In Vitro Evaluation of Experimental Self-Adhesive Orthodontic Composites Used to Bond Ceramic Brackets
Abstract
:1. Introduction
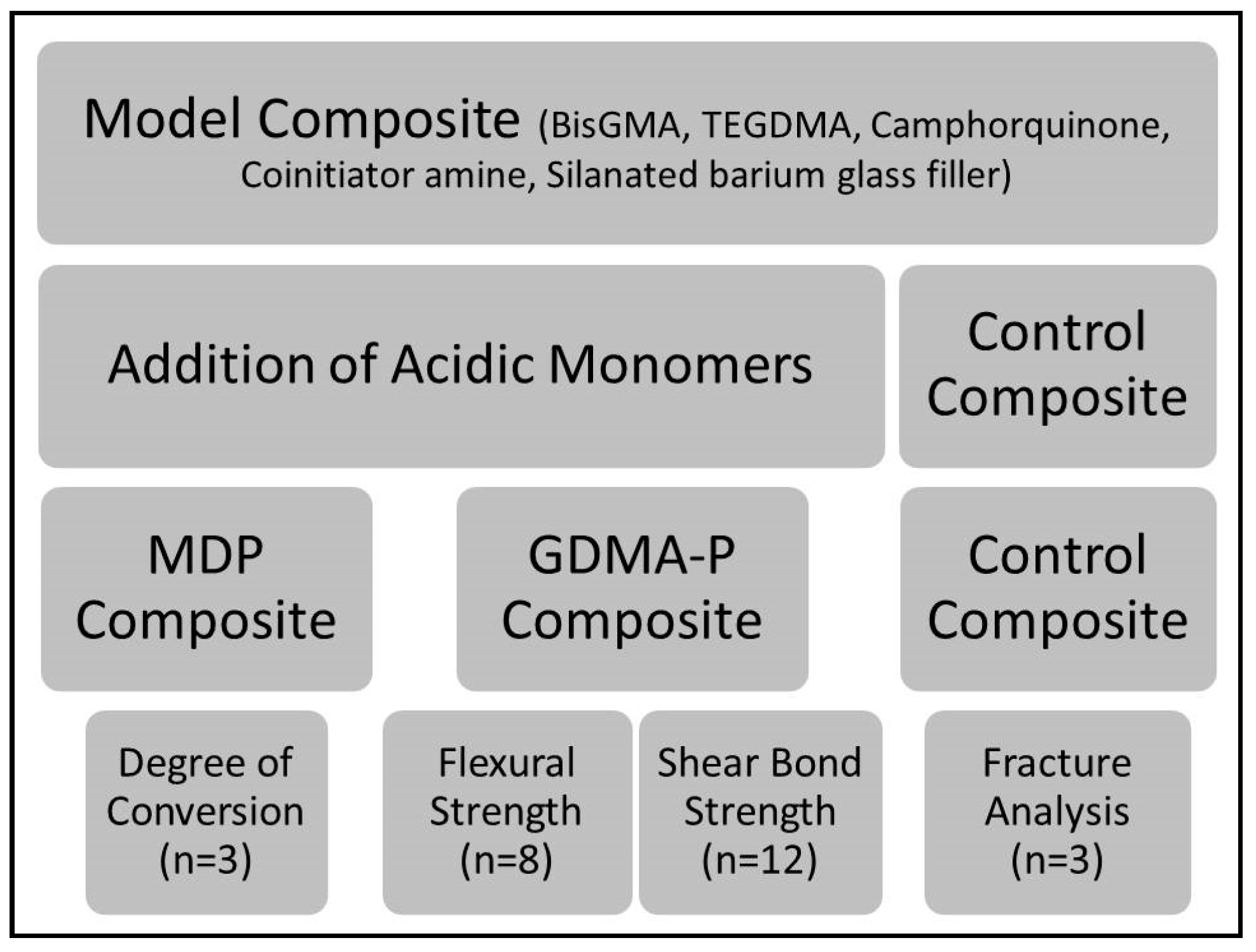
2. Materials and Methods
2.1. Formulation of Experimental Composites
2.2. Degree of Conversion
2.3. Three-Point Bending Test
2.4. Shear Bond Strength (SBS)
2.4.1. Selection and Preparation of Samples
2.4.2. Shear Bond Strength Test
2.5. Clinical Fracture Pattern
2.6. Statistical Analysis
3. Results
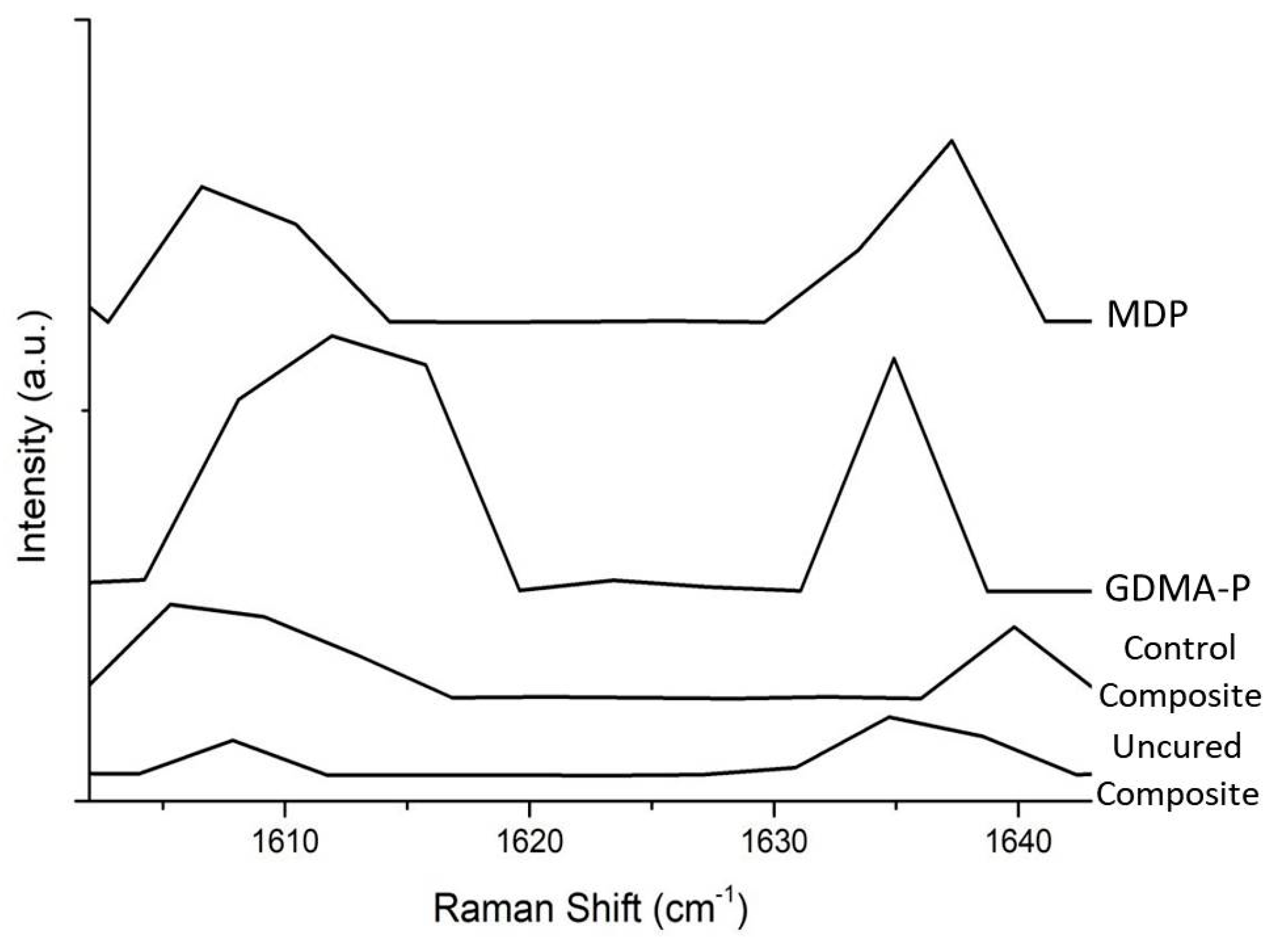
3.1. Degree of Conversion
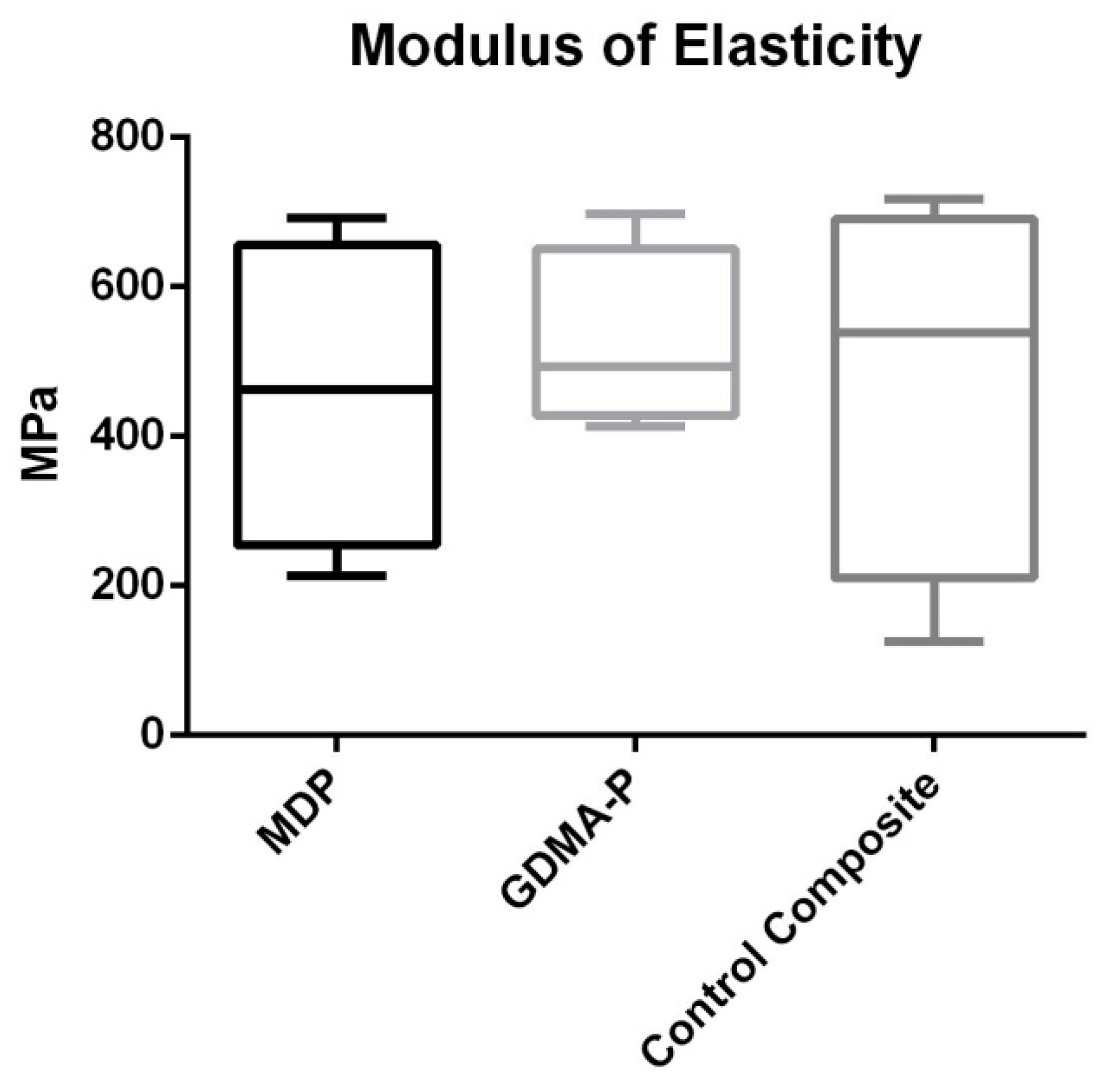
3.2. Three-Point Bending Test
3.3. Shear Bond Strength Test
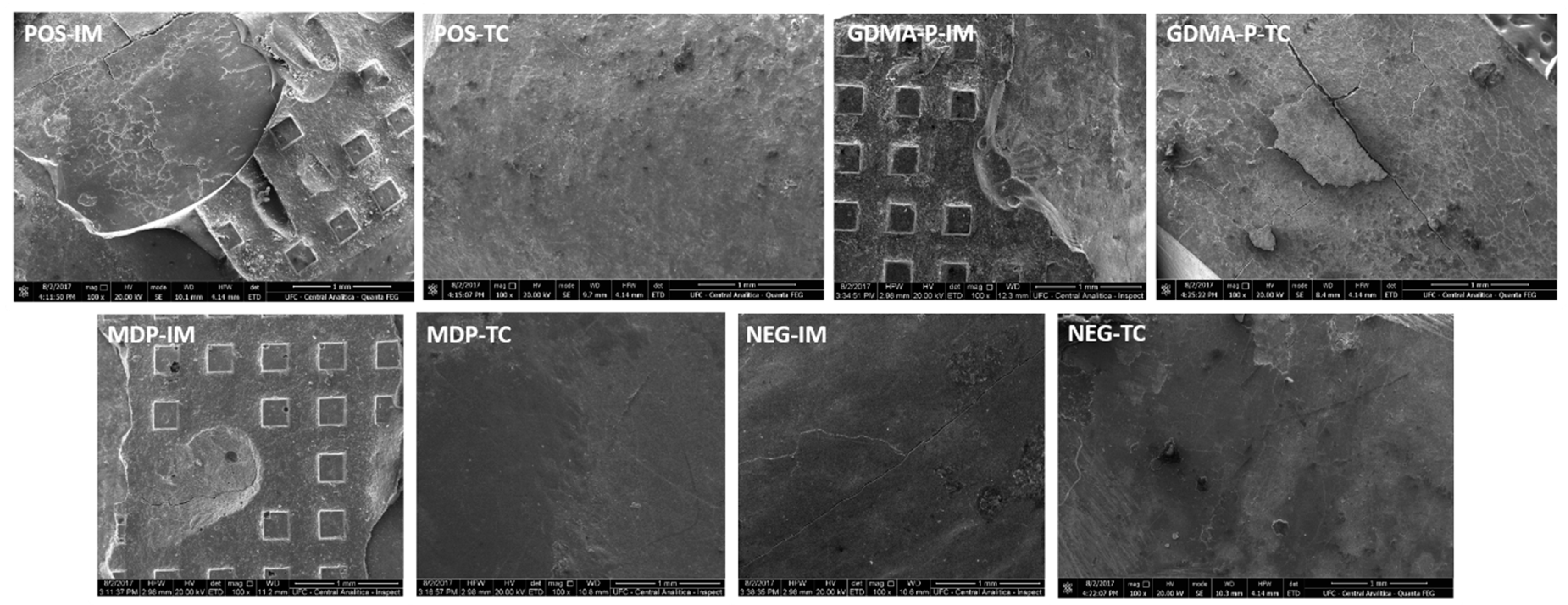
3.4. Clinical Fracture Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajagopal, R.; Padmanabhan, S.; Gnanamani, J. A comparison of shear bond strength and debonding characteristics of conventional, moisture-insensitive, and self-etching primers in vitro. Angle Orthod. 2004, 74, 264–268. [Google Scholar] [PubMed]
- Bishara, S.E.; Oonsombat, C.; Soliman, M.M.; Warren, J.J.; Laffoon, J.F.; Ajlouni, R. Comparison of bonding time and shear bond strength between a conventional and a new integrated bonding system. Angle Orthod. 2005, 75, 237–242. [Google Scholar] [PubMed]
