 (28.0–35.9) |
| Overweight BMI = 25–29.9 kgm2 | 27.7 (23.6–31.8) | 20.4 (16.5–24.3) | 25.4 (22.4–28.5) | |
| Obese BMI ≥ 30 kgm2 | 39.1 (35.8–42.3) | 50.6 (43.0–58.1) | 42.6 (39.4–45.8) | |
| Hunger Scale [25] | ||||
| Total score = 0: No risk | 57.9 (49.5–66.3) | 48.8 (38.9–58.7) | 0.1483 | 54.9 (48.5–61.3) |
| 1–4: At risk of hunger | 22.1 (17.2–27.0) | 28.9 (23.0–34.9) | 24.4 (20.6–28.2) | |
| 5–8: Food shortage in house | 20.0 (14.8–25.1) | 22.3 (16.5–28.0) | 20.7 (16.8–24.6) |
| Age (years) | 1–<3 | 3–<6 | 6–<10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Province | GTG | WC | All | GTG | WC | All | GTG | WC | All |
| Sample size | 185 | 148 | 333 | 282 | 232 | 514 | 266 | 213 | 479 |
| Total energy (kJ) | 4813 (4366–5260) | 5220 (4105–6335) | 4944 (4465–5424) | 5389 * (4847–5931) | 6105 (5860–6350) | 5626 (5264–5987) | 6537 (6340–6735) | 6515 (6150–6880) | 6530 (6329–6730) |
| Carbohydrate (g) | 165.1 (156.2–173.9) | 160.7 (114.9–206.5) | 163.7 (148.7–178.6) | 182.0 * (172.5–191.6) | 197.9 (192.6–203.2) | 187.3 (181.5–193.1) | 214.0 ** (208.6–219.4) | 199.3 (195.0–203.6) | 209.0 (205.0–213.0) |
| %E from carbohydrate | 60.5 * (58.8–62.2) | 54.3 (49.6–59.0) | 58.5 (55.9–61.0) | 61.2 * (59.0–63.3) | 57.9 (56.5–59.3) | 60.1 (58.6–61.5) | 58.5 * (57.5–59.5) | 54.5 (52.0–57.0 | 57.1 (56.0–58.3) |
| Total protein(g) | 32.0 * (27.5–36.5) | 40.2 (35.7–44.7) | 34.6 (30.9–38.4) | 37.5 ** (32.9–42.1) | 46.1 (45.6–46.7) | 40.3 (37.4–43.2) | 44.9 (41.6–48.2) | 48.7 (43.0–54.4) | 46.2 (43.0–49.4) |
| %E from protein | 11.2 * (10.5–11.9) | 13.1 (11.6–14.5) | 11.8 (10.9–12.7) | 11.8 * (11.6–12.1) | 12.9 (12.2–13.6) | 12.2 (11.8–12.5) | 11.7 * (11.5–12.0) | 12.8 (11.9–13.7) | 12.1 (11.7–12.4) |
| Animal protein(g) | 15.3 ** (13.4–17.2) | 23.2 (20.4–26.0) | 17.8 (15.4–20.3) | 18.7 ** (15.8–21.6) | 25.9 (24.8–27.0) | 21.0 (19.5–22.6) | 22.1 * (19.8–24.4) | 28.1 (23.7–32.4) | 24.1 (21.8–26.5) |
| %E from animal protein | 5.6 (4.9–6.4) | 7.8 (5.2–10.5) | 6.3 (4.9–7.7) | 5.9 ** (5.7–6.2) | 7.3 (6.5–8.1) | 6.4 (6.0–6.7) | 5.9 ** (5.5–6.2) | 7.5 (6.7–8.3) | 6.4 (6.0–6.8) |
| Plant protein (g) | 13.2 ** (12.8–13.5) | 10.4 (9.2–11.5) | 12.3 (11.6–12.9) | 17.8 ** (16.5–19.1) | 15.0 (14.7–15.4) | 16.9 (15.9–17.9) | 21.9 *** (21.6–22.2) | 18.7 (18.0–19.3) | 20.8 (20.5–21.2) |
| %E from plant protein | 4.6 ** (4.2–5.0) | 3.3 (2.9–3.8) | 4.2 (3.9–4.6) | 5.6 *** (5.4–5.8) | 4.2 (3.9–4.4) | 5.1 (4.9–5.3) | 5.6 ** (5.3–5.8) | 4.7 (4.6–4.8) | 5.3 (5.2–5.4) |
| Fibre (g) | 10.8 * (10.5–11.1) | 8.7 (7.0–10.5) | 10.1 (9.4–10.9) | 13.0 (11.9–14.1) | 12.2 (12.0–12.3) | 12.7 (12.0–13.4) | 14.7 ** (14.2–15.3) | 12.8 (12.3–13.3) | 14.1 (13.6–14.5) |
| Age (years) | 1–<3 | 3–<6 | 6–<10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Province | GTG | WC | All | GTG | WC | All | GTG | WC | All |
| Sample size | 185 | 148 | 333 | 282 | 232 | 514 | 266 | 213 | 479 |
| Total fat | 36.7 * (31.5–41.9) | 45.1 (41.3–48.8) | 39.4 (34.7–44.0) | 41.1 (33.9–48.4) | 49.7 (45.0–54.4) | 44.0 (38.8–49.1) | 54.9 (52.0–57.9) | 59.0 (52.9–65.0) | 56.3 (53.2–59.4) |
| %E fat | 29.0 (28.2–29.7) | 33.2 (28.4–38.1) | 30.3 (28.7–32.0) | 28.1 (26.5–29.7) | 30.0 (27.9–32.0) | 28.7 (27.6–29.9) | 31.4 * (30.6–32.2) | 33.8 (31.9–35.8) | 32.2 (31.3–33.2) |
