Stability of Executive Functioning of Moderately-Late Preterm and Full-Term Born Children at Ages 11 and 19: The TRAILS Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
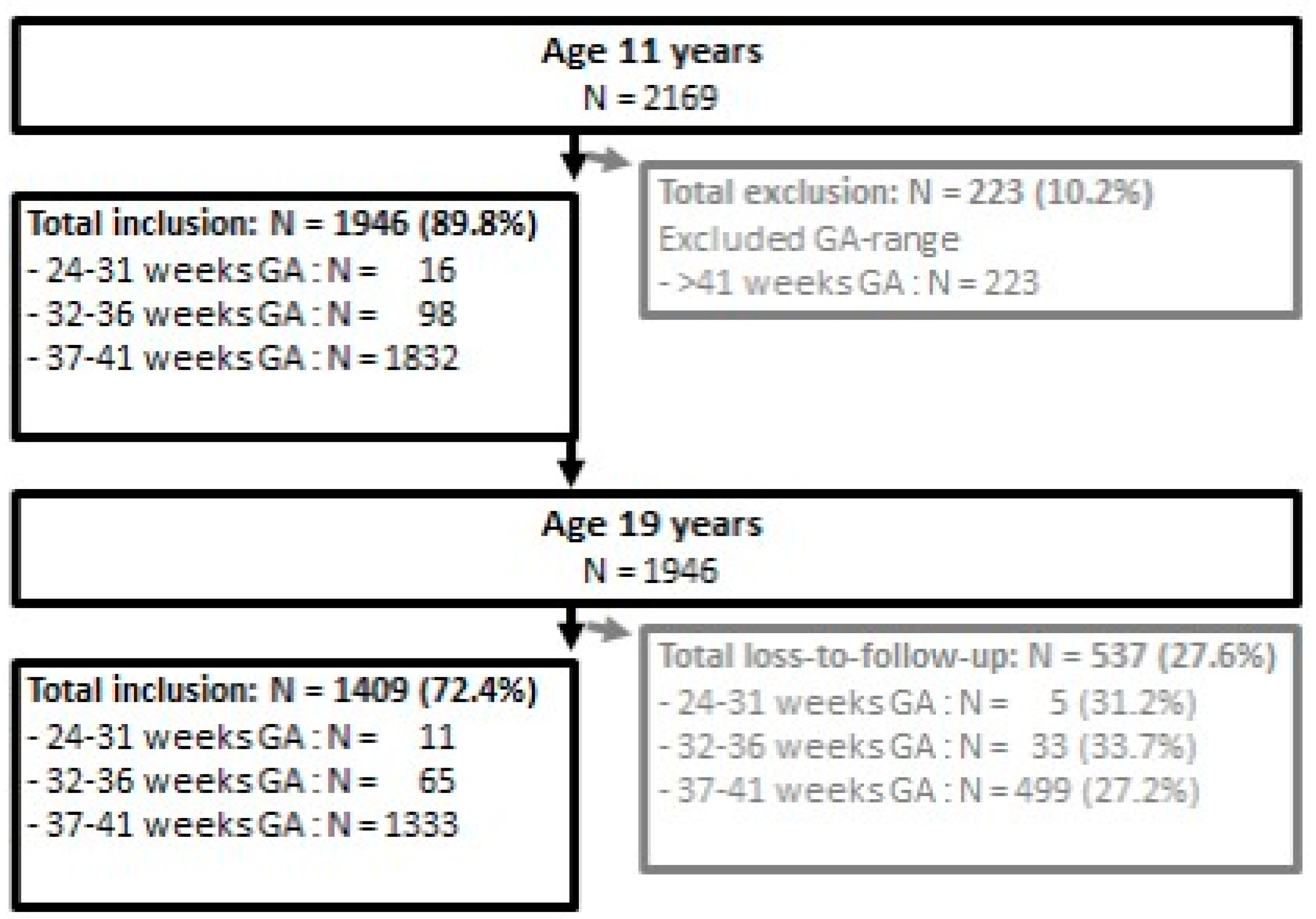
2.1. Participants
2.2. Procedure and Measures
2.2.1. Procedure
2.2.2. Executive Functioning by the Amsterdam Neuropsychological Tasks
2.2.3. Background Characteristics
2.3. Statistical Analysis
3. Results
3.1. Background Characteristics
3.2. Executive Functioning at Age 11 and Age 19
3.3. Change in Executive Functioning between Age 11 and Age 19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, C.E.; Cheong, J.L.Y.; Fam, L.G.; Leemans, A.; Seal, M.L.; Doyle, L.W.; Anderson, P.J.; Spittle, A.J.; Thompson, D.K. Moderate and late preterm infants exhibit widespread brain white matter microstructure alterations at term-equivalent age relative to term-born controls. Brain Imaging Behav. 2016, 10, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; Doyle, L.W.; Anderson, P.J.; Lee, K.J.; Cheong, J.L.Y. Moderate and late preterm birth: Effect on brain size and maturation at term-equivalent age. Radiology 2014, 273, 232–240. [Google Scholar] [CrossRef]
- Nosarti, C.; Giouroukou, E.; Healy, E.; Rifkin, L.; Walshe, M.; Reichenberg, A.; Chitnis, X.; Williams, S.C.R.; Murray, R.M. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 2008, 131, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marret, S.; Marchand-Martin, L.; Picaud, J.C.; Hascoët, J.-M.; Arnaud, C.; Rozé, J.-C.; Truffert, P.; Larroque, B.; Kaminski, M.; Ancel, P.-Y.; et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: The EPIPAGE cohort study. PLoS ONE 2013, 8, e62683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitcher, J.B.; Riley, A.M.; Doeltgen, S.H.; Kurylowicz, L.; Rothwell, J.C.; McAllister, S.M.; Smith, A.E.; Clow, A.; Kennaway, D.J.; Ridding, M.C. Physiological evidence consistent with reduced neuroplasticity in human adolescents born preterm. J. Neurosci. 2012, 32, 16410–16416. [Google Scholar] [CrossRef] [Green Version]
- Latal, B. Prediction of neurodevelopmental outcome after preterm birth. Pediatric Neurol. 2009, 40, 413–419. [Google Scholar] [CrossRef]
- Van Houdt, C.A.; Oosterlaan, J.; van Wassenaer-Leemhuis, A.G.; van Kaam, A.H.; Aarnoudse-Moens, C.S.H. Executive function deficits in children born preterm or at low birthweight: A meta-analysis. Dev. Med. Child Neurol. 2019, 61, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Gyamfi-Bannerman, C.; Ananth, C.V. Trends in spontaneous and indicated preterm delivery among singleton gestations in the United States, 2005–2012. Obstet. Gynecol. 2014, 124, 1069–1074. [Google Scholar] [CrossRef]