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1995, 34, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Rensch, J.A. Direct cementation of orthodontic attachments. Am. J. Orthod. Dentofac. Orthop. 1973, 63, 156–160. [Google Scholar] [CrossRef]
- Olsen, M.E.; Bishara, S.E.; Boyer, D.B.; Jakobsen, J.R. Effect of varying etching times on the bond strength of ceramic brackets. Am. J. Orthod. Dentofac. Orthop. 1996, 109, 403–409. [Google Scholar] [CrossRef]
- Russell, J.S. Aesthetic orthodontic brackets. J. Orthod. 2005, 32, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.P.; Kommi, P.B.; Kumar, M.S. Evaluating the Type of Light Transmittance in Mono Crystalline, Poly Crystalline and Sapphire Brackets-An Invitro Spectrofluorometer Study. J. Clin. Diagn. Res. 2016, 10, ZC18. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, J. Problems associated with ceramic brackets suggest limiting use to selected teeth. Angle Orthod. 1922, 62, 145–152. [Google Scholar]
- Crooks, M.; Hood, J.; Harkness, M. Thermal debonding of ceramic brackets: An in vitro study. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 163–172. [Google Scholar] [CrossRef]
- Al-Saleh, M.; El-Mowafy, O. Bond strength of orthodontic brackets with new self-adhesive resin cements. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.; Segner, D. Shear bond strength of metal brackets compared with a new ceramic bracket. Am. J. Orthod. Dentofac. Orthop. 1988, 94, 201–206. [Google Scholar] [CrossRef]
