1. Introduction
Geothermal energy is attracting increasing interest among industrial investors, public administrations, and research institutions worldwide thanks to its wide range of development possibilities for heat production and storage and power production, as demonstrated by the many projects carried out globally. In Geneva, the GEothermies program (
www.geothermies.ch, accessed on 9 May 2022) has been running since 2014, intending to implement a portfolio of geothermal projects at different depths with a primary focus on heating and cooling applications. Understanding the circulation paths and the mineral-water interactions controlling the final geochemical composition of the deep geothermal fluid is crucial to identifying drilling targets and designing the appropriate surface installations, gathering, and distribution systems [
1,
2,
3].
The groundwaters in the Geneva basin have been investigated in the past because of the scientific interests in understanding their flow in the different karstic and fractured aquifers and more recently to reconstruct the hydro-geochemical setting of the basin in a geothermal exploration perspective [
4,
5].
Cold springs discharging from karstic aquifers are located on the northern part of the basin, along the Jura Mountains, and springs showing hypo to low thermal features are associated with fault-controlled piston flow systems and are located along the Saleve Ridge in the southern part of the basin [
6]. Additionally, geothermal waters have been identified by the Thonex-01 geothermal exploration deep well, drilled in 1992, and by the GEo-01 wells drilled in 2018, providing direct insights about the geochemical reservoir fluid composition in the Geneva Basin.
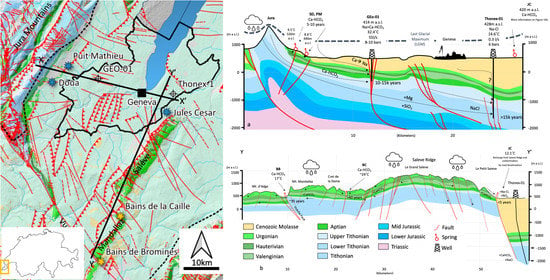
In this study, data from previous studies have been combined with new data collected in 2018 and 2020 from five springs (Source de la Doua SD, Puits Mathieu PM, Jules Cesar JC, Bains de la Bormines BB, and Bains de la Caille BC) and two deep boreholes (GEo-01 and Thonex-01). The goal is to provide constraints and contribute to a better understanding of the geothermal resources circulating in the Upper Mesozoic carbonates, which represent, at present, the main geothermal target for industrial projects (
Figure 1).
SD and PM springs are in the northern sector of the study area along the Jura Mountains foothills. These springs discharge from the lower Cretaceous karstified carbonates in lateral contact with the Quaternary and Oligocene sediments, the latter mainly acting as an aquiclude. In the same sector of the GB, the geothermal well GEo-01 was drilled in 2018 by Services Industriels de Geneve (SIG) in the framework of the ongoing GEothermies geothermal exploration program. The well enters the Mesozoic carbonates at 411 m TVD and reaches a total depth of 744 m (
Figure 2). Several water inflows were observed in the open-hole section while drilling the Mesozoic carbonates for a total flow rate of 55.6 L/s at 10 bars of wellhead pressure and 34 °C wellhead temperature [
4]. In all, 76% of the water flow was observed in the karstified and fractured Lower Cretaceous units and the remaining 24% from the fractured upper Jurassic (
Table 1). Literature data from chemostratigraphic and petrologic analysis on cuttings collected during drilling operations to reconstruct a stratigraphic log [
10,
11] reveal that the Molasse is composed mainly by intercalations of siliciclastic rocks that vary between shales and sandstones. Calcite (41.7% average), Quartz (20.2% average), Illite (9% average), Plagioclase, Dolomite, and Chlorite (5.4%, 4.88%, and 4.47% average respectively) are the main mineralogical phases observed. Calcite is the dominant mineralogical phase in the Lower Cretaceous carbonates (81.23% average), combined with Quartz (10.4% average). In the Upper Jurassic, Calcite (77% average) is partially replaced by Dolomite (18.4%) due to dolomitization processes linked to the diagenetic evolution, and Quartz accounts for a minor part (2.8% average).
Moving towards the SE part of the study, the Thonex-01 well is the first geothermal well drilled in Geneva in 1993 and reaches a TVD of 2530 m. Before entering the Lower Cretaceous carbonates, the well penetrated 1330.7 m of Cenozoic Molasse sediments. Water inflow with a temperature of 70 °C was observed between 2110 and 2136 m TVD and 88 °C bottomhole temperature was recorded at 2530 m TVD (
Figure 2). The well is characterized by the low artesian flow of 0.3–0.5 L/s and wellhead temperature between 15 and 20 °C, indicating conductive cooling during water upflow. Recent borehole seismic surveys aimed at characterizing the Mesozoic reservoir also revealed a high level of seismic noise in the upper section of the well in the Molasse section, most probably associated with bad cementing and casing conditions, potentially draining some water from this unit [
12]. The water discharged from the Thonex-01 well has been studied in the past. Muralt, in his study [
13], combined new data from samplings carried out after drilling and acidification operations aimed at enhancing reservoir permeability [
14]. The study showed that at that time, reservoir water was still strongly contaminated by acidification. Nevertheless, the author was able to constrain the purely meteoric origin of the discharged waters; this catchment area was identified in the Jura Mountains, about 18–20 km towards NW at an elevation of about 1000 m. The water-rock interactions modeling indicated a dominant flow from the Upper Jurassic limestone, giving a Na-Cl composition. Additionally, the same study presented
13C and
87Sr/
86Sr analysis performed on a core sample from the Kimmeridgian (Upper Jurassic) limestone, revealing concertation in
13C of −0.2‰ and a Sr isotopic ratio of 0.70726 (± 0.000014). In 2008, wellbore inspections were performed by SIG, revealing the presence of deposit along the cased section between 900 and 1100 m in depth. Semi-quantitative chemical analysis revealed a dominant composition of iron (40%), Sulfur (20%), and Calcium (10%) interpreted as linked to bacterial activity. An additional temperature log was performed, reporting a maximal temperature of 68 °C at 1724 m TVD. Vuataz and Giroud [
5] carried out a sampling campaign, and the results support Muralt’s hypothesis about the origin and the recharge zone of the discharged waters, which are in thermal equilibrium with the reservoir at 2000–2500 m in depth at an average temperature of about 85 °C. Furthermore, according to Carbon isotopes, these authors estimated a residence time of about 15,000 years. More recently, a study was carried out by BRGM [
15] in the framework of the FP7 IMAGE project, focusing on the development of auxiliary geothermometers, providing further constraints to the estimated reservoir temperature of 70 ± 20 °C.