- Rose, G. Sick Individuals and Sick Populations. Int. J. Epidemiol. 2001, 30, 427–432. [Google Scholar] [CrossRef]
- Mulder, H.; Pitchford, N.J.; Marlow, N. Processing speed and working memory underlie academic attainment in very preterm children. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F267–F272. [Google Scholar] [CrossRef]
- Boelema, S.R.; Harakeh, Z.; Ormel, J.; Hartman, C.A.; Vollebergh, W.A.M.; van Zandvoort, M.J.E. Executive functioning shows differential maturation from early to late adolescence: Longitudinal findings from a TRAILS study. Neuropsychology 2014, 28, 177. [Google Scholar] [CrossRef]
- Burnett, A.C.; Scratch, S.E.; Anderson, P.J. Executive function outcome in preterm adolescents. Early Hum. Dev. 2013, 89, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Jisa, K.A.; Clarey, D.D.; Peeples, E.S. Magnetic resonance imaging findings of term and preterm hypoxic-ischemic encephalopathy: A review of relevant animal models and correlation to human imaging. Open Neuroimaging J. 2018, 12, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, I.S.; Kerns, K.A.; Müller, U.; Ahronovich, M.D.; Litman, F.R. Executive functions in extremely low birth weight and late-preterm preschoolers: Effects on working memory and response inhibition. Child Neuropsychol. 2012, 18, 586–599. [Google Scholar] [CrossRef]
- Cserjesi, R.; Van Braeckel, K.N.J.A.; Butcher, P.R.; Kerstjens, J.M.; Reijneveld, S.A.; Bouma, A.; Geuze, R.H.; Bos, A.F. Functioning of 7-year-old children born at 32 to 35 weeks’ gestational age. Pediatrics 2012, 130, e838–e846. [Google Scholar] [CrossRef] [Green Version]
- Curtis, W.J.; Lindeke, L.L.; Georgieff, M.K.; Nelson, C.A. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain 2002, 125, 1646–1659. [Google Scholar] [CrossRef] [Green Version]
- Suikkanen, J.; Miettola, S.; Heinonen, K.; Vääräsmäki, M.; Tikanmäki, M.; Sipola, M.; Matinolli, H.-M.; Järvelin, M.-R.; Räikkönen, K.; Hovi, P.; et al. Reaction times, learning, and executive functioning in adults born preterm. Pediatric Res. 2021, 89, 198–204. [Google Scholar] [CrossRef]
- Blackwell, K.L.; Trzesniewski, K.H.; Dweck, C.S. Implicit theories of intelligence predict achievement across an adolescent transition: A longitudinal study and an intervention in child. Child Dev. 2007, 78, 246–263. [Google Scholar] [CrossRef]
- Luu, T.M.; Ment, L.; Allan, W.; Schneider, K.; Vohr, B.R. Executive and memory function in adolescents born very preterm. Pediatrics 2011, 127, e639–e646. [Google Scholar] [CrossRef]
- Ritter, B.C.; Nelle, M.; Perrig, W.; Steinlin, M.; Everts, R. Executive functions of children born very preterm-deficit or delay? Eur. J. Pediatrics 2013, 172, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, A.; Adamsson, M.; Serenius, F.; Hägglöf, B. Executive Functioning and learning skills of adolescent children born at fewer than 26 weeks of gestation. PLoS ONE 2016, 11, e0151819. [Google Scholar] [CrossRef]