| Total SF (g) | 10.5 ** (8.4–12.7) | 14.9 (14.4–15.5) | 12.0 (10.0–13.9) | 11.9 * (8.8–15.0) | 15.6 (13.6–17.7) | 13.1 (10.8–15.5) | 14.8 ** (13.8–15.9) | 17.9 (16.6–19.2) | 15.9 (15.0–16.7) |
| %E from SF | 8.1 (7.2–9.0) | 10.6 (8.1–13.2) | 8.9 (7.5–10.3) | 8.1 * (7.2–9.0) | 9.4 (8.4–10.4) | 8.5 (7.9–9.1) | 8.5 ** (8.2–8.9) | 10.3 (10.1–10.6) | 9.1 (8.9–9.4) |
| Cholesterol (mg) | 83.9 ** (60.6–107.2) | 154.8 (118.0–191.5) | 106.8 (82.5–131.2) | 115.4 (86.2–144.6) | 146.9 (130.8–163.0) | 125.8 (105.7–146.0) | 139.7 ** (126.5–152.9) | 178.7 (164.8–192.7) | 153.0 (145.5–160.4) |
| MUFA (g) | 10.6 * (9.3–11.9) | 13.1 (11.2–15.0) | 11.4 (10.0–12.8) | 12.9 (10.2–15.6) | 15.3 (14.0–16.7) | 13.7 (11.9–15.5) | 16.9 ** (16.3–17.4) | 19.5 (18.1–20.8) | 17.7 (17.1–18.4) |
| %E MUFA | 8.6 (8.2–9.0) | 9.8 (6.3–13.4) | 9.0 (7.7–10.3) | 8.8 (8.1–9.5) | 9.2 (8.7–9.8) | 9.0 (8.6–9.3) | 9.7 ** (9.4–10.0) | 11.2 (10.9–11.6) | 10.2 (9.9–10.5) |
| PUFA | 9.6 (8.7–10.6) | 9.4 (8.9–9.80 | 9.5 (8.8–10.3) | 12.3 (11.7–12.9) | 12.0 (11.5–12.6) | 12.2 (11.9–12.5) | 17.7 (16.3–19.1) | 16.2 (13.5–18.8) | 17.2 (15.9–18.5) |
| %E PUFA | 8.0 (6.6–9.5) | 7.2 (5.6–8.7) | 7.8 (6.7–8.8) | 8.4 ** (7.9–9.0) | 7.3 (7.1–7.4) | 8.1 (7.7–8.5) | 9.9 (9.4–10.5) | 9.0 (7.7–10.3) | 9.6 (9.0–10.2) |
| Trans fats (g) | 0.7 (0.4–1.0) | 1.0 (0.7–1.3) | 0.8 (0.5–1.1) | 0.8 ** (0.7–1.0) | 1.2 (1.1–1.2) | 0.9 (0.9–1.0) | 1.0 * (1.0–1.1) | 1.4 (1.1–1.7) | 1.2 (1.0–1.3) |
| %E Trans fats | 0.5 (0.3–0.7) | 0.7 (0.3–1.0) | 0.6 (0.3–0.8) | 0.6 ** (0.5–0.7) | 0.7 (0.7–0.8) | 0.6 (0.6–0.7) | 0.6 *(0.5–0.6) | 0.8 (0.6–0.9) | 0.7 (0.6–0.7) |
| Free sugars (g) | 29.6 (25.9–33.2) | 33.8 (28.9–38.7) | 30.9 (27.5–34.4) | 32.4 (26.2–38.7) | 36.9 (35.9–37.9) | 33.9 (30.0–37.9) | 39.0 (34.9–43.1) | 42.6 (36.9–48.3) | 40.2 (37.0–43.5) |
| %E Free sugars | 9.9 (8.5–11.3) | 10.3 (7.8–12.9) | 10.0 (8.6–11.4) | 10.1 (9.6–10.7) | 10.2 (9.0–11.4) | 10.2 (9.5–10.8) | 10.0 (8.7–11.3) | 11.0 (8.4–13.5) | 10.3 (8.9–11.8) |
| 1–<3 -Years- Old (N = 333) | 3–<6 -Years-Old (N = 514) | 6–<10-Years-Old (N = 479) | ||||||
|---|---|---|---|---|---|---|---|---|
| Food Contributing to kJ | Contribution to KJ Per Capita | % of total kJ | Food Contributing to KJ | Contribution to KJ Per Capita g | % of Total KJ | Food Contributing to KJ | Contribution to KJ Per Capita g | % of Total kJ |
| Maize porridge | 1265.3 | 25.6 | Maize porridge | 1248.6 | 22.3 | Maize porridge | 1206.5 | 18.5 |
| Salty snacks | 275.3 | 5.6 | Potato/sw. potato | 324.2 | 5.8 | White bread | 466.7 | 7.2 |
| Potato/sw. potato | 238.4 | 4.8 | Salty snacks | 323.2 | 5.8 | Salty snacks | 423.1 | 6.5 |
| Whole milk | 236.6 | 4.8 | Chicken | 293.9 | 5.2 | Potato/sw. potato | 370.9 | 5.7 |
| Food Contributing to CHO | Contribution to CHO Per Capita g | % of Total CHO | Food Contributing to CHO | Contribution to CHO Per Capita g | % of Total CHO | Food Contributing to CHO | Contribution to CHO Per Capita g | % of Total CHO |
| Maize porridge | 60.18 | 36.8 | Maize porridge | 59.4 | 32.0 | Maize porridge | 56.9 | 27.0 |
| Granulated sugar | 10.13 | 6.2 | Granulated sugar | 12.9 | 7.0 | White bread | 20.5 | 9.7 |
| Potato/sw. potato | 7.35 | 4.5 | White bread | 10.8 | 5.8 | Granulated sugar | 14.5 | 6.9 |
| BMS | 6.53 | 4.0 | White rice | 10.4 | 5.6 | Cold drink | 11.8 | 5.6 |