The Jules Cesar spring is located at NE tip of the Saleve Ridge and discharges from the Quaternary-Molasse sediments. The BC and BB springs, located at the extremities of the Mandallaz mountain, discharge waters under hypo to low thermal conditions. A geochemical study on a set of springs in the GB, including BC and BB, was carried out by Nissile in the 1980s [
6] focusing on major ions, stable isotopes of Oxygen and Hydrogen, and Tritium. Springs at BC showed a temperature up to 24.4 °C with a Ca > Na-HCO
3 composition. The primary fluid flow mechanism controlling these springs has been described as having its origin in the Malm carbonates, which are located at about 200 m b.g.l. and extend down to 1900 m b.g.l. in this area [
15,
16]. Thermal waters naturally flow towards the surface along the main NW-SE trending strike-slip fault system cutting the area. The BB spring is at the SW boundary of Mandallaz along the southern tip of the Vuache fault, the most tectonically active fault structure of the entire Geneva basin [
17,
18,
19], as demonstrated by the M 5.3 earthquake in 1996. The BB spring typically discharges water at 16–17 °C in temperature, and, interestingly, a few days after the 1996 seismic event, the spring reacted with a significant increase of flow rate and temperature, clearly suggesting the strong control of the Vuache fault on the fluid flow of this spring [
20].
2. Geologic and Geothermal Settings of the Geneva Basin
The Geneva Basin (GB) is the westernmost part of the North Alpine Foreland Basin that extends from France’s Savoy region to Linz in Austria [
21]. The GB covers an area of about 2000 km
2 extending from Nyon to the NE, down to Vuache Mountain to the SW; the Jura Haute-Chaine limits it to the NW and the Saleve Ridge on the SE. Four major lithological units are recorded at depth [
21,
22,
23]: the crystalline basement, whose erosive cover shows a 1–3° dip towards S-SE, and whose tectonic depressions are filled with Permo-Carboniferous clastic sediments [
24], represents a potential future deep geothermal target for combined generation of heat, power and metals. Its sedimentary cover is composed of Mesozoic carbonate units spanning from Triassic to Lower Cretaceous. Tertiary and Quaternary sediments [
8,
25,
26] show favorable geothermal potential for heat production and storage at different temperatures (
Figure 1).
Triassic units only crop out locally in the inner Jura chain, and are represented by the German stratigraphic units consisting of the siliciclastic Buntsandstein, dolomitic and evaporitic Muschelkalk, then varying alternations of evaporitic and dolomitic to a marno-dolomitic sequence up to the Rhaetian shales and sandstones, which were identified in hydrocarbon wells in the region [
16,
25,
26]. The Jurassic sequence is mostly dominated by carbonates deposition accumulated in different depositional environments, including shallow to deep platform environments, reef and peri-reefal settings, and tidal systems. Similarly, the lower Cretaceous sequence is also dominated by carbonate sedimentation but marked by an increase in intercalations of siliciclastic material likely associated with the nearby development of a subaerial landscape during the lowermost Cretaceous regional sea-level fall in the Valanginian. The Upper Jurassic and Lower Cretaceous carbonates are broadly exposed on the Jura, Saleve-Mandallaz, and Vuache mountains. A hiatus lasting about 72 million years defines the erosional surface of the Lower Cretaceous that marks the transition towards the overlying Cenozoic sediments. The sub-aerial exposure of the Lower Cretaceous is represented by the Siderolithic (Upper Eocene in age) formation composed by a karstic pocket, heterogeneous in geometry and irregularly distributed across the entire area, and formed in response of intense and extended karstification processes. The karst system, along with compartmentalization of a tectonic origin, plays a dominant role in the hydrogeological drainage of the Cretaceous and Jurassic limestones across the entire basin.
The Cenozoic interval consists only of siliciclastic Lower Freshwater Molasse deposits that comprise alternations of sandstones and marl of Oligocene-early Miocene age with overlain Quaternary glacial and glacio-lacustrine and lacustrine deposits hosting the main freshwater resources of the Geneva area.
The tectonic evolution of the GB is associated with the alpine compressional phase that caused the decoupling of the sedimentary succession from the basement by a detachment surface occurring on the Triassic evaporites [
27,
28,
29,
30]. In response to the alpine compression, the Mesozoic and Cenozoic sedimentary cover of the GB underwent some shortening locally coupled to a rotational motion. This shortening was absorbed through the structuration of the fold and thrust reliefs of the Jura arc mountains during the late Miocene and Early Pliocene [
31], and by the coeval formation of accommodation of strike-slip faults. Recent interpretations of available 2D seismic lines allowed defining the regional structural framework of the area, highlighting the presence of a set of SW-NE oriented thrust fault system and two main strike-slip fault systems, oriented NNW-SSE and WNW-ESE respectively. The first dominates the southern part of the basin and its most evident expression is the Vuache fault. Similar structures cut through the Saleve-Mandallaz mountain ridge, where the BB and BC springs are located. The second is observed in the northern part of the study area on the shores of the Leman Lake and in the Jura Mountains [
16].
The entire Geneva Basin was covered by an ice sheet during the last glacial period around 24,000 years ago, reaching a maximum elevation of about 2000 m in the Jura mountains and 1000–1200 m in the Geneva area [
32], suggesting that the entire area was under about 40–50 bar of hydrostatic pressure during that period that could have enabled the infiltration of waters through the Molasse sediments down to the Upper Mesozoic carbonates.
The geothermal conditions over the Geneva Basin (GB) have been constrained by combining different sets of data from boreholes and exploration wells [
33]. In their study, the authors reconstructed the distribution of the geothermal gradient, which ranges between 25–30 °C/km, but areas of enhanced advective thermal regime could be located where permeable fault corridors allow flow towards the surface of deep groundwater. These results, coupled to analysis of borehole petrophysics and hydraulic data (Moscariello et al., 2020), allowed a sequence of potential targets for geothermal energy to be identified in faulted and karstic reservoirs in the Mesozoic units, which can show high flow rate (as GEo-01 well), temperatures up to 120 °C in the Triassic formations, and up to 140–150 °C in the Permo-Carboniferous troughs at about 4000 m in depth.
3. Materials and Methods
The company Hydroisotop GmbH was assigned by the University of Geneva to perform hydrochemical and isotopic analyses on the water samples collected in 2018 and 2020.
3.1. Water Sampling
The parameters temperature, pH, dissolved oxygen, specific electrical conductivity (EC), and redox value were measured in the field. Being very sensitive, these parameters were measured in flowing water. An adequate measurement was achieved by using a flow-through cell, which constantly renews the water inside and, hence, the water measured while sampling. A 10 L bucket was used as a functional but straightforward flow-through cell. Alkalinity (acid capacity at pH 4.3) and the base capacity at pH 8.2 were also determined in the field. The determination is performed by titration using 0.1-mol HCl for the titration of alkalinity and 0.01-mol NaOH for the titration of the base capacity. The base capacity complies with the dissolved CO2 and the alkalinity-/acid capacity of the amount of HCO3−, assuming that there is no main influence of contained organic acids on the titration.
Before sampling, the two geothermal wells were circulated to discharge the entire volume of water stored in the well. In 2018, Geo-01 was circulated for 72 h before sampling and the Thonex-01 for 193 h; in 2020, the Thonex-01 well was circulated for 487 h. At both wells, H2S odor was perceived, and at Thonex-01, a black deposit started forming at the wellhead a few minutes after it was opened.