- Wehrle, F.M.; Kaufmann, L.; Benz, L.D.; Huber, R.; O’Gorman, R.L.; Latal, B.; Hagmann, C.F. Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Hum. Dev. 2016, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, K.; Lahti, J.; Sammalathi, S.; Wolke, D.; Lano, A.; Andersson, S.; Pesonen, A.-K.; Eriksson, J.G.; Kajantie, E.; Raikkonen, K. Neurocognitive outcome in young adults born late-preterm. Dev. Med. Child Neurol. 2018, 60, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Winter, A.F.; Oldehinkel, A.J.; Veenstra, R.; Brunnekreef, J.A.; Verhulst, F.C.; Ormel, J. Evaluation of non-response bias in mental health determinants and outcomes in a large sample of pre-adolescents. Eur. J. Epidemiol. 2005, 20, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunnekreef, J.A.; De Sonneville, L.M.J.; Althaus, M.; Minderaa, R.B.; Oldehinkel, A.J.; Verhulst, F.C.; Ormel, J. Information processing profiles of internalizing and externalizing behavior problems: Evidence from a population-based sample of preadolescents. J. Child Psychol. Psychiatry 2007, 48, 185–193. [Google Scholar] [CrossRef] [PubMed]
- De Sonneville, L.M.J. Amsterdam neuropsychological tasks: A computer-aided assessment program. Cogn. Ergon. Clin. Assess. Comput. Assist. Learn. Comput. Psychol. 1999, 6, 204–217. [Google Scholar]
- De Sonneville, L.M.J.; Verschoor, C.A.; Njiokiktjien, C.; Veld, V.O.H.; Toorenaar, N.; Vranken, M. Facial identity and facial emotions: Speed, accuracy, and processing strategies in children and adults. J. Clin. Exp. Neuropsychol. 2002, 24, 200–213. [Google Scholar] [CrossRef]
- De Sonneville, L.M.J. Amsterdam neuropsychological tasks: Scientific and clinical applications. Tijdschr. Voor Neuropsychol. 2005, 1, 27–41. [Google Scholar]
- Stins, J.F.; de Sonneville, L.M.J.; Groot, A.S.; Polderman, T.C.; van Baal, C.G.C.M.; Boomsma, D.I. Heritability of selective attention and working memory in preschoolers. Behav. Genet. 2005, 35, 407–416. [Google Scholar] [CrossRef]
- Luu, T.M.; Vohr, B.R.; Allan, W.; Schneider, K.C.; Ment, L.R. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics 2011, 128, 313–322. [Google Scholar] [CrossRef]
- Ganzeboom, H.B.G.; Treiman, D.J. Internationally comparable measures of occupational status for the 1988 international standard classification of occupations. Soc. Sci. Res. 1996, 25, 201–239. [Google Scholar] [CrossRef]
