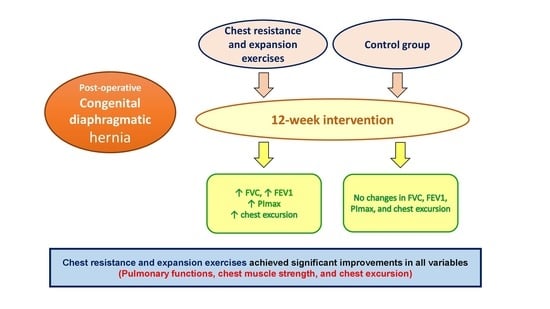
Effect of Chest Resistance and Expansion Exercises on Respiratory Muscle Strength, Lung Function, and Thoracic Excursion in Children with a Post-Operative Congenital Diaphragmatic Hernia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
Allocation, Randomization, and Blinding
2.3. Sample Size Estimation
2.4. Outcome Measures
2.4.1. Respiratory Muscle Strength
2.4.2. Lung Functions
2.4.3. Thoracic Excursion
2.4.4. Intervention
2.4.5. The Power Breathe KH2 Resistance Exercise
2.4.6. Chest Expansion Exercise
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackerman, K.G.; Pober, B.R. Congenital diaphragmatic hernia and pulmonary hypoplasia: New insights from developmental biology and genetics. Am. J. Med. Genet. C Semin. Med. Genet. 2007, 145C, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hu, J.; Druschel, C.M.; Kirby, R.S. Twenty-five-year survival of children with birth defects in New York State: A population-based study. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.; Bower, C.; Dickinson, J.E.; Sokol, J. Outcomes of congenital diaphragmatic hernia: A population-based study in Western Australia. Pediatrics 2005, 116, e356–e363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallot, D.; Boda, C.; Ughetto, S.; Perthus, I.; Robert-Gnansia, E.; Francannet, C.; Laurichesse-Delmas, H.; Jani, J.; Coste, K.; Deprest, J.; et al. Prenatal detection and outcome of congenital diaphragmatic hernia: A French registry-based study. Ultrasound Obstet. Gynecol. 2007, 29, 276–283. [Google Scholar] [CrossRef]
- McGivern, M.R.; Best, K.E.; Rankin, J.; Wellesley, D.; Greenlees, R.; Addor, M.C.; Arriola, L.; De Walle, H.; Barisic, I.; Beres, J.; et al. Epidemiology of congenital diaphragmatic hernia in Europe: A register-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F137–F144. [Google Scholar] [CrossRef]
- Van den Hout, L.; Schaible, T.; Cohen-Overbeek, T.E.; Hop, W.; Siemer, J.; van de Ven, K.; Wessel, L.; Tibboel, D.; Reiss, I. Actual outcome in infants with congenital diaphragmatic hernia: The role of a standardized postnatal treatment protocol. Fetal Diagn. Ther. 2011, 29, 55–63. [Google Scholar] [CrossRef]
- Spoel, M.; Laas, R.; Gischler, S.J.; Hop, W.J.; Tibboel, D.; de Jongste, J.C.; Ijsselstijn, H. Diagnosis-related deterioration of lung function after extracorporeal membrane oxygenation. Eur. Respir. J. 2012, 40, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Spoel, M.; Marshall, H.; IJsselstijn, H.; Parra-Robles, J.; van der Wiel, E.; Swift, A.J.; Rajaram, S.; Tibboel, D.; Tiddens, H.A.; Wild, J.M. Pulmonary ventilation and micro-structural findings in congenital diaphragmatic hernia. Pediatr. Pulmonol. 2016, 51, 517–524. [Google Scholar] [CrossRef]
- Rygl, M.; Rounova, P.; Sulc, J.; Slaby, K.; Stranak, Z.; Pycha, K.; Pýcha, K.; Svobodova, T.; Pohunek, P.; Škába, R. Abnormalities in pulmonary function in infants with high-risk congenital diaphragmatic hernia. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2015, 159, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Kassim, Z.; Moxham, J.; Davenport, M.; Nicolaides, K.; Greenough, A.; Rafferty, G. Respiratory muscle strength in healthy infants and those with surgically correctable anomalies. Pediatr. Pulmonol. 2015, 50, 71–78. [Google Scholar]