- Gwinnett, A.J. A comparison of shear bond strength of metal and ceramic brackets. Am. J. Orthod. Dentofac. Orthop. 1988, 93, 346–348. [Google Scholar] [CrossRef]
- Goracci, C.; Margvelashvili, M.; Giovannetti, A.; Vichi, A.; Ferrari, M. Shear bond strength of orthodontic brackets bonded with a new self-adhering flowable composite. Clin. Oral Investig. 2013, 17, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Neto, V.G.; Nobre, C.F.A.; De Paula, D.M.; Souza, L.C.; Silva, J.C.; Moreira, M.M.; Picanço, P.R.B.; Feitosa, V.P. Glycerol-dimethacrylate as alternative hydrophilic monomer for HEMA replacement in simplified adhesives. J. Mech. Behav. Biomed. Mater. 2018, 82, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Yoshihara, K.; Yoshida, Y.; Okihara, T.; Yamamoto, T.; Momoi, Y.; Van Meerbeek, B. Interference of functional monomers with polymerization efficiency of adhesives. Eur. J. Oral Sci. 2016, 124, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zope, A.; Zope-Khalekar, Y.; Chitko, S.S.; Kerudi, V.V.; Patil, H.A.; Bonde, P.V.; Dolas, S.G. Comparison of Self-Etch Primers with Conventional Acid Etching System on Orthodontic Brackets. J. Clin. Diagn. Res. 2016, 10, ZC19–ZC22. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Stansbury, J.W.; Carpenter, G.H.; Watson, T.F.; Sinhoreti, M.A.; Correr, A.B. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 2014, 42, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracane, J.L.; Stansbury, J.W.; Burke, F.J.T. Self-adhesive composite cements–chemistry, properties and clinical considerations. J. Oral Rehabil. 2011, 38, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.R. A review of direct bonding. Br. J. Orthod. 1975, 2, 171–180. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Yoshida, Y.; Hirata, I.; Snauwaert, J.; De Munck, J.; Okazaki, M.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Influence of the chemical structure of functional monomers on their adhesive performance. J. Dent. Res. 2008, 87, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Munck, J.D.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Adhesion to Enamel and Dentin: Current Status and Future Challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, H.M.; Alves, T.S.; Bresciani, E.; Niu, L.N.; Tay, F.R.; Pucci, C.R. Can long-term dentine bonding created in real life be forecasted by parameters established in the laboratory? Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; Yoshida, Y.; Van Meerbeek, B. Chemical interaction of glycero-phosphate dimethacrylate (GPDM) with hydroxyapatite and dentin. Dent. Mater. 2018, 34, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Zecin-Deren, A.; Sokolowsky, J.; Szczesio-Wlodarczyk, A.; Piwonski, I.; Lukomska-Szymanska, M.; Lapinska, B. Multi-layer application of self-etch and universal adhesives and the effect on dentin bond strength. Molecules 2019, 24, 345. [Google Scholar] [CrossRef] [PubMed]
- Fujita Nakajima, K.; Nikaido, T.; Arita, A.; Hirayama, S.; Nishiyama, N. Demineralization capacity of commercial 10-methacryloyloxydecyl dihydrogen phosphate-based all-in-one adhesive. Dent. Mater. 2018, 34, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
| Groups | Procedure Description | |
|---|---|---|
| Positive control immediate (POS-IM) | Positive control after thermocycling (POS-TC) | Enamel acid-etching + adhesive application + control composite insertion |
| Negative control immediate (NEG-IM) | Negative control after thermocycling (NEG-TC) | Control composite insertion |
| MDP immediate (MDP-IM) | MDP after thermocycling (MDP-TC) | Insertion of composite with MDP acidic monomer |
| GDMA-P immediate (GDMA-P-IM) | GDMA-P after thermocycling (GDMA-P-TC) | Insertion of composite with GDMA-P acidic monomer |
| Groups | Degree of Conversion (%) |
|---|---|
| Control composite | 84.6 ± 4.3% a |
| GDMA-P | 58.9 ± 4.0% b |
| MDP | 23.0 ± 5.2% c |