- Jaspers, M.; de Meer, G.; Verhulst, F.C.; Ormel, J.; Reijneveld, S.A. Limited validity of parental recall on pregnancy, birth and early childhood at child-age 10 years. J. Clin. Epidemiol. 2010, 63, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloosterman, G. On intrauterine growth: The significance of prenatal care. Int. J. Gynecol. Obstet. 1970, 8, 895–912. [Google Scholar] [CrossRef]
- Silverstein, A.B. Validity of WISC-R short forms. J. Clin. Psychol. 1975, 31, 696–697. [Google Scholar] [CrossRef]
- VanderSteene, G.; Van Haasen, P.P.; De Bruyn, E.E.J.; Pijl, Y.L.; Poortinga, Y.H.; Lutje Spelberg, H.C.; Spoelders-Claes, R.; Stinissen, J. Wechsler Intelligence Scale for Children–Revised (Dutch edn); Swets & Zeitlinger: Lisse, The Netherlands, 1986. [Google Scholar]
- Sattler, J.M. Assessment of Children (Revised and Updated ), 3rd ed.; Jerome, M., Ed.; Sattler: San Diego, CA, USA, 1992. [Google Scholar]
- Tideman, E. Longitudinal follow-up of children born preterm: Cognitive development at age 19. Early Hum. Dev. 2000, 58, 81–90. [Google Scholar] [CrossRef]
- Ritter, B.C.; Perrig, W.; Steinlin, M.; Everts, R. Cognitive and behavioral aspects of executive functions in children born very preterm. Child Neuropsychol. 2014, 20, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Ayyavoo, A.; Derraik, J.G.B.; Hofman, P.L.; Cutfield, W.S. Postterm births: Are prolonged pregnancies too long? J. Pediatrics 2014, 164, 647–651. [Google Scholar] [CrossRef]
- Bennet, L.; Van Den Heuij, L.; Dean, J.M.; Drury, P.; Wassink, G.; Gunn, A.J. Neural plasticity and the Kennard principle: Does it work for the preterm brain? Clin. Exp. Pharmacol. Physiol. 2013, 40, 774–784. [Google Scholar] [CrossRef]
- Mulder, H.; Pitchford, N.J.; Hagger, M.S.; Marlow, N. Development of executive function and attention in preterm children: A systematic review. Dev. Neuropsychol. 2009, 34, 393–421. [Google Scholar] [CrossRef]
- Aarnoudse-Moens, C.S.H.; Weisglas-Kuperus, N.; van Goudoever, J.B.; Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and / or very low birth weight children. Pediatrics 2009, 124, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Hallin, A.-L.; Hellström-Westas, L.; Stjernqvist, K. Follow-up of adolescents born extremely preterm: Cognitive function and health at 18 years of age. Acta Paediatrics 2010, 99, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.C.; Scratch, S.E.; Lee, K.J.; Cheong, J.; Searle, K.; Hutchinson, E.; De Luca, C.; Davey, M.A.; Roberts, G.; Doyle, L.W.; et al. Executive Function in Adolescents Born <1000 g or <28 Weeks: A Prospective Cohort Study. Pediatrics 2015, 135, e826–e834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luciana, M.; Lindeke, L.; Georgieff, M.; Mills, M.; Nelson, C.A. Neurobehavioral evidence for working-memory deficits in with histories of prematurity. Dev. Med. Child Neurol. 1999, 41, 521–533. [Google Scholar] [CrossRef] [PubMed]