3.2. Sample Analysis and Data Interpretation
Measurements of the physico-chemical parameters pH, EC, and redox were done in the field using WTW probes. The determination of pH and EC, and the analysis of the alkalinity and acidity, which were carried out by titration, and were repeated in the laboratory. Major anions and cations were analyzed by ionic chromatography using a Dionex ICS 1500 system. Trace elements and metals were determined using a Thermo Fischer ICP-MS system. The Excel spreadsheet of Powell and Cumming [
34] was used to generate the database combining all data sources. Geochemical facies were determined via Piper [
35] and Shoeller plots [
36] to represent major ion analyses results and the occurrence of mixing processes was constrained by binary plots.
Cl/Br molar ratio was also computed as a useful indicator to define the origin of the salinity of groundwater [
37,
38,
39,
40,
41], focusing on those samples rich in Chloride (such as Jules Cesar Spring) and/or showing Na-Cl facies (Thonex-01 well).
Stable water isotopes of Oxygen δ
18O and Hydrogen δ D were determined by cavity ring-down spectroscopy using a Picarro L 2130-I Analyser. Stable isotopes data from three weather stations (Thonon, Chancy, and Nyon) have been collected from the publicly available IAEA WISER database (
https://nucleus.iaea.org/wiser/index.aspx, accessed on 9 May 2022) to provide a baseline dataset for the data from the study samples and reconstruct the local meteoric water line and constrain the origin of the sampled waters and the elevation of the main catchment area.
Sulfur and Oxygen isotopes were analyzed by Isotope ratio mass spectrometry using ThermoScientific Delta V. The origin and redox reactions of Sulfur (Sulfur cycle) were assessed by the ratio of the stable isotopes δ34S and δ32S. All Sulfur isotope ratios in this paper are given against the Standard Vienna Canyon Diablo Troilit (V-CDT), and 18.4‰ δ18O-SO4, and all oxygen isotope ratios are given against the Standard Vienna Standard Mean Ocean Water.
The Strontium Isotopes ratio
87Sr/
86Sr ratio of groundwater was compared to the Sr isotopic ratio of the Upper Malm (Kimmeridigian Unit) available from literature data [
12,
42] and the δ
18O composition of the sampled waters to constrain water-rock interactions and mixing processes affecting the discharged water chemical composition [
43,
44,
45,
46,
47].
Tritium, Sulfur hexafluoride (SF6), and Carbon isotopes were used to constrain the residence time of the studies waters. Tritium was analyzed after electrolytic enrichment by liquid scintillation counting, using a Perkin Elmer Quantulus 6220 low level liquid counter. Carbon isotopes were analyzed using isotope ratio mass spectrometry, which used DANI HS 86.50 ThermoScientific Delta V.
Reservoir temperature was constrained by a set of geothermometers and by saturation indexes and alkaline geothermometers. The geothermometers of Na-K and Na-K-Ca with Mg correction were evaluated and compared to the other Na-K-Ca equations [
48,
49] as it is demonstrated that the Na-K geothermometer, developed by Fournier [
50] and Giggenbach [
51], highly overestimates the temperature for low enthalpy fluids [
52] and for waters high in Ca.
Saturation indexes were computed using fluid-mineral equilibria equations in PHREEQC code [
53], selecting the most representative samples in terms of temperature and mineralization.
4. Results
4.1. Hydrogeochemical Typing
The pH of the study area varies from 6.4 to 8.2, with an average of 7.3, which indicates the predominant alkaline nature of the groundwater typical of carbonate environments. The EC values vary from 358 to 2500 μS, with an average 963 μS/cm. Higher values up to 10,880 μS are recorded in 1993 for the Thonex-01 well, and are due to the HCl acidification operations carried out in 1994 [
12,
13]. Major cations display a dominance in the order Ca > Mg > Na > K for the waters collected from springs with Calcium concentrations from 1.35 to 5.49 meq/L. Calcium in Thonex-01 shows a decrease in time from up to 170.12 meq/L in 1993 to down to less than 4.5 meq/L measured since 2010 [
4,
5,
14]. Sodium is the dominant cation for Thonex-01 and Geo-01 wells, with concentrations up to 14.4 meq/L and 1.4 meq/L respectively, followed by Ca > Mg > K. Sodium concentration at Thonex-01, which does not display major variations even after 1993’s acidifications. Values of magnesium vary from 0.16 to 2.47 meq/L for all samples except Thonex-01, where values up to 21.81 meq/L were recorded in 1993. Concentration of potassium shows a range from 0.01 to 0.31 meq/L and, as Sodium, did not show any major increase in response to acidification. The full dataset is available in the
supplementary materials.
Anions show abundance in the order of HCO
3 > SO
4 > Cl. Bicarbonate is the dominant cation in groundwater; its concentration varies between 1.86 and 19.6 meq/L and, as Sodium does not show any major fluctuations in response to acidifications in Thonex-01. Sulfate ranges from 0.05 to 1.27 meq/L and Chloride from 0.03 to 11.56 mg/L with a peak up to 189.69 meq/L in 1993 at Thonex-01, and as Calcium, it displays a progressive decrease in concentration with time down to below 19 meq/L. The linear correlation between Cl and Ca can be explained by the dissolution of carbonates due to injection of HCl according to the equation:
which can also explain the relative low pH and high HCO
3 concentration observed in 1994. Moreover, magnesium and Strontium are correlated to Chloride for the 1993 samples, indicating the direct effect of the acidification on the dissolution of those two elements as well.
The analyzed waters can be classified into four main types (
Figure 3 and
Figure 4):
Ca-HCO
3: SD, PM, BB, BC, JC. This fingerprint is typical of a calcareous environment as observed for the Malm thermal waters in the Jura [
54].
Na > Ca-HCO3: GEo-01 well.
Na-Cl: Thonex-01 at present-day conditions/
Ca-Cl: Thonex-01 after 1993 acidification operations.
4.2. Water-Rock Interactions
All sampled waters show predominant geochemical facies compatible with fluid-mineral interactions within the Mesozoic carbonates that compose the main geothermal reservoirs in the Geneva Basin.
An evolution from a pure Ca-HCO
3 composition (SD and PM springs) to Na>Ca-HCO
3 (Geo-01 well), to eventually Na-Cl (Thonex-01 well) facies is observed along the North-South direction. On the SW-NE direction, BB and BC springs show mild thermal conditions and pure Ca-HCO
3 composition (
Figure 5).
The evolution observed on the NW-SE profile is a typical behavior in sedimentary basins, which is also observed in other Molasse Basins regions such as is Bavaria [
55] and in other geologically similar regions [
56,
57,
58]. The dominant process controlling this behavior in sedimentary rock aquifer systems is the activating of ion exchange with increasing residence time, depth, and temperature. Favorable conditions for the occurrence of ion exchange are usually a recharged source of groundwater with elevated Ca concentrations and the presence of minerals with both high cation exchange capacities (CEC) and Na on their exchange sites [
58,
59]. The occurrence of ion exchange in the analyzed waters is confirmed by the relation between Na-Cl and Ca + Mg–HCO
3-SO
4 (
Figure 6A). If ion exchange occurs in the groundwater, the slope of the trend line must be −1, which can be explained by the following reaction [
60]:
The trend line of all the samples shows a slope of −0.8915, and the line from the samples, collected in 2018–2020 under homogeneous sampling and analysis conditions, shows a slope of −0.9638, confirming that Ca2+, Mg2+ and Na+ are interrelated through ion exchange processes.