- Lally, K.P.; Bagolan, P.; Hosie, S.; Lally, P.A.; Stewart, M.; Cotten, C.M.; Van Meurs, K.P.; Alexander, G.; Congenital Diaphragmatic Hernia Study Group. Corticosteroids for fetuses with congenital diaphragmatic hernia: Can we show benefit? J. Pediatr. Surg. 2006, 41, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Snoek, K.G.; Capolupo, I.; van Rosmalen, J.; HoutLde, J.; Vijfhuize, S.; Greenough, A.; Wijnen, R.M.; Tibboel, D.; Reiss, I.K.; CDH EURO Consortium. Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia: A randomized clinical trial (the VICI-trial). Ann Surg. 2016, 263, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Russell, K.W.; Yoder, B.A.; Fenton, S.J. Congenital diaphragmatic hernia: A narrative review of controversies in neonatal management. Transl. Pediatr. 2021, 10, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Moawd, S.A.; Azab, A.R.; Ibrahim, Z.M.; Verma, A.; Abdelbasset, W.K. Impacts of respiratory muscle training on respiratory functions, maximal exercise capacity, functional performance, and quality of life in school-aged children with postoperative congenital diaphragmatic hernia. Dis. Markers 2020, 2020, 8829373. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.M.; Elnegamy, T.E.H. Effect of chest physical therapy on pediatrics hospitalized with pneumonia. Int. J. Health Rehabil. Sci. 2015, 4, 219–226. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Alsubaie, S.F.; Tantawy, S.A.; Abo Elyazed, T.I.; Kamel, D.M. Evaluating pulmonary function, aerobic capacity, and pediatric quality of life following a 10-week aerobic exercise training in school-aged asthmatics: A randomized controlled trial. Patient Prefer Adherence 2018, 12, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- Kido, S.; Nakajima, Y.; Miyasaka, T.; Maeda, Y.; Tanaka, T.; Yu, W.; Maruoka, H.; Takayanagi, K. Effects of combined training with breathing resistance and sustained physical exertion to improve endurance capacity and respiratory muscle function in healthy young adults. J. Phys. Ther. Sci. 2013, 25, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Dean, E. Mobilization and exercise. In Cardiovascular and Pulmonary Physical Therapy, 4th ed.; Frownfelter, D., Dean, E., Eds.; Mosby: St. Louis, MO, USA, 2006; pp. 263–306. [Google Scholar]
- Winkelmann, E.R.; Chiappa, G.R.; Lima, C.O.; Viecili, P.R.; Stein, R.; Ribeiro, J.P. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am. Heart J. 2009, 158, 768.e1–768.e7. [Google Scholar] [CrossRef]
- Minahan, C.; Sheehan, B.; Doutreband, R.; Kirkwood, T.; Reeves, D.; Cross, T. Repeated-sprint cycling does not induce respiratory muscle fatigue in active adults: Measurements from the powerbreathe® inspiratory muscle trainer. J. Sports Sci. Med. 2015, 14, 233–238. [Google Scholar]
- Langer, D.; Jacome, C.; Charususin, N.; Scheers, H.; McConnell, A.; Decramer, M.; Gosselink, R. Measurement validity of an electronic inspiratory loading device during a loaded breathing task in patients with COPD. Respir. Med. 2013, 107, 633–635. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.A.; Whitelaw, W.A. The assessment of maximal respiratory mouth pressures in adults. Respir. Care 2009, 54, 1348–1359. [Google Scholar] [PubMed]
- Bockenhauer, S.E.; Chen, H.; Julliard, K.N.; Weedon, J. Measuring thoracic excursion: Reliability of the cloth tape measure technique. J. Am. Osteopat. Assoc. 2007, 107, 191–196. [Google Scholar]
- LaPier, T.K.; Cook, A.; Droege, K.; Oliverson, R. Intertester and intratester reliability of chest excursion measurements in subject without impairment. Cardiopulm. Phys. Ther. 2000, 3, 94–98. [Google Scholar] [CrossRef]