| Domain Information Processing |
| Component 1 Baseline Speed: Simple visuomotor time. |
 |
|
| Domain Attention Control |
| Component 2 Sustained Attention |
 |
|
| Component 3 Inhibition: Inhibition of prepotent responses. |
 |
|
| Domain Cognitive Flexibility |
| Component 4 Pattern Search: Automatic and controlled visuospatial pattern recognition. |
 |
|
| Component 5 Working Memory: Working memory capacity |
 |
|
| Full-Term | Moderately-Late Preterm | ||
|---|---|---|---|
| N (%)/Mean (SD) | N (%)/Mean (SD) | p * | |
| Participants at age 11 years | 1832 | 98 | |
| Participants at age 19 years (% of age 11) | 1333 (72.8) | 65 (66.3) | 0.165 |
| Male | 904 (49.3) | 45 (45.9) | 0.509 |
| Gestational age (weeks) | 39.69 (1.05) | 34.87 (1.42) | <0.001 |
| Low socioeconomic status | 461 (25.2) | 22 (22.4) | 0.546 |
| Ethnicity-Dutch | 1583 (86.4) | 88 (89.8) | 0.789 |
| Moroccan/Turkish | 23 (1.3) | 0 (0.0) | |
| Other | 227 (12.4) | 10 (10.2) | |
| Birth weight (grams) | 3435 (490) | 2435 (605) | <0.001 |
| Small for gestational age <10th percentile | 245 (13.4) | 19 (19.4) | 0.091 |
| Postnatal days in hospital | 4.05 (8.4) | 15.27 (14.6) | <0.001 |
| School problems # | 402 (21.9) | 25 (25.5) | 0.407 |
| Verbal and performance score, based on: | 97.39 (14.92) | 97.39 (15.02) | 0.997 |
| WISC-R vocabulary test (vocabulary score) | 9.09 (2.84) | 9.49 (2.81) | 0.172 |
| WISC-R block design test (spatial score) | 10.05 (3.10) | 9.64 (2.97) | 0.206 |
| Median age time point 11 years (N = 1946) | 11.10 (0.55) | 11.07 (0.54) | 0.628 |
| Median age time point 19 years (N = 1409) | 19.19 (0.57) | 19.11 (0.53) | 0.295 |
| Measures | RT FT Mean (SD) | RT MLP Mean (SD) | RT Z-Score MLP Mean (SD) | Effect Size Beta (95% CI) | p Crude | p Adjusted * | p Adjusted for Age 11 # |
|---|---|---|---|---|---|---|---|
| Age 11 years | |||||||
| Baseline Speed | 309 (39) | 307 (40) | −0.10 (0.95) | −0.09 (−0.29 to 0.12) | 0.39 | 0.37 | |
| Pattern search | 1469 (485) | 1523 (534) | 0.11 (1.09) | 0.11 (−0.10 to 0.31) | 0.31 | 0.28 | |
| Working memory | 470 (259) | 488 (268) | 0.10 (1.06) | 0.09 (−0.11 to 0.30) | 0.38 | 0.37 | |
| Sustained attention | 1.73 (0.90) | 1.89 (0.94) | 0.18 (1.07) | 0.19 (−0.02 to 0.39) | 0.07 | 0.06 | |
| Inhibition | 199 (161) | 185 (136) | −0.9 (0.89) | −0.08 (−0.28 to 0.12) | 0.43 | 0.45 | |
| Attentional flexibility | 557 (221) | 562 (205) | −0.03 (0.91) | 0.02 (−0.19 to 0.22) | 0.88 | 0.87 | |
| Age 19 years | |||||||
| Baseline Speed | 237 (22) | 235 (19) | −0.06 (−0.31 to 0.19) | 0.65 | 0.76 | 0.94 | |
| Pattern search | 815 (269) | 829 (286) | 0.05 (1.05) | 0.05 (−0.20 to 0.30) | 0.70 | 0.43 | 0.44 |
| Working memory | 236 (147) | 231 (142) | −0.02 (0.94) | −0.02 (−0.27 to 0.23) | 0.86 | 0.92 | 0.81 |