| Food Contributing to Protein | Contribution to Protein (Per Capita g) | % of Total Protein | Food Contributing to Protein | Contribution to Protein (Per Capita g) | % of Total Protein | Food Contributing to Protein | Contribution to Protein (Per Capita g) | % of Total Protein |
| Chicken | 5.9 | 16.6 | Chicken | 7.8 | 16.6 | Chicken | 7.6 | 16.1 |
| Maize porridge | 5.6 | 15.7 | Maize porridge | 5.5 | 17.7 | Maize porridge | 5.5 | 11.6 |
| Whole milk | 2.9 | 8.2 | Beef | 2.5 | 8.2 | White bread | 4.0 | 8.4 |
| Beef | 1.8 | 5.2 | Whole milk | 2.3 | 5.2 | Beef | 3.4 | 7.1 |
| Food Contributing to Fat | Contribution to Fat (Per Capita g) | % Total Fat | Food Contributing to Fat | Contribution to Fat (Per Capita g) | % Total Fat | Food Contributing to Fat | Contribution to Fat (Per Capita g) | % Total Fat |
| Salty snacks | 4.1 | 10.4 | Salty snacks | 4.8 | 10.8 | Salty snacks | 6.2 | 11.4 |
| Whole milk | 3.1 | 7.9 | Chicken | 4.0 | 9.0 | Med fat margarine | 5.7 | 10.4 |
| Chicken | 3.1 | 7.8 | Med fat margarine | 3.7 | 8.3 | Processed meat | 4.8 | 8.7 |
| BMS | 2.4 | 6.3 | Processed meat | 3.5 | 8.0 | Chicken | 4.1 | 7.5 |
| Foods Contributing to Saturated Fat | Contribution to SF (Per Capita g) | % of total SF | Foods Contributing to Saturated Fat | Contribution to SF (Per Capita g) | % of SF | Foods Contributing to Saturated Fat | Contribution to SF (Per Capita g) | % of SF |
| Whole milk | 1.8 | 14.5 | Salty snacks | 1.7 | 12.7 | Salty snacks | 2.2 | 13.9 |
| Salty snacks | 1.4 | 11.9 | Whole milk | 1.4 | 10.6 | Processed meat | 1.7 | 11.2 |
| Processed meat | 0.8 | 6.9 | Processed meat | 1.3 | 9.8 | Whole milk | 1.3 | 8.3 |
| Chicken | 0.8 | 6.5 | Chicken | 1.0 | 7.9 | Med fat margarine | 1.2 | 7.4 |
| Foods Contributing to Added Sugar | Contribution to AS (Per Capita g) | % of Total AS | Foods Contributing to Added Sugar | Contribution to AS (Per Capita g) | % of AS | Foods Contributing to AS | Contribution to AS (Per Capita g) | % of AS |
| Granulated sugar | 10.1 | 39.6 | Granulated sugar | 12.9 | 42.0.4 | Granulated sugar | 14.5 | 39.7 |
| Candy | 4.7 | 18.5 | Candy | 6.1 | 19.9 | Candy | 6.8 | 18.6 |
| Cold drinks | 2.1 | 8.3 | Cold drinks | 3.3 | 10.7 | Cold drinks | 6.5 | 17.7 |
| Yoghurt | 1.9 | 7.3 | Cookies | 1.7 | 5.4 | Cookies | 2.2 | 6.0 |
| Foods Contributing to fibre | Contribution to Fibre (Per Capita g) | % of Total Fibre | Foods Contributing to Fibre | Contribution to Fibre (Per Capita g) | % of Total Fibre | Foods Contributing to Fibre | Contribution to Fibre (Per Capita g) | % of Total Fibre |
| Maize porridge | 3.8 | 37.9 | Maize porridge | 3.7 | 30.2 | Maize porridge | 3.8 | 27.5 |
| High fibre cereal | 1.1 | 10.5 | High fibre cereal | 1.3 | 10.3 | White bread | 1.4 | 10.2 |
| Potato/sw. potato | 0.7 | 7.1 | Brown bread | 1.0 | 7.9 | Brown bread | 1.4 | 9.7 |
| Fresh fruit | 0.5 | 5.3 | Potato/sw. potato | 0.9 | 7.4 | Potato/sw. potato | 1.1 | 7.7 |
| Gauteng | Western Cape | All | ||||
|---|---|---|---|---|---|---|
| 1–< 3- Years | 3–<6-Years | 1–<3- Years | 3–< 6- Years | 1–<3- Years | 3–<6- Years | |
| Number of children sampled | 185 | 282 | 148 | 232 | 333 | 514 |
| Number breastfeeding | 17 | 1 | 22 | 4 | 39 | 5 |
| % Infants breastfed | 9.2 | 0.4 | 14.9 | 1.7 | 11.7 | 1.0 |