The identification of these reactions was made by plotting Ca + Mg vs. HCO
3 + SO
4 with a 1:1 mixing line [
60,
61] that defines the charge balance equilibrium between Ca, Mg, SO
4 and HCO
3 due to simple dissolution of calcite and dolomite, which are also the dominant mineralogical phases in the carbonate reservoirs in the GB, and gypsum. In
Figure 6B, some of the samples (SD, PM, BB) plot on the mixing line; JC spring is slightly shifted towards the Ca-HCO
3 domain, whereas Geo-01, Thonex-01, and BC spring plot towards the HCO3-SO4 field indicating a deficiency of (Ca + Mg) relative to (SO
4 + HCO
3). In the case of JC spring, the excess of Ca and Mg can be generated by the clay minerals in the Molasse sediments. One of the mains sources of clay minerals can be silica weathering. The Molasse analyzed in the Geneva basin reveals a relatively rich concentration in silicate minerals, particularly Quartz and Plagioclase. Despite being the dominant component identified, Quartz cannot contribute significantly to the groundwater composition due to its high resistance to weathering. However, Al-silicate minerals weathering, such as the decomposition of plagioclase, can strongly contribute to groundwater composition. It leads to the formation of secondary mineral phases such as clays (illite, montmorillonite, and kaolinite), broadly present in the Molasse sequence (11). In the case of Geo-01, Thonex-01 and BC spring, the excess of negative charge of HCO3 and SO4 must be balanced by Na, the only other major cation, whose progressive enrichment observed from along the NW-SE section can be explained by ion-exchange reactions where calcium in solution is exchanged for sodium. To support this interpretation, sample points below the 1:1 line in Na vs. Cl scatter diagram (
Figure 6C,D) show that most points fall on the Na field rather than Cl, indicating that sodium ion concentration is high in the study area. Similarly, the Ca + Mg vs. Total cations (TC) scatter diagram (
Figure 6E,F) indicates that most of the samples lie above the 1:1 line.
4.3. Fluid-Minerals Equilibria
Fluid-mineral equilibria of the main mineralogical phases were computed with PHREEQC [
53], code choosing the most representative samples in terms of temperature and mineralization.
All the springs are close to equilibrium with Calcite and Aragonite and SD and PM undersaturated in Dolomite and evaporitic minerals, suggesting a circulation in the calcite-rich Lower Cretaceous karstified carbonates. All springs are close to saturation with respect to Quartz and slightly undersaturated with Chalcedony. None of the springs is in equilibrium with the analyzed clay minerals, K-feldspar, Pyrite and Fe-oxides.
GEo-01 water is in equilibrium with Calcite, Anorthite and Dolomite, confirming a dual contribution to the total flow from the calcite-rich limestone Lower Cretaceous and the Dolomite-rich carbonates from the Upper Jurassic. The sample is undersaturated with respect to Gypsum, Anhydrite, and Amorphous Silica, whereas it is close to equilibrium with Quartz and Chalcedony, suggesting a secondary source from the siliciclastic Molasse sediments. GEo-01 waters are not close to saturation with respect to clay minerals, except for Illite, nor with Pyrite and Fe-oxides.
Regarding the Thonex-01 well, the rather heterogeneous analysis results over time made identifying unique mineral-fluid equilibria patterns difficult. Therefore, only the most recent results from 2010 up to 2020 are presented as they can be considered the more representative of natural fluid-flow and reservoir conditions. Saturation Indexes for the Thonex-01 well were computed for the wellhead temperature, and increasing temperature up to the bottomhole temperature of 88 °C was recorded after drilling operations [
13]. The Thonex-01 well is close to saturation with Calcite and Aragonite and slightly oversaturated in Dolomite at wellhead and bottomhole temperatures. It is not close to equilibrium with Gypsum or Anhydrite; it is slightly undersaturated with respect to amorphous Silica and close to saturation with Chalcedony and Quartz. Thonex-01 waters are close to saturation with Kaolinite and K-Feldspar at wellhead temperature, whereas they are far from saturation with the other mineralogical phases.
The results are reported in
Table 2.
To better constrain the evolution with the temperature of the mineral-fluid equilibria, binary plots for the main mineralogical phases are presented in
Figure 7. The results also provide insights into the equilibrium temperature in the reservoirs for the thermal waters. At GEo-01, the equilibrium with Calcite, Aragonite, and Quartz is attained at about 35–40 °C, which is close to wellhead temperature and possibly excludes any inputs from deeper aquifers. At Thonex-01, the equilibrium with Quartz and Chalcedony is attained at about 65–95 °C for the modeled samples, which are oversaturated with respect to Calcite, Aragonite, and Dolomite at the measured reservoir temperature of 88 °C. The BC samples are in equilibrium with Calcite, Aragonite, Dolomite, and Quartz at temperatures between 35–45 °C, which are slightly higher than the measured surface temperature, indicating an upflow from the carbonate reservoir at about 750–1000 m in depth.
4.4. Sulfur Isotopes
The analysis of the Sulfur34 isotope in dissolved Sulfate and H
2S varies significantly between the data available (
Figure 8). In low-temperature geothermal systems, the coexistence of Sulfates and Sulfides in the aqueous solution can be due to several factors, including thermal decomposition of organic Sulfur [
62], dissolution of pyrite, bacterial Sulfate reduction (BSR), and thermochemical Sulfate reduction (TSR) [
62]. Thermal decomposition of organic Sulfur compounds requires reservoir temperatures greater than 200 °C, and it can be excluded for the samples of the Geneva Basin. Pyrite can be dissolved from the Malm limestones containing pyrite, which is known to have δ
34S negative values in limestones [
63], leading to the generation of H
2S depleted in δ
34S values in the range of −10 to −25‰ [
64]. BSR is recognized as one of the essential processes controlling Sulfate partitioning of SO
4 to H
2S or HS
− or S
2− (depending on pH), accompanied by oxidation of organic matter [
65]. The importance of bacterial SO
4 reduction depends upon the rate of supply and reactivity of organic matter and yields a wide range of δ
34S–H
2S values between +20 and −40‰ [
66]. By contrast, the δ
34S-SO
4 values of residual SO
4 and biogenic H
2S are controlled by the kinetic isotopic fractionation factor and by the open or closed nature of the system with respect to SO
4 and H
2S.