| Sustained attention | 0.88 (0.45) | 0.91 (0.41) | 0.13 (1.01) | 0.09 (−0.16 to 0.34) | 0.47 | 0.22 | 0.99 |
| Inhibition | 169 (141) | 147 (128) | −0.13 (0.96) | −0.16 (−0.41 to 0.09) | 0.20 | 0.34 | 0.24 |
| Attentional flexibility | 337 (142) | 371 (187) | 0.15 (1.18) | 0.22 (−0.03 to 0.48) | 0.09 | 0.047 | 0.07 |
| Measures | % Errors FT Mean (SD) | % Errors MLP Mean (SD) | % Errors z-Score MLP Mean (SD) | Effect Size Beta (95% CI) | p Crude | p Adjusted * | p Adjusted for Age 11 # |
|---|---|---|---|---|---|---|---|
| Age 11 years | |||||||
| Pattern search | 3.78 (9.06) | 3.37 (7.28) | −0.05 (0.79) | −0.05 (−0.25 to 0.15) | 0.649 | 0.653 | |
| Working memory | −3.36 (7.78) | −3.05 (5.90) | 0.03 (0.75) | 0.04 (−0.16 to 0.24) | 0.714 | 0.710 | |
| Sustained attention | 5.09 (3.15) | 4.61 (2.98) | −0.14 (0.98) | −0.14 (−0.35 to 0.06) | 0.172 | 0.111 | |
| Inhibition | 5.88 (9.03) | 5.89 (7.79) | 0.00 (0.86) | 0.00 (−0.20 to 0.20) | 0.988 | 0.817 | |
| Attentional flexibility | 9.01 (13.01) | 9.92 (15.01) | 0.06 (1.19) | 0.06 (−0.14 to 0.27) | 0.553 | 0.841 | |
| Age 19 years | |||||||
| Pattern search | 2.92 (9.48) | 1.77 (5.41) | −0.11 (0.60) | −0.12 (−0.36 to 0.13) | 0.917 | 0.462 | 0.463 |
| Working memory | −4.05 (7.35) | −3.83 (6.98) | 0.02 (0.95) | 0.13 (−0.22 to 0.27) | 0.843 | 0.818 | 0.853 |
| Sustained attention | 4.17 (3.66) | 3.83 (2.28) | −0.06 (0.78) | −0.05 (−0.30 to 0.19) | 0.671 | 0.677 | 0.846 |
| Inhibition | 5.51 (9.76) | 5.92 (10.12) | 0.04 (1.07) | 0.04 (−0.21 to 0.29) | 0.756 | 0.373 | 0.579 |
| Attentional flexibility | 1.63 (5.67) | 2.21 (7.57) | 0.06 (1.23) | 0.06 (-0.19 to 0.32) | 0.622 | 0.692 | 0.419 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reijneveld, S.A.; Hornman, J.; Boelema, S.R.; de Winter, A.F. Stability of Executive Functioning of Moderately-Late Preterm and Full-Term Born Children at Ages 11 and 19: The TRAILS Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4161. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18084161
Reijneveld SA, Hornman J, Boelema SR, de Winter AF. Stability of Executive Functioning of Moderately-Late Preterm and Full-Term Born Children at Ages 11 and 19: The TRAILS Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(8):4161. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18084161
Chicago/Turabian StyleReijneveld, Sijmen A., Jorijn Hornman, Sarai R. Boelema, and Andrea F. de Winter. 2021. "Stability of Executive Functioning of Moderately-Late Preterm and Full-Term Born Children at Ages 11 and 19: The TRAILS Cohort Study" International Journal of Environmental Research and Public Health 18, no. 8: 4161. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18084161