| Mean (SD) intake of breast milk per day(g) (only consumers) | 335.3 (177) | 200.0 (-) | 272.7 (172) | 175.0 (96) | 300.0 (175) | 180.0 (84) |
| Mean (SD) frequency of feeds | 3.4 (1.8) | 2.0 (-) | 2.7 (1.7) | 1.8 (1.0) | 3.0 (1.7) | 1.8 (0.8) |
| % Contribution to energy intake (consumers of breast milk only) | 21.4 | 8.8 | 16.7 | 7.8 | 18.7 | 8.0 |
| Mean (SD) kJ portion p/day | 995.8 (524) | 594.0 (-) | 810.0 (512) | 519.8 (284) | 891.0 (519) | 534.6 (249) |
| Bivariate Logistic Regression | Multivariate Logistic Regression (Adjusted for Gender and Ethnicity) | |||||
|---|---|---|---|---|---|---|
| Children 1–<3-Yrs Energy < EER N = 333 (n = 145) OR (95% CI) | Children 3–<6-Yrs Energy < EER N = 514 (n = 349) OR (95% CI) | Children 6–<10-Yrs Energy < EER N = 479 (n = 351) OR (95% CI) | Children 1–<3-Yrs Energy < EER N = 333 (n = 145) OR (95% CI)b | Children 3–<6-Yrs Energy < EER N = 514 (n = 349) OR (95% CI)c | Children 6–<10-Yrs Energy < EER N = 479 (n = 351) OR (95% CI) d | |
| Primary caregiver a | ||||||
| Mother | Ref | Ref | Ref | |||
| Grandparent | 1.50 (0.59–3.84) | 1.50 (0.76–2.96) | 1.72 (0.82–3.62) | |||
| Other (e.g., father, aunt) | 1.40 (0.46–4.20) | 2.13 (0.89–5.08) | 1.37 (0.67–2.79) | |||
| Gender | ||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref |
| Female | 0.75 (0.45–1.25) | 0.77 (0.49–1.23) | 0.69 (0.41–1.18) | 0.64 (0.37–1.11) | 0.74 (0.47–1.17) | 0.66 (0.38–1.16) |
| Head of household | ||||||
| Father | Ref | Ref | Ref | Ref | ||
| Mother | 1.11 (0.34–3.60) | 0.65 (0.32–1.33) | 0.90 (0.42–1.95) | 0.90 (0.27–2.96) | ||
| Grandparent | 1.93 (1.05–3.56) * | 0.95 (0.57–1.58) | 0.69 (0.40–1.21) | 1.89 (0.94–3.82) | ||
| Other (e.g., aunt, uncle) | 3.82 (1.19–12.25) * | 0.82 (0.36–1.85) | 0.75 (0.24–2.36) | 4.77 (1.63–13.91) ** | ||
| Marital status of mother | ||||||
| Married | Ref | Ref | Ref | |||
| Other (e.g., unmarried, divorced) | 1.67 (0.77–3.64) | 1.11 (0.63–1.97) | 1.06 (0.56–1.98) | |||
| Mother’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | Ref | Ref | |
| Completed grade 12 | 0.33 (0.17–0.62) ** | 1.33 (0.79–2.26) | 0.44 (0.25–0.75) ** | 0.27 (0.14–0.51) ** | 0.52 (0.29–0.92) * | |
| Qualification after grade 12 | 0.27 (0.09–0.81) * | 1.05 (0.58–1.91) | 0.79 (0.38–1.68) | 0.26 (0.08–0.91) * | 0.84 (0.34–2.03) | |
| Father’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | |||
| Completed grade 12 | 0.62 (0.31–1.24) | 0.87 (0.49–1.54) | 0.76 (0.36–1.59) | |||
| Qualification after grade 12 | 0.39 (0.13–1.24) | 0.66 (0.31–1.41) | 0.50 (0.23–1.11) | |||
| Mother’s employment status | ||||||
| Not employed/do not know | Ref | Ref | Ref | |||
| Employed | 0.47 (0.22–1.01) | 1.06 (0.65–1.73) | 1.44 (0.80–2.61) | |||
| Father’s employment status | ||||||
| Not employed/do not know | Ref | Ref | Ref | |||
| Employed | 0.73 (0.37–1.42) | 1.06 (0.59–1.89) | 0.78 (0.42–1.44) | |||
| Wealth index quintiles | ||||||
| One/two/three | Ref | Ref | Ref | Ref | ||
| Four/five | 0.74 (0.39–1.41) | 0.67 (0.38–1.18) | 0.56 (0.35–0.89) * | 0.73 (0.41–1.28) | ||
| Ethnicity | ||||||
| Black African | Ref | Ref | Ref | Ref | Ref | Ref |
| Mixed Ancestry | 1.06 (0.52–2.16) | 0.61 (0.34–1.11) | 1.40 (0.66–2.98) | 1.21 (0.59–2.48) | 0.63 (0.33–1.20) | 1.35 (0.62–2.93) |
| Province | ||||||