At BB δ
34S–SO
4 is 20.1‰, similar to GEo-01, where it is 23‰, whereas ad SD and SJC is it 3.6‰ and 6.6‰, respectively (
Figure 9). The values from BB and GEo-01 are compatible with the dissolution of Tertiary sediments and Malm carbonates (Pearson et al., 1991), whereas the values from SD and SJC are most probably related to oxidation of organic matter. In the Thonex-01 well, it shows a drastic shift towards very positive values, ranging from 22.2‰ in 1993 to 87.5‰ in 2010. Based on the 1993 analysis [
12], the origin of the dissolved Sulfate in the geothermal fluid was explained as due to the dissolution of evaporites from layers other than the Upper Kimmeridgian, as the analysis of the cuttings did not reveal Sulfate minerals. Possible evaporites can be found in the Cenozoic sediments and in the Triassic units. The δ
34S–SO
4 value of 87.5‰ reported in 2010s analysis is abnormally high and shows that samples analyzed in previous studies were strongly disturbed by acid injections of 1993. According to NAGRA data [
67], the highest values of δ
34S–SO
4 (20 to 35‰) would come from the Tertiary formations of the Molasse and Malm levels, which are close to the values from BB, GEo-01 and Thonex-01 (1993 samples), but they are far from the 2010’s value. The isotopic values of Triassic waters show δ
34S–SO
4 values of less than 20‰. A study of geothermal reservoir fluids in the Dogger of the Paris Basin shows δ
34S–SO
4 between 23 and 49‰ [
68]. The higher values observed at Thonex-01 could be related to the combination of dissolved Sulfides in the water, and to bacterial reduction of Sulfate to Sulfides and precipitation of the latter on the well casings. An alternative explanation could be that the Sulfate dissolved in the fluid comes from the oxidation of Sulfides in the rock during the underground journey until all the initial oxygen is consumed. At present, it is unclear at what point in the underground flow this process could occur. Regarding δ
34S–H
2S, dilution, and acidification had caused it to disappear from the 1993 analysis in Thonex-01. However, in 2010, δ
34S–H
2S shows a high value of 52.3‰. Similar values could not be found in the literature about similar geologic settings, which generally give values between 0 and 10‰. Analysis from 2018 reveals values of −40.1‰ for BB, most probably due to bacteria reduction, as cyanobacterial deposits were observed while sampling in 2010, and were also recognized in previous sampling surveys [
6]. BC, GEo-01, and Thonex-01 (2010 and 2020 samples) show values of 14‰, 19.2‰, 52.3 and 23.9‰, respectively, suggesting the formation of secondary Sulfate and oxidation of Sulfides.
Based on the current state of understanding, it is impossible to fully discriminate the origin of the Sulfates without further modeling and sampling. However, under reservoir conditions, all analyzed waters are undersaturated with respect to both anhydrite and gypsum, suggesting the hypothesis that the SO4 contribution from evaporites is clearly subordinated with respect to the SO4 derived from the oxidation of Sulfides minerals.
4.5. Chloride vs. Bromide
Cl/Br ratio observations were also carried out in this study, as it is an effective method to decipher the origin of the salinity in groundwater with focus on the distribution of the Chloride content across the Geneva basin. Assuming Cl
− and Br
− as conservative elements in solution, the molar Cl/Br ratio can be used as a natural tracer, as it is close to 655 for the seawater (mass ratio around 290), is lower for residual brines, and is greater in waters dissolving halite mineral [
37,
38,
39,
40]. Chloride deriving from atmospheric and waste sources [
37,
69] can be neglected compared to the leaching of brines, and the potential dissolution of halite for those deep fluids in the Geneva Basin having Cl->10% of the Total Dissolved Solids (TDS), such as Thonex-01 wells (~32%). With respect to salt domes dissolution, waters can be significantly depleted in bromide as shown in the German Basin, with Cl/Br exceeding 1000 and situated in the typical domain of halite dissolution. In the Paris Basin, where geothermal waters are produced from the mid- to lower Mesozoic carbonates, the Cl/Br ratio allowed identifying brines <655 ratio in the Dogger and Keuper ratios, whereas the Trias ratio is higher [
39]. In the mid-Jurassic case, high salinity waters are related to a primary brine, whereas in the Trias case mixing of brines of primary brine and secondary brine formed by salt dissolution is responsible for the observed salinity. Over the Swiss Molasse plateau, such analysis on the geothermal waters circulating in the sedimentary units has never been performed before. Michard et al. [
70] investigated the chemical evolution of waters circulating in the Hercynian crystalline basement in NE Switzerland and identified the contribution of Permo-Carboniferous sediments dissolution in samples with high Na-Cl concentrations Cl/Br > 655. In the Geneva Basin, detectable Br
− concentrations, representative of reservoir conditions, are available for the GEo-01 (0.03 meq/L) and Thonex-01 (0.02–0.07 meq/L), thanks to the recent sampling campaigns presented in this study, and the Cl/Br molar ratio ranges from 9 at GEo-01 to 145–168 at Thonex-01 (
Figure 10). These results show that the contributions of halite dissolution from Tertiary or Triassic units, as well as the mixing with connate seawater, can be excluded for two main reasons:
Chemostratigraphic analysis on GEo-01 did not reveal the presence of evaporitic lithologies at GEo-01
The presence of the Triassic evaporites below the Thonex-01 well has never been constrained. Furthermore, the Triassic units are separated from the Upper Jurassic carbonates by the Mid and Lower Jurassic carbonate sequence, which might act as an impermeable barrier for fluid upflow from the Triassic towards the Upper Jurassic. Additionally, no major and permeable tectonic accidents are known to cut the Thonex-01 area.
Therefore, the Na-Cl facies in Thonex-01 can be explained by a contribution of residual Chloride from acidification tests and Na-Ca ion exchange processes as described in
Section 4.2.
4.6. Strontium Isotopes as Tracers for Water-Rock Interactions
All sampled waters have
87Sr/
86Sr values above the average values of the Upper Mesozoic rocks, which is 0.707308 (
Figure 11A–C), ranging from 0.707105 and 0.707537.
87Sr/
86Sr in groundwater samples are relatively constant with respect to strontium content (
Figure 11B), being the Thonex-01 wells enriched in Sr but with
87Sr/
86Sr in the lower boundary, like the SD and PM springs. The
87Sr/
86Sr measured on a core sample from the Kimmeridgian unit at 2034–2118 m in Thonex-01 is 0.707276 [
12]. The higher Sr
2+ concentrations corresponding with low
87Sr/
86Sr ratios of the Thonex-01 waters may indicate dissolution of carbonates, most likely from Upper Mesozoic rocks, where the water flow was observed in the well (
Figure 2). The JC, BB, and BC springs have low Sr concentration but higher values in Sr isotopic ratio, whereas GEo-01 well shows an intermediate Sr concentration with high values of
87Sr/
86Sr ratio.