- Malaguti, C.; Rondelli, R.R.; de Souza, L.M.; Domingues, M.; Dal Corso, S. Reliability of chest wall mobility and its correlation with the pulmonary function in patients with chronic obstructive pulmonary disease. Respir. Care 2009, 24, 1703. [Google Scholar]
- Basso, R.P.; Regueiro, E.M.G.; Jamami, M.; Di Lorenzo, V.A.P.; Costa, D. Relationship of the measure of the amplitude thoracoabdominal in asthmatics and healthy adolescents with the physical performance. Fisioter. Mov. 2011, 24, 107–114. [Google Scholar] [CrossRef] [Green Version]
- De Sá, R.B.; Pessoa, M.F.; Cavalcanti, A.G.L.; Camposa, C.S.L.; Amorimb, A.C.; de Andradea, A.D. Immediate effects of respiratory muscle stretching on chest wall kinematics and electromyography in COPD patients. Respir. Physiol. Neurobiol. 2017, 242, 1–7. [Google Scholar] [CrossRef]
- Adigüzel, H.; Eğilmez, M.; Sarikabadayi, U.; Elbasan, B.; Demirgüç, A.; Ergun, N. Chest physiotherapy in a neonatal infant after congenital diaphragmatic hernia surgery. Turk. Klin. J. Health Sci. 2021, 6, 179–186. [Google Scholar] [CrossRef]
- Christian, P.S. Chest physiotherapy for infants. Int. J. Physiother. Res. 2014, 2, 699–705. [Google Scholar]
- Arend, M.; Kivastik, J.; Mäestu, J. Maximal inspiratory pressure is influenced by intensity of the warm-up protocol. Respir. Physiol. Neurobiol. 2016, 230, 11–15. [Google Scholar] [CrossRef]
- Okuyama, H.; Kubota, A.; Kawahara, H.; Oue, T.; Kitayama, Y.; Yagi, M. Correlation between lung scintigraphy and long-term outcome in survivors of congenital diaphragmatic hernia. Pediatr. Pulmonol. 2006, 41, 882–886. [Google Scholar] [CrossRef]
- Romer, L.M.; McConnell, A.K. Specificity and reversibility of inspiratory muscle training. Med. Sci. Sports Exerc. 2003, 35, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Downey, A.E.; Chenoweth, L.M.; Townsend, D.K.; Ranum, J.D.; Ferguson, C.S.; Harms, C.A. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2007, 156, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.V.; Lima, W.L.; Nobre, A.; dos Santos, A.M.; Brito, L.M.; Costa, M.d.R. Inspiratory muscle training and respiratory exercises in children with asthma. J. Bras. Pneumol. 2008, 34, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moawd, S.A.; Azab, A.R.; Alrawaili, S.M.; Abdelbasset, W.K. Inspiratory muscle training in obstructive sleep apnea associating diabetic peripheral neuropathy: A randomized control study. BioMed Res. Int. 2020, 2020, 5036585. [Google Scholar] [CrossRef]
- Langer, D.; Charususin, N.; Jácome, C.; Hoffman, M.; McConnell, A.; Decramer, M.; Gosselink, R. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys. Ther. 2015, 95, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Fregonezi, G.; Sarmento, A.; Pinto, J.; LoMauro, A.; Resqueti, V.; Aliverti, A. Thoracoabdominal asynchrony contributes to exercise limitation in mild asthmatic subjects. Front. Physiol. 2018, 9, 719. [Google Scholar] [CrossRef]
- Schwede, M.; Lee, R.Y.; Zhuo, H.; Kangelaris, K.N.; Jauregui, A.; Vessel, K.; Belzer, A.; Deiss, T.; Matthay, M.A.; Liu, K.D.; et al. Clinician recognition of the acute respiratory distress syndrome: Risk factors for under-recognition and trends over time. Crit. Care Med. 2020, 48, 830–837. [Google Scholar] [CrossRef]
- Hernández-Álvarez, E.D.; Guzmán-David, C.A.; Ruiz-González, J.C.; Ortega-Hernández, A.M.; Ortiz-González, D.C. Effect of a respiratory muscle training program on lung function, respiratory muscle strength and resting oxygen consumption in sedentary young people. Rev. Fac. Med. 2018, 66, 605–609. [Google Scholar] [CrossRef]