| Western Cape | Ref | Ref | Ref | |||
| Gauteng | 1.77 (0.91–3.47) | 1.63 (0.92–2.90) | 0.75 (0.44–1.27) | |||
| Type of residence | ||||||
| Urban formal | Ref | Ref | Ref | |||
| Urban informal | 1.32 (0.65–2.69) | 1.45 (0.77–2.73) | 0.61 (0.34–1.08) | |||
| Rural | 1.18 (0.62–2.25) | 1.00 (0.57–1.76) | 0.91 (0.55–1.49) | |||
| Mother’s BMI | ||||||
| Underweight/normal weight | Ref | Ref | Ref | Ref | ||
| Overweight | 0.78 (0.34–1.79) | 0.99 (0.58–1.68) | 1.04 (0.45–2.39) | 1.28 (0.53–3.09) | ||
| Obese | 0.53 (0.25–1.15) | 0.99 (0.56–1.76) | 0.45 (0.22–0.90) * | 0.55 (0.26–1.18) | ||
| Hunger scale | ||||||
| Total score = 0: No risk | Ref | Ref | Ref | Ref | ||
| 1–4: At risk of hunger | 0.73 (0.37–1.45) | 0.54 (0.34–0.87) * | 1.14 (0.55–2.38) | 0.54 (0.33–0.86) * | ||
| 5–8: Food shortage in house | 1.61 (0.63–4.12) | 0.56 (0.33–0.93) * | 1.05 (0.50–2.20) | 0.53 (0.32–0.88) * | ||
| Bivariate Logistic Regression | Multivariate Logistic Regression (Adjusted for Gender and Ethnicity) | |||||
|---|---|---|---|---|---|---|
| Children 1–<3-Yrs %E from Protein < AMDR N = 333 (n = 5) OR (95% CI) Nothing Significant | Children 3–<6-Yrs %E from Protein < AMDR N = 514 (n = 89) OR (95% CI) | Children 6–<10-Yrs %E from Protein < AMDR N = 479 (n = 131) OR (95% CI) | Children 1–<3-Yrs %E from Protein< AMDR N = 333 (n = 5) OR (95% CI) Nothing Significant | Children 3–<6-Yrs %E from Protein< AMDR N = 514 (n = 89) OR (95% CI) b | Children 6–<10-Yrs %E from Protein< AMDR N = 479 (n = 131) OR (95% CI) c | |
| Primary Caregiver a | ||||||
| Mother | Ref | Ref | ||||
| Grandparent | 0.70 (0.33–1.47) | 0.84 (0.43–1.66) | ||||
| Other (father, sibling, aunt) | 0.60 (0.21–1.72) | 1.31 (0.71–2.42) | ||||
| Age in years | ||||||
| Gender | ||||||
| Male | Ref | Ref | Ref | Ref | ||
| Female | 0.98 (0.60–1.60) | 0.86 (0.49–1.49) | 1.06 (0.63–1.77) | 0.74 (0.42–1.33) | ||
| Head of household | ||||||
| Father | Ref | Ref | Ref | |||
| Mother | 2.50 (1.15–5.44) * | 2.03 (0.97–4.25) | 2.38 (1.10–5.17) * | |||
| Grandparent | 1.02 (0.46–2.25) | 1.10 (0.62–1.95) | 1.05 (0.46–2.42) | |||
| Other (e.g., aunt, uncle, friend) | 1.04 (0.37–2.91) | 2.12 (0.95–4.73) | 1.02 (0.36–2.90) | |||
| Marital status of mother | ||||||
| Married | Ref | Ref | ||||
| Other e.g., unmarried, divorced | 1.21 (0.73–2.02) | 1.32 (0.71–2.48) | ||||
| Mother’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | |||
| Completed grade 12 | 0.58 (0.34–1.00) * | 0.96 (0.56–1.64) | 0.54 (0.30–0.96) * | |||
| Qualification after grade 12 | 0.80 (0.36–1.78) | 0.75 (0.35–1.62) | 0.91 (0.41–2.04) | |||
| Father’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | ||||
| Completed grade 12 | 1.08 (0.62–1.89) | 0.97 (0.61–1.52) | ||||
| Qualification after grade 12 | 0.94 (0.40–2.19) | 0.82 (0.34–1.98) | ||||
| Mother’s employment status | ||||||
| Not employed/do not know | Ref | Ref | ||||
| Employed | 0.88 (0.46–1.69) | 0.70 (0.38–1.31) | ||||
| Father’s employment status | ||||||
| Not employed/do not know | Ref | Ref | ||||
| Employed | 0.85 (0.48–1.51) | 1.08 (0.67–1.75) | ||||
| Wealth index quintiles | ||||||
| One/two/three | Ref | Ref | ||||
| Four/five | 1.46 (0.81–2.64) | 0.80 (0.49–1.30) | ||||
| Ethnicity | ||||||
| Black African | Ref | Ref | Co-linear with province | Co-linear with province | ||