Figure 11C shows the correlation between
87Sr/
86Sr and
18δO in the sampled groundwaters with the cold springs of SD, PM, and JC being richer in
18δO, whereas thermal waters show higher depletion in
18δO. The GEo-01 shows one of the highest
87Sr/
86Sr and is the depleted in
18δO, which could indicate that the sampled waters derive from a mixing between endmembers facing different mineral-water interactions: one endmember purely interacting with the Upper Mesozoic carbonates, and a second endmember enriched in
87Sr. The second endmember can be associated with fluid flow within the Molasse sediments, which are rich in Sr-bearing minerals such as feldspars, notably Plagioclase and K-Feldspar. These minerals compose in average 5.5% and 1.5% respectively of the total entire mineralogical phases identified at GEo-1. Micas including muscovite and biotite represent 2% and 1.5% of the total entire mineralogical phases identified in the Molasse, and clay minerals such as Illite and Chlorite compose 9.5% and 4.5% of the Lower Freshwater Molasse sediments in the GB [
9].
4.7. Reservoir Temperature
One of the significant applications of water geochemistry in exploring the potential geothermal reservoirs involves estimating their temperature condition in the reservoir using chemical and isotopic geothermometers on the sampled fluids. A wide variety of geothermometers such as Na-K, Na-K-Ca, Na-K-Ca-Mg, K-Mg, SiO
2, δ
18O
H2O-SO4 have been developed for all types of geological environments [
1,
51,
71,
72]. However, reservoir temperature estimates are always impacted by chemical or isotopic equilibrium reactions between water and minerals of reservoir, physical conditions in the subsurface, mixing processes between different endmembers, and re-equilibration processes that can occur in fluid flow towards the surface.
In the Geneva Basin, only the Thonex-01 well has been investigated in the past to assess its reservoir temperature. Muralt [
12] used the Silica and δ
18O
H2O-SO4 of dissolved Sulfate geothermometers, resulting in a reservoir temperature of 76 °C and 58 °C respectively, also identifying a partial re-equilibration of the Silica with respect to Chalcedony during the upflow. Vuataz and Giroud [
5] applied the classical geothermometers (Na-K, Na-K-Ca, K-Mg, Chalcedony, and δ
18O
H2O-SO4) to the 2010’s fluid composition and estimated temperatures between 60 and 85 °C, being coherent with the bottomhole measured temperatures and excluding a contribution of fluid from deeper levels. Sanjuan et al. [
14] applied auxiliary geothermometers to the waters sampled in 2016 water, highlighting how the Na-Li and K-F geothermometers give over-estimated temperature values (129 and 127 °C, respectively), and the Na-Cs and K-Mn geothermometers yield under-estimated values (32 °C and 38 °C, respectively). All the other auxiliary geothermometers (Mg-Li, Na-Rb, K-Sr, K-Fe and K-W) give temperature values ranging from 53 to 84 °C, in agreement with the measured temperature and to those estimated using the classical geothermometers in previous studies and concluding that a temperature close to 70 ± 20 °C can be selected.
In this study, we applied classic and auxiliary geothermometers to the new data collected focusing on the BB and BC thermal springs and to the GEo-01, and Thonex-01 well (see full results in the
supplementary materials). Results from BC highlight that the Silica and alkaline, particularly K
2/Mg, geothermometers are suitable for constraining reservoir temperature and are coherent with the results from the saturation indexes (30–40 °C). By contrast, the Na-K-Ca formulation underestimates temperature with values below the measured temperature. For GEo-01 Quartz, Na-K-Ca and K
2/Mg provide reliable results of temperature around 35 °C, in agreement with the results from saturation indexes and the borehole measured temperature. The classical geothermometers (Na-K, Na-K-Ca, K-Mg, chalcedony, Quartz, etc.) applied to the chemical composition of the Thônex-1 deep water indicate a relative concordance of the estimated temperatures between 60 and 85 °C, with the measured temperatures in the borehole and confirm the chemical equilibrium of the fluid with a fractured system at a depth of 2000–2500 m. A temperature close to 75 ± 5 °C can be selected using these geothermometers, which corresponds to the bottomhole measured temperature, suggesting that the geothermal fluid does not quickly come from deeper levels than those known in the borehole, nor it is in equilibrium state with lithologies different than the Malm carbonates.
4.8. Origin of the Groundwaters and Elevation of the Recharge Zone
Stable isotopes of Oxygen δ
18O and Hydrogen δD have been used to constrain the origin of the sampled water, the elevation of the recharge zone, and the climatic condition of the infiltration area. A Local Meteoric Water Line was reconstructed from the interpolation of data available from three weather stations located in Geneva, Thonon, and Nyon cities. The Local Meteoric Water Line (LMWL) resulted from the following equation:
As shown in
Figure 12, all samples plot close to the Global Meteoric Water Line (GMWL) and the LMWL, indicating a pure meteoric origin. However, the boreholes GEO-01 and Thonex-01 reveal a depletion in stable isotopes, suggesting that the discharged water infiltrated in colder climatic conditions compatible with those during the late glacial period between ~16,000 and 11,700 years ago [
73,
74,
75].
Being temperature closely related to the moisture content of the precipitation, the lower the temperature the lower is the capacity of water to hold moisture. Air with low moisture content is expected to be depleted in δD and δ
18O, allowing interpretations on the origin of the fractionation of the stable isotopes in the samples. Fractionation due to temperature conditions is associated with elevations, latitude, and temperature of the recharge area. Within a geothermal system, recharge and discharge are usually close to each other (in the order of max few tens of kilometers). Therefore, effects due to latitude can be excluded. Specifically for the Geneva Basin, the main recharge units are commonly considered to be the Upper Mesozoic carbonates that are exposed in the Jura Mountains, between 900 and 1500 m a.s.l., and along the Saleve Ridge, between 800 and 1300 m a.s.l. The altitude of the recharge zone was constrained by the different relationships between stable isotopes and elevation available in the literature for the Franco-Swiss region (
Figure 13). Knowing that the elevation of the Jura and Saleve areas remained unchanged for the past 20,000 years, the results show that the elevation of the catchment area for the boreholes’ samples is systematically higher than the springs. This supports the hypothesis of an infiltration in colder climatic conditions for the two wells due to the depletion of δD and δ
18O associated with cold climate.
4.9. Groundwater Residence Time
The groundwater residence time was approached through the Tritium and SF6 environmental traces, and the Carbon isotopes. The 3H and SF6 dating methods were applied to constrain residence time for the young groundwater with ages covering the range of recent to ~60 a. 14C was tested for dating thermal waters with an expected residence time of thousands of years.
4.9.1. Tritium
Present-day Tritium content in precipitations in the study area varies between 4 and 15 T.U. according to the data retrieved from the IAEA WISER database for the Thonon weather station (
Figure 14). The data from the samples collected in the Geneva basin reveal that Tritium concentrations from the cold springs SD, PM and JC are coherent with present-day meteoric waters, revealing a residence time of a few years. BC tritium data suggest a residence time of about 30–35 years, and BC spring reveal values lower than the T.U. at the time of sampling. This suggests a residence time larger than 40 years. The shift towards higher values in the 1988 samples suggests the occurrence of mixing with young and rather shallow meteoric waters [
6]. The same can be inferred from the 1993 Thonex-01 samples, which show the effect of the young freshwater used for drilling fluids and the acidification operations on the samples, which contain variable percentages of the reservoir endmember. The effect of acidification decreases in time as shown from the distribution of the samples from 2010 and 2018 and visible in
Figure 5. Thonex-01 and Geo-01 samples from 2018 show Tritium concentration below detection limit, indicating that recharge occurred before 1950s nuclear tests. Based on Tritium data, we can assess that SD PM and JC discharge young water compatible with a residence time of 5–10 years, whereas the thermal springs BB and BC in the southern part of the GB have a longer residence time of about 35 and 50 years respectively.
