Elephant Endotheliotropic Herpesvirus Is Omnipresent in Elephants in European Zoos and an Asian Elephant Range Country
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression of Recombinant EEHV Proteins
2.2. Sera
2.3. ELISAs
3. Results
3.1. Production and Purification of Recombinant EEHV1A gB, gH/gL and gL
3.2. Development of EEHV-Specific ELISAs Using Purified EEHV1A gB, gH/gL, and gL Proteins
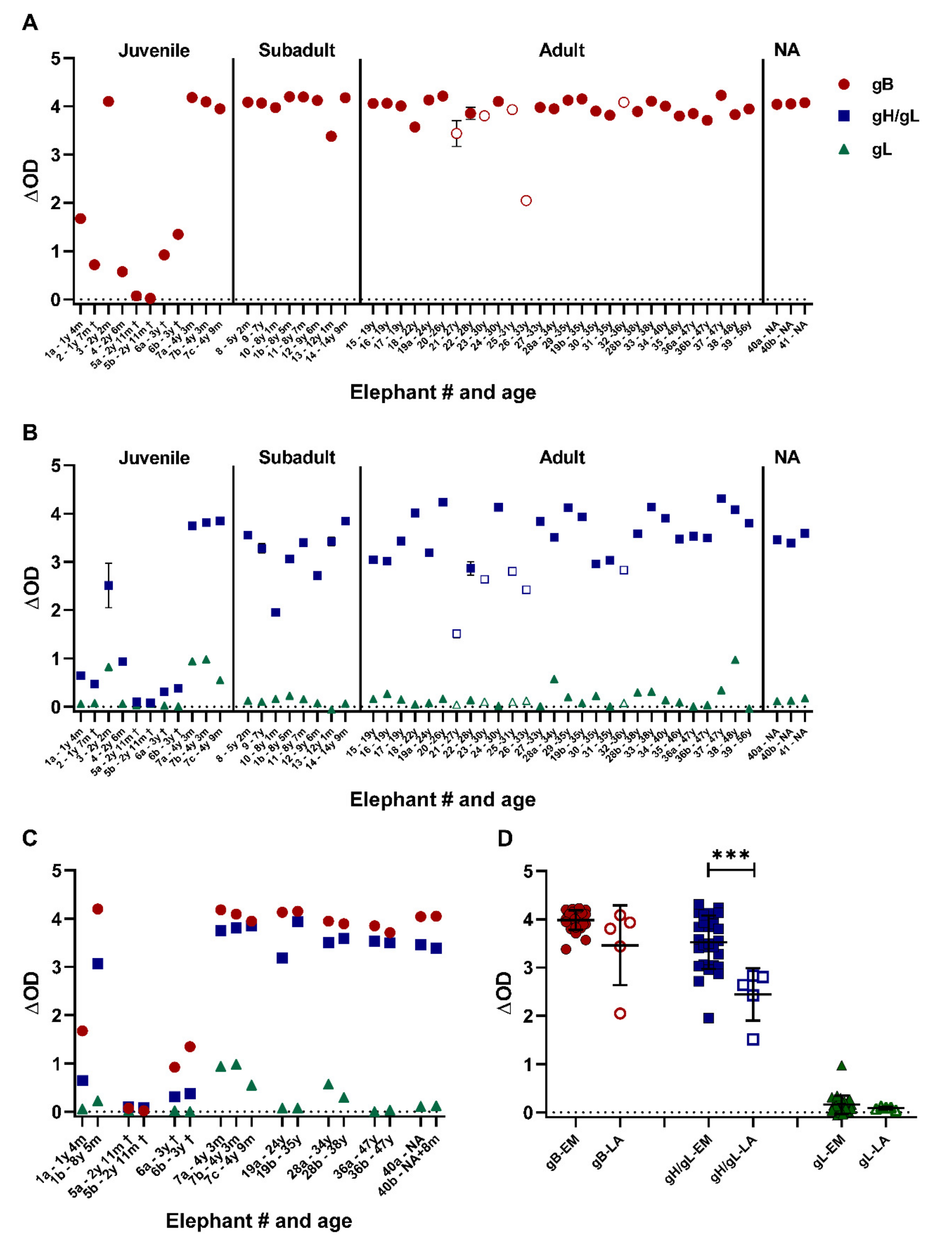
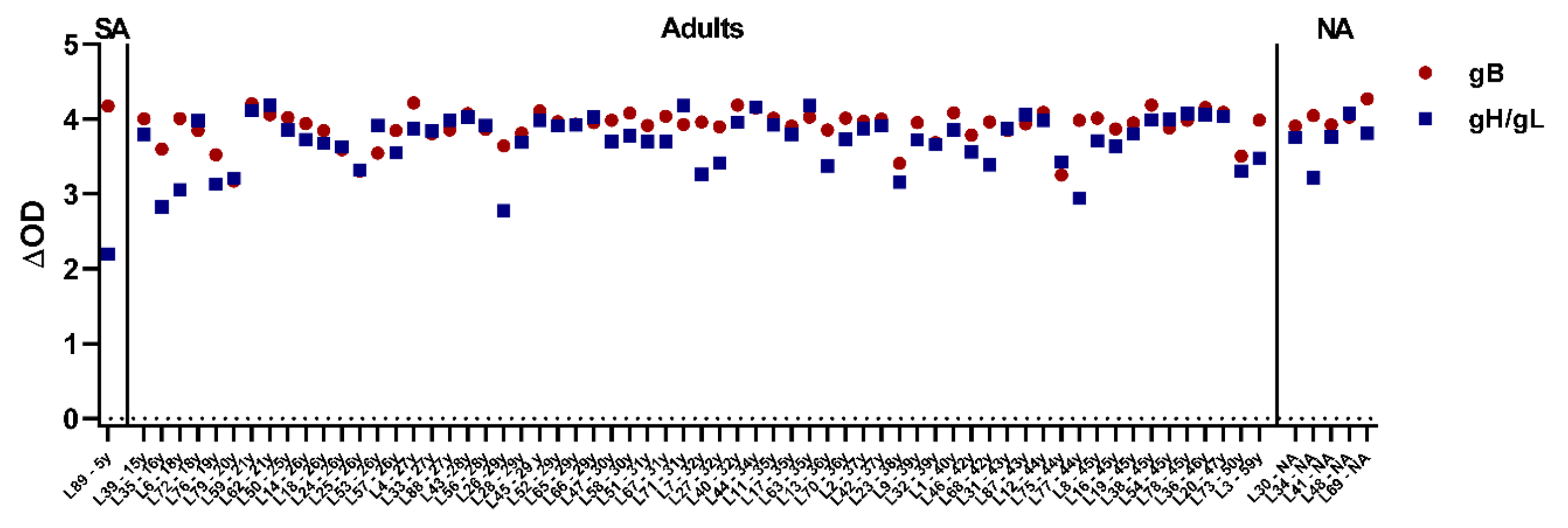
3.3. Close to All Tested Zoo Elephants Are EEHV Seropositive