| Mixed ancestry | 0.64 (0.30–1.34) | 0.55 (0.31–0.98) * | ||||
| Province | ||||||
| Western Cape | Ref | Ref | Ref | Ref | ||
| Gauteng | 2.03 (1.10–3.77) * | 2.25 (1.30–3.90) ** | 2.07 (1.08–4.00) * | 2.23 (1.33–3.72) ** | ||
| Type of residence | ||||||
| Urban formal | Ref | Ref | ||||
| Urban informal | 1.44 (0.86–2.43) | 1.19 (0.73–1.94) | ||||
| Rural | 0.60 (0.28–1.26) | 0.47 (0.24–0.92) | ||||
| Mother’s BMI | ||||||
| Underweight/normal weight | Ref | Ref | Ref | |||
| Overweight | 0.61 (0.27–1.40) | 2.53 (1.15–5.54) * | 2.30 (1.06–5.01) * | |||
| Obese | 0.96 (0.48–1.90) | 2.15 (1.13–4.07) * | 2.23 (1.18–4.24) * | |||
| Hunger scale | ||||||
| Total score = 0: No risk | Ref | Ref | ||||
| 1–4: At risk of hunger | 1.53 (0.77–3.03) | 1.09 (0.54–2.22) | ||||
| 5–8: Food shortage in house | 1.52 (0.83–2.78) | 1.86 (0.89–3.89) | ||||
| Bivariate Logistic Regression | Multivariate Logistic Regression (Adjusted for Gender and Ethnicity) | |||||
|---|---|---|---|---|---|---|
| Children 1–<3-Yrs %E from Fat < AMDR OR (95% CI) N = 333 (n = 178) | Children 3–<6-Yrs %E from Fat > AMDR N = 514 (n = 98) OR (95% CI) | Children 6–<10-Yrs %E from Fat > AMDR N = 479 (n = 159) OR (95% CI) | Children 1–<3- Yrs %E from Fat < AMDR OR (95% CI) N = 333 (n = 178) No Results b | Children 3–<6-Yrs %E from Fat > AMDR N = 514 (n = 98) OR (95% CI) c | Children 6–<10- Yrs %E from Fat > AMDR N = 479 (n = 159) OR (95% CI) d | |
| Primary Caregiver a | ||||||
| Mother | Ref | Ref | Ref | |||
| Grandparent | 1.85 (0.80–4.29) | 1.13 (0.59–2.15) | 1.10 (0.50–2.40) | |||
| Other (e.g., father, sibling, aunt) | 2.40 (0.75–7.73) | 0.58 (0.19–1.73) | 0.72 (0.33–1.57) | |||
| Gender | ||||||
| Male | Ref | Ref | Ref | Ref | Ref | |
| Female | 0.75 (0.47–1.21) | 1.29 (0.76–2.18) | 1.07 (0.64–1.78) | 1.15 (0.65–2.03) | 1.10 (0.65–1.84) | |
| Head of household | ||||||
| Father | Ref | Ref | Ref | |||
| Mother | 0.45 (0.20–1.04) | 1.41 (0.68–2.93) | 0.92 (0.43–1.95) | |||
| Grandparent | 0.97 (0.56–1.67) | 1.68 (0.81–3.49) | 1.21 (0.66–2.23) | |||
| Other (e.g., aunt, uncle) | 1.17 (0.48–2.88) | 1.78 (0.70–4.53) | 1.03 (0.41–2.62) | |||
| Marital status of mother | ||||||
| Married | Ref | Ref | Ref | |||
| Other (e.g., unmarried, divorced) | 1.75 (0.88–3.47) | 1.23 (0.65–2.35) | 0.67 (0.39–1.13) | |||
| Mother’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | |||
| Completed grade 12 | 0.95 (0.50–1.80) | 0.94 (0.45–1.94) | 1.88 (0.94–3.78) | |||
| Qualification after grade 12 | 1.25 (0.55–2.86) | 0.98 (0.43–2.23) | 1.54 (0.67–3.52) | |||
| Father’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | Ref | ||
| Completed grade 12 | 1.09 (0.55–2.17) | 0.43 (0.21–0.86) * | 1.00 (0.60–1.66) | 0.43 (0.21–0.87) * | ||
| Qualification after grade 12 | 0.47 (0.14–1.53) | 1.19 (0.64–2.24) | 2.02 (0.77–5.27) | 0.95 (0.45–2.02) | ||
| Mother’s employment status | ||||||
| Not employed/do not know | Ref | Ref | Ref | |||
| Employed | 1.15 (0.53–2.52) | 1.34 (0.81–2.22) | 1.35 (0.82–2.22) | |||
| Father’s employment status | ||||||
| Not employed/do not know | Ref | Ref | Ref | |||
| Employed | 1.25 (0.66–2.36) | 1.43 (0.81–2.54) | 1.39 (0.91–2.13) | |||
| Wealth Index Quintiles | ||||||
| One/two/three | Ref | Ref | Ref | Ref | Ref | |