Tritium data have also been coupled to SF
6 to further provide constraints for the residence time (
Figure 15). In case of the springs PM, SD and JC, the measured
3H and SF
6 consistently indicate young groundwater based on lumped parameter models [
76]. The elevation of the sampling points is approximately identical (about 450 m a.s.l.), the measured temperature while sampling varies between 8.6 to 12.1 °C, and the average elevation of the recharge has been set to 700 m.
The residence time for the cold springs SD, PM, and JC confirm the estimation from Tritium data, for short residence time in the order of 5–10 years. As 3H and SF6 are above the detection limit but below the regional/actual input for the sampling points for BB and BC, they indicate mixing of young and old components. The mixing rate has been quantified in about 35–40% for BB and in less than 20% for BC.
4.9.2. Carbon Isotopes
The absence of Tritium in the groundwater sampled in the two exploration wells, allowed assessing a recharge prior the nuclear test. However, to better assess the age of the groundwaters discharged from the two geothermal wells, we applied carbon isotopes which data are resumed in
Figure 16.
Thermal water sampled in the GEo-01 borehole has a δ
14C concentration of 2.24 pmC and a δ
13C isotope ratio of −4.4‰ V-PDB, suggesting a shorter residence time than for the Thonex-01 well. Muralt [
12] was the first to analyze carbon isotopes of the waters discharged from Thonex-01 and on a rock sample collected at depth (2034–2118 m in the Upper Jurassic Kimmeridgian Fm.). At that time, results clearly showed the contamination from waters used for acidification, as the water showed a concentration of 28 pcm in δ
14C, while the δ
13C is 0.43 pcm, more positive than the value of −0.2 measured for the aquifer rock. After calculating the effect of dilution, Muralt proposed and then rejected a groundwater age of about 13,000 years (during the last glacial period), suggesting the acidification and air-lift activities as the process generating the enrichment of δ
14C. More recently, Vuataz and Giroud [
5] determined a residence time between 10,000 and 15,000 years for Thonex-01 well. δ
13C value changed between 1993 and 2010, from 0.43 to −2.3‰, and it can be observed how the concentration of δ
14C decreased between the first analysis in 1993 and that in 2010. Unlike Tritium, δ
14C is strongly influenced by carbonates dissolved from the Mesozoic host-rocks, which age is much higher than the possible dating range with δ
14C. In 1993, the δ
14C was 28 pcm, whereas in 2010 it was 2.2 pcm. The δ
13C of a geothermal fluid at equilibrium in its reservoir should be close to that of the carbonate rock. In 1993 this was the case, but the sampling conditions during an air-lift test probably created degassing of CO
2 and then partial precipitation of calcite, resulting in a series of isotopic changes that were impossible to quantify. As an effect, the value in δ
13C measured in 2010 has moved further away from that of the rock to become more negative.
New isotopic data about the Kimmeridgian carbonate collected on outcrops in the Jura mountains allowed reconstructing the diagenetic evolution reporting δ
13C values ranging between −4.9 and 2.2 pmC, with negative values associated with de-dolomitization phases [
42]. The results also indicate a rather broad value range associated with the diagenetic process, hence the depositional environment that characterizes the Geneva Basin’s different portions. Based on these new data, the analysis performed on the water samples of Thonex-01 well in 2020 resulted to be similar to the 2010 values with δ
13C = 0.4 pmC and δ
14C = 1.42 pmC.
Based on collected research data of the region and by applying methods for the determination of the initial setup of A° and
14C-DIC model ages according to Eichinger [
77] and DIC development according to Pearson [
78,
79] and Mook [
80,
81,
82,
83], an adapted model representation was developed (
Figure 16). The blue line of development is adapted to the groundwater recharge in carbonate-rich soil/substrate lithologies (δ
13C-DICA° ca. −12‰ VPDB and
14C-DIC
A° 65 pmC). The yellow line takes carbonate-poor soil/substrate lithologies (
13C-DIC
A° ca. −18‰ VPDB und 14C-DIC
A° 75 pmC) into account. Starting from the different A
0 conditions (
13C/
14C-DIC
A°), the underlying model of the curves visualizes the radioactive decay during groundwater aging. Further, it shows the adaption of “open system” with an increase of DIC of about 30 to 50% or 2 mmol/L, respectively and in parallel the isotope exchange with marine carbonate (δ
13C-CaCO
3 ca. 0‰) until a complete alignment of the carbon isotopic composition of the aquifer rock is attained. The results provide new constraints for estimating the residence time for the GEo-01 waters, being between 10–15,000 years, and are coherent with the results from previous studies, suggesting a residence time of more than 15,000 years for the Thonex-01 water.
5. Discussion
Based on the analytical results on the groundwater samples in the Geneva Basin, interpretations have been developed, which have been combined and summarized by two geothermal conceptual fluid flow models.
The first model X-X′ runs on the NW-SE direction from the Jura Mountain to the Saleve ridge and passed by the SD, PM, GEo-1, JC and Thonex-01 samples (
Figure 17a). The main recharge area is identified in the Jura Mountains, where meteoric waters infiltrate through the exposed Upper Mesozoic units as constrained by the stable isotopes of Oxygen and Hydrogen. Here two aquifers control the fluid flow:
The Urgonian Lower Cretaceous carbonates, locally karstified and fractured, allowing a short residence time (<5 years, as constrained by Tritium and SF6 data) at the Jura foothills, as demonstrated by the SD and PM cold springs. Groundwater circulating in this aquifer are characterized by a Ca-HCO3 composition, typical of carbonate environments.
The Upper Malm carbonates are generally tight in terms of permeability but show enhanced conditions where fault corridors, particularly strike-slip faults, cut the Lower Cretaceous and Malm sequence. This is the case of the GEo-1 and the Thonex-02 wells, which discharge from the same Upper Mesozoic units but with opposite productivities due to the heterogeneous permeability distribution across the Geneva Basin. Moreover, the BB and BC springs discharge from major fault structures activating piston flow systems predominantly from deeper levels of the Upper Jurassic carbonates.