| Group | Immediate | After Thermocycling | Fracture Pattern | ||
|---|---|---|---|---|---|
| Mixed | Cohesive | Adhesive | |||
| Positive | 8.47 ± 0.88 MPa aA | 1.12 ± 0.52 Mpa aB | 7/12 | 3/12 | 2/12 |
| GDMA-P | 6.17 ± 1.46 Mpa bA | 1.34 ± 1.04 Mpa aB | 4/12 | - | 8/12 |
| MDP | 7.07 ± 2.69 Mpa abA | 1.45 ± 0.60 Mpa aB | 6/12 | 4/12 | 2/12 |
| Negative | 2.37 ± 1.81 Mpa cA | 0.11 ± 0.27 Mpa aB | 1/12 | - | 11/12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.C.; Sabóia, V.; Marçal, F.; Sena, N.; De Paula, D.; Ribeiro, T.; Feitosa, V. In Vitro Evaluation of Experimental Self-Adhesive Orthodontic Composites Used to Bond Ceramic Brackets. Materials 2019, 12, 419. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12030419
Costa AC, Sabóia V, Marçal F, Sena N, De Paula D, Ribeiro T, Feitosa V. In Vitro Evaluation of Experimental Self-Adhesive Orthodontic Composites Used to Bond Ceramic Brackets. Materials. 2019; 12(3):419. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12030419
Chicago/Turabian StyleCosta, Ana Carolina, Vicente Sabóia, Felipe Marçal, Nara Sena, Diego De Paula, Thyciana Ribeiro, and Victor Feitosa. 2019. "In Vitro Evaluation of Experimental Self-Adhesive Orthodontic Composites Used to Bond Ceramic Brackets" Materials 12, no. 3: 419. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12030419