| Four/five | 1.06 (0.59–1.91) | 1.87 (1.00–3.49) * | 1.64 (1.02–2.62) * | 1.71 (0.82–3.59) | 1.36 (0.81–2.28) | |
| Ethnicity | ||||||
| Black African | Ref | Ref | Ref | Ref | Ref | |
| Mixed ancestry | 0.18 (0.10–0.33) *** | 3.08 (1.69–5.62) ** | 1.91 (1.18–3.08) ** | 2.65 (1.38–5.09) ** | 1.96 (1.22–3.13) ** | |
| Province | ||||||
| Western Cape | Ref | Ref | Ref | Co-linear with ethnicity | ||
| Gauteng | 2.65 (1.37–5.13) ** | 0.46 (0.24–0.86) * | 0.77 (0.46–1.27) | |||
| Type of residence | ||||||
| Urban formal | Ref | Ref | Ref | Ref | ||
| Urban informal | 1.43 (0.81–2.54) | 0.52 (0.26–1.04) | 0.32 (0.16–0.65) ** | 0.44 (0.20–0.96) * | ||
| Rural | 0.93 (0.50–1.74) | 1.33 (0.74–2.38) | 0.82 (0.46–1.44) | 0.65 (0.38–1.12) | ||
| Mother’s BMI | ||||||
| Underweight/normal weight | Ref | Ref | Ref | |||
| Overweight | 0.99 (0.55–1.79) | 0.96 (0.47–1.97) | 0.71 (0.37–1.38) | |||
| Obese | 0.76 (0.43–1.34) | 0.98 (0.49–2.00) | 1.29 (0.79–2.10) | |||
| Hunger scale | ||||||
| Total score = 0: No risk | Ref | Ref | Ref | Ref | ||
| 1–4: At risk of hunger | 1.46 (0.80–2.68) | 1.06 (0.56–2.02) | 0.59 (0.29–1.19) | 0.58 (0.29–1.15) | ||
| 5–8: Food shortage in house | 1.32 (0.64–2.73) | 0.56 (0.28–1.12) | 0.50 (0.28–0.87)* | 0.54 (0.30–0.97) * | ||
| Bivariate Logistic Regression | Multivariate Logistic Regression (Adjusted for Gender and Ethnicity) | |||||
|---|---|---|---|---|---|---|
| Children 1–<3-Yrs %E from Total Free Sugars >10%E N = 333 (n = 126) OR (95% CI) | Children 3–<6-Yrs %E from Total free Sugars >10%E N = 514 (n = 225) OR (95% CI) | Children 6–<10- Yrs %E from Total Free Sugars >10%E N = 479 (n = 214) OR (95% CI) | Children 1–<3-Yrs %E from Total Free Sugars >10%E N = 333 (n = 126) OR (95% CI) b | Children 3–<6-Yrs %E from Total Free Sugars >10%E N = 514 (n = 225) OR (95% CI) c | Children 6–<10- Yrs %E from Total Free Sugars >10%E N = 479 (n = 214) OR (95% CI) d | |
| Primary caregiver a | ||||||
| Mother | Ref | Ref | Ref | |||
| Grandparent | 1.16 (0.64–2.10) | 0.97 (0.59–1.57) | 1.03 (0.57–1.86) | |||
| Other (e.g., father, sibling, aunt) | 0.83 (0.28–2.44) | 0.86 (0.41–1.82) | 1.30 (0.68–1.50) | |||
| Gender | ||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref |
| Female | 1.43 (0.80–2.57) | 0.92 (0.58–1.47) | 0.85 (0.53–1.38) | 1.52 (0.77–3.00) | 0.89 (0.57–1.40) | 0.90 (0.54–1.51) |
| Head of household | ||||||
| Father | Ref | Ref | Ref | Ref | Ref | |
| Mother | 3.28 (1.28–8.45) * | 1.35 (0.76–2.41) | 0.67 (0.32–1.37) | 3.31 (1.22–8.94) * | 1.44 (0.75–2.73) | |
| Grandparent | 2.89 (1.53–5.43) ** | 1.49 (0.95–2.34) | 0.92 (0.58–1.45) | 2.83 (1.51–5.32) ** | 1.45 (0.88–2.39) | |
| Other (e.g., aunt, uncle) | 2.80 (0.98–8.00) | 2.92 (1.22–7.02) * | 0.76 (0.33–1.77) | 2.70 (0.95–7.70) | 2.79 (1.04–7.46) * | |
| Marital status of mother | ||||||
| Married | Ref | Ref | Ref | |||
| Other (e.g., unmarried, divorced) | 1.74 (0.89–3.39) | 1.04 (0.68–1.57) | 0.74 (0.44–1.25) | |||
| Mother’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | |||
| Completed grade 12 | 1.40 (0.70–2.80) | 1.11 (0.60–2.06) | 1.03 (0.60–1.76) | |||
| Qualification after grade 12 | 1.13 (0.47–2.71) | 1.15 (0.65–2.03) | 1.44 (0.65–3.18) | |||
| Father’s highest education | ||||||
| Did not complete grade 12 | Ref | Ref | Ref | |||