Geothermal waters discharged by the two exploration wells enter thermal equilibrium with the host rock by conduction along their circulation path at increasing depth. The residence time varies between 10–15,000 years for GEo-01 and >15,000 for Thonex-01 as constrained by Carbon isotopes. Interestingly, GEo-01 shows geochemical facies that differs from the classic carbonate composition. Ion exchange processes activate with mineralogical phases of the Molasse sediments (Plagioclase and clay minerals in particular), resulting in partial substitution of Ca with Na and an increase in Strontium isotopes ratio due to radioactive Strontium uptake, giving a Na > Ca-HCO3 composition. For the GEo-01 well, the relatively long residence time, compared to the SD and PM springs, suggests that the discharged waters’ source is meteoric waters that infiltrated during the last glacial period, where an ice-sheet cover of about 500–600 m covered the Geneva Basin. This could have contributed to the percolation of waters through the low permeability Molasse sediments before entering the karstified/fractured Lower Cretaceous system which contributed for more than 70% to the total flow rate of the well. Wellhead pressure is about 8–10 bars hydrostatic pressure in equilibrium with the springs located at the Jura foothills. The Thonex-01 wells discharge a Na-Cl water that derives possibly from a combination of subglacial input from the waters slowly circulating in the Molasse sediment, as possible considering the bad cementing and casing conditions along the molasse section observed in the well and storage in the Upper Mesozoic carbonates. Additionally, fault corridors between GEo-01 and Thonex-01 could enable infiltration of Na-rich waters from the Lower Cretaceous to the Upper Jurassic, where the only water inflow evidence have been observed in the well. As residence time depth and temperature increase, mineral-water interactions re-equilibration can occur. The high Chlorine content can still be due to some residual effect of the HCl acidification operations carried out in the 1990 s to enhance the permeability reservoir. The low flow rate and significant difference between bottomhole temperature (88 °C) and wellhead temperature (~15 °C) suggest a slow artesian flow, conductive cooling, and subsequent re-equilibration of the mineral-water equilibria with partial precipitation of calcite. For both wells, there is no clear evidence of inputs from deeper level than the Upper Mesozoic as also constrained by mineral-fluid equilibria and geothermometric calculations.
On the same profile X-X′, the JC cold spring discharges a Ca-HCO3 type of water, suggesting the carbonates exposed along the Saleve ridge as main catchment area. However, the high Strontium isotope ratio suggests a partial contribution of a shallow endmember circulating in the upper section of the Molasse sediments. This is further supported by the high concentration of Na, Cl and NO3, suggesting contamination by anthropic activity, such as the use of salt on the road during the winter season and agricultural activities.
The second model Y-Y′ runs along the Saleve-Mandallaz ridge where the two thermal springs BB and BC are located (
Figure 17b). The two springs discharge a Ca-HCO
3 type of water, with main recharge area localized along the Saleve-Mandallaz Ridge, mainly where the karstified Lower Cretaceous carbonates are exposed, acting as preferential infiltration paths. The discharged waters at BB show hypo-thermal characteristics and is located along the Vuache Fault, the most seismogenic structure of the entire region and known to be associated with deep fluid flows. Residence time is about 35 years, and the sampled water is in equilibrium with the carbonate minerals of the Upper Mesozoic. BC springs discharge water at up to 24 °C and are also associated with the strike-slip fault corridors cutting the Saleve-Mandallaz ridge in the NW-SE direction. Alkaline geothermometers applied to the BC waters indicate an equilibrium temperature of about 35–40 °C, suggesting a piston flow from the fractured Upper Jurassic carbonates, which reach a thickness of more than 1500 m in that region. Residence time at BC is in the order of >40 years, according to Tritium and SF
6 data. The Strontium isotopic signature suggests a potential contribution from the Molasse sediments which surround the Saleve ridge, supporting the hypothesis of mixing between a deep thermal carbonate endmember with a shallow siliciclastic endmember from the Molasse sediments.
6. Conclusions
This paper presents the results of the interpretation of hydrochemical data from a set of sampling campaigns carried out in the past 30 years on different springs and geothermal exploration wells in the Geneva Basin. This study focuses on the analyses of major ions, trace elements, stable isotopes of Oxygen and Hydrogen, Tritium, SF6, Carbon, Sulfur, and Strontium isotopes. Analytical data have been combined to constrain the main element of the fluid flow from a geothermal perspective. These include the recharge areas, the origin of the sampled groundwaters, the mineral-water interactions and the mixing processes occurring at depth, and the residence time.
As results, some interpretations are proposed which need to be challenged, validated, or discharged by future studies, including new data acquisition and modelling. Nevertheless, we arrived at several practical interpretations that are useful for the fluid flow characterization of low to medium enthalpy geothermal resources in the Geneva Basin. The groundwaters show a geochemical evolution in the NW-SE direction, where cold springs located in the Jura mountains foothills, on the NW side of the Geneva Basin, discharge from the Lower Cretaceous karstic carbonates giving the typical Ca-HCO3 signature. Their circulation path is relatively short and rapid, with the main catchment area of meteoric waters in the Jura mountains. Moving towards SW, the GEo-01 well discharges geothermal waters having a Na > Ca-HCO3 signature, indicating that the discharged waters the catchment area are not limited to the Jura Mountains, but a partial recharge from waters from the Molasse sediments might occur as suggested by the identified ion exchange processes. This is further constrained by the stable isotopes of Oxygen and Hydrogen, which reveal that recharge occurred in colder climatic conditions, coherent with the estimated residence time of about 10–15,000 years. On the SE side of the GB, the Thonex-01 wells discharges Na-Cl water that derives from mixing between an endmember circulating in the Molasse sediments and a second one flowing in the Upper Jurassic carbonates. The low flow rate and significant difference between bottomhole temperature (88 °C) and wellhead temperature (~15 °C) is linked to conductive cooling, and subsequent re-equilibration of the mineral-water equilibria with partial precipitation of calcite. Residence time is estimated to be >15,000 years. In the same area the JC cold spring discharges a Ca-HCO3 type of water, with a main catchment area along the Saleve ridge and strong contamination by anthropic activities as suggested by the high concentration of Na, Cl, and NO3. On the Southern part of the Basin, two springs showing mild thermal features have been studied. BB and BC discharge a Ca-HCO3 type of water, with the main recharge area along the Saleve-Mandallaz Ridge where the karstified Lower Cretaceous carbonates are exposed. Residence time is about 35 years for BB, and water is in equilibrium with the carbonate minerals of the Upper Mesozoic. BC springs discharge water up to 24 °C with a reservoir temperature close 35–40 °C, suggesting a piston flow from the fractured Upper Jurassic carbonates. Residence time at BC is in the order of >40 year.
In this study, the results of a comprehensive hydrochemical analysis on groundwaters over the entire Geneva basin are presented. The new data collected in 2018–2020 proved new insights about the major role of the karstified and fractured Lower Cretaceous carbonates, and of fault structures in the Upper Jurassic in controlling deep fluid flow in the GB. Additionally, the identified ion exchange processes reveal new potential scenarios to constrain recharge processes and fluid flow mechanisms for the geothermal waters discharged from the two deep geothermal wells. This study provides valuable constraints to understand the temporal and spatial evolution of geothermal resources in the GB to support the development of geothermal projects for heat production and storage from deep reservoirs in Molasse sediments and Upper Mesozoic carbonates.