- ParkS, J. Effects of inspiratory muscles training plus rib cage mobilization on chest expansion, inspiratory accessory muscles activity and pulmonary function in stroke patients. Appl. Sci. 2020, 10, 5178. [Google Scholar] [CrossRef]
- Rehman, A.; Ganai, J.; Aggarwal, R.; Alghadir, A.H.; Iqbal, Z.A. Effect of passive stretching of respiratory muscles on chest expansion and 6-minute walk distance in copd patients. Int. J. Environ. Res. Public Health. 2020, 17, 6480. [Google Scholar] [CrossRef]

| Characteristics | Control Group (n = 16) | Study Group (n = 16) | p-Value |
|---|---|---|---|
| Age, years | 12.2 ± 1.4 | 12.4 ± 1.5 | 0.699 |
| Gender, m/f | 9/7 | 10/6 | 0.719 |
| Body mass index, Kg/m2 | 20.8 ± 3.5 | 21.2 ± 3.3 | 0.742 |
| Hospitalization period, months | 2.1 ± 0.5 | 1.9 ± 0.7 | 0.359 |
| Classification of CDH defect, n (%) | |||
| B-defect | 9 (56.25) | 11 (68.75) | 0.465 |
| C-defect | 7 (43.75) | 5 (31.25) | |
| Affected side, n (%) | |||
| Left side | 11 (68.75) | 12 (75) | 0.694 |
| Right side | 5 (31.25) | 4 (25) | |
| Medical TTT, n (%) | |||
| Inhaled corticosteroid | 3 (18.75) | 5 (31.25) | 0.414 |
| Inhaled corticosteroid+ long-acting β2-agonist | 13 (81.25) | 11 (68.75) |
| Measures | Control Group (n = 16) | Study Group (n = 16) | Mean Difference (95% CI) | Group × Time Interaction | |
|---|---|---|---|---|---|
| p-Value | η2 | ||||
| FVC, pred. | |||||
| Pre- | 77.2 ± 10.7 | 77.7 ± 11.2 | −0.5 (−8.41 to 7.41) | 0.005 | 0.16 |
| Post- | 81.3 ± 10.5 | 89.6 ± 9.8 | −8.3 (−15.6 to −0.97) | ||
| p-value | 0.265 | 0.001 | |||
| FEV1, pred. | |||||
| Pre- | 70.6 ± 7.4 | 70.8 ± 7.8 | −0.2 (5.7 to 5.3) | ˂0.001 | 0.14 |
| Post- | 74.1 ± 7.2 | 81.5 ± 5.4 | −7.4 (−11.99 to −2.8) | ||
| p-value | 0.172 | ˂0.001 | |||
| PImax, cmH2O | |||||
| Pre- | 39.4 ± 7.8 | 39.8 ± 8.1 | −0.4 (−6.14 to 5.34) | ˂0.001 | 0.15 |
| Post- | 41.2 ± 7.6 | 51.7 ± 6.8 | −10.5 (−15.71 to −5.3) | ||
| p-value | 0.411 | ˂0.001 | |||
| Thoracic excursions, cm | |||||
| Pre- | 6.5 ± 1.3 | 6.7 ± 1.5 | −0.2 (−1.21 to 8.1) | 0.036 | 0.17 |
| Post- | 6.7 ± 1.4 | 7.8 ± 1.2 | −1.1 (−2.04 to −0.16) | ||
| p-value | 0.581 | 0.022 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, A.R.; Abdelbasset, W.K.; Alrawaili, S.M.; Elsayed, A.E.A.; Hajelbashir, M.I.; Kamel, F.H.; Basha, M.A. Effect of Chest Resistance and Expansion Exercises on Respiratory Muscle Strength, Lung Function, and Thoracic Excursion in Children with a Post-Operative Congenital Diaphragmatic Hernia. Int. J. Environ. Res. Public Health 2022, 19, 6101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19106101
Azab AR, Abdelbasset WK, Alrawaili SM, Elsayed AEA, Hajelbashir MI, Kamel FH, Basha MA. Effect of Chest Resistance and Expansion Exercises on Respiratory Muscle Strength, Lung Function, and Thoracic Excursion in Children with a Post-Operative Congenital Diaphragmatic Hernia. International Journal of Environmental Research and Public Health. 2022; 19(10):6101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19106101
Chicago/Turabian StyleAzab, Alshimaa R., Walid Kamal Abdelbasset, Saud M. Alrawaili, Abbas Elbakry A. Elsayed, Mohammed Ibrahim Hajelbashir, FatmaAlzahraa H. Kamel, and Maged A. Basha. 2022. "Effect of Chest Resistance and Expansion Exercises on Respiratory Muscle Strength, Lung Function, and Thoracic Excursion in Children with a Post-Operative Congenital Diaphragmatic Hernia" International Journal of Environmental Research and Public Health 19, no. 10: 6101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19106101