| Completed grade 12 | 1.42 (0.70–2.87) | 1.06 (0.69–1.64) | 1.36 (0.81–2.28) | |||
| Qualification after grade 12 | 0.71 (0.27–1.88) | 1.08 (0.62–1.88) | 0.95 (0.43–2.08) | |||
| Mother’s employment status | ||||||
| Not employed/do not know | Ref | Ref | Ref | Ref | Ref | |
| Employed | 0.99 (0.47–2.08) | 1.66 (1.02–2.70) * | 2.09 (1.23–3.56) ** | 1.31 (0.80–2.14) | 1.86 (1.08–3.22) * | |
| Father’s employment status | ||||||
| Not employed/do not know | Ref | Ref | Ref | Ref | ||
| Employed | 0.84 (0.52–1.33) | 0.70 (0.44–1.12) | 1.60 (1.10–2.35) * | 1.57 (1.02–2.42) * | ||
| Wealth index quintiles | ||||||
| One/two/three | Ref | Ref | Ref | |||
| Four/five | 0.95 (0.51–1.76) | 1.22 (0.73–2.02) | 0.99 (0.63–1.57) | |||
| Ethnicity | ||||||
| Black African | Ref | Ref | Ref | Ref | Ref | Ref |
| Mixed ancestry | 1.50 (0.80–2.81) | 1.40 (0.77–2.55) | 2.73 (1.64–4.56) ** | 1.21 (0.59–2.48) | 1.28 (0.70–2.36) | 2.59 (1.50–4.49) ** |
| Province | ||||||
| Western Cape | Ref | Ref | Ref | |||
| Gauteng | 0.99 (0.53–1.87) | 0.82 (0.47–1.42) | 0.56 (0.34–0.91) * | Co-linear with ethnicity | ||
| Type of residence | ||||||
| Urban formal | Ref | Ref | Ref | Ref | ||
| Urban informal | 0.43 (0.23–0.81) * | 1.01 (0.62–1.66) | 0.67 (0.43–1.04) | 0.50 (0.25–1.02) | ||
| Rural | 0.79 (0.42–1.47) | 0.95 (0.62–1.45) | 0.99 (0.65–1.49) | 0.73 (0.36–1.45) | ||
| Mother’s BMI | ||||||
| Underweight/normal weight | Ref | Ref | Ref | Ref | ||
| Overweight | 1.33 (0.63–2.78) | 0.42 (0.20–0.88) * | 1.25 (0.71–2.22) | 0.50 (0.23–1.11) | ||
| Obese | 1.00 (0.52–1.95) | 0.87 (0.51–1.48) | 1.38 (0.76–2.49) | 1.04 (0.58–1.86) | ||
| Hunger scale | ||||||
| Total score = 0: No risk | Ref | Ref | Ref | Ref | ||
| 1–4: At risk of hunger | 0.62 (0.30–1.29) | 0.50 (0.29–0.84) ** | 0.64 (0.33–1.26) | 0.47 (0.28–0.79) ** | ||
| 5–8: Food shortage in house | 0.82 (0.33–2.01) | 0.45 (0.26–0.77) ** | 0.69 (0.39–1.19) | 0.49 (0.27–0.86) * | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steyn, N.P.; Nel, J.H.; Malczyk, S.; Drummond, L.; Senekal, M. Provincial Dietary Intake Study (PDIS): Energy and Macronutrient Intakes of Children in a Representative/Random Sample of 1–<10-Year-Old Children in Two Economically Active and Urbanized Provinces in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1717. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051717
Steyn NP, Nel JH, Malczyk S, Drummond L, Senekal M. Provincial Dietary Intake Study (PDIS): Energy and Macronutrient Intakes of Children in a Representative/Random Sample of 1–<10-Year-Old Children in Two Economically Active and Urbanized Provinces in South Africa. International Journal of Environmental Research and Public Health. 2020; 17(5):1717. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051717
Chicago/Turabian StyleSteyn, Nelia P., Johanna H. Nel, Sonia Malczyk, Linda Drummond, and Marjanne Senekal. 2020. "Provincial Dietary Intake Study (PDIS): Energy and Macronutrient Intakes of Children in a Representative/Random Sample of 1–<10-Year-Old Children in Two Economically Active and Urbanized Provinces in South Africa" International Journal of Environmental Research and Public Health 17, no. 5: 1717. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051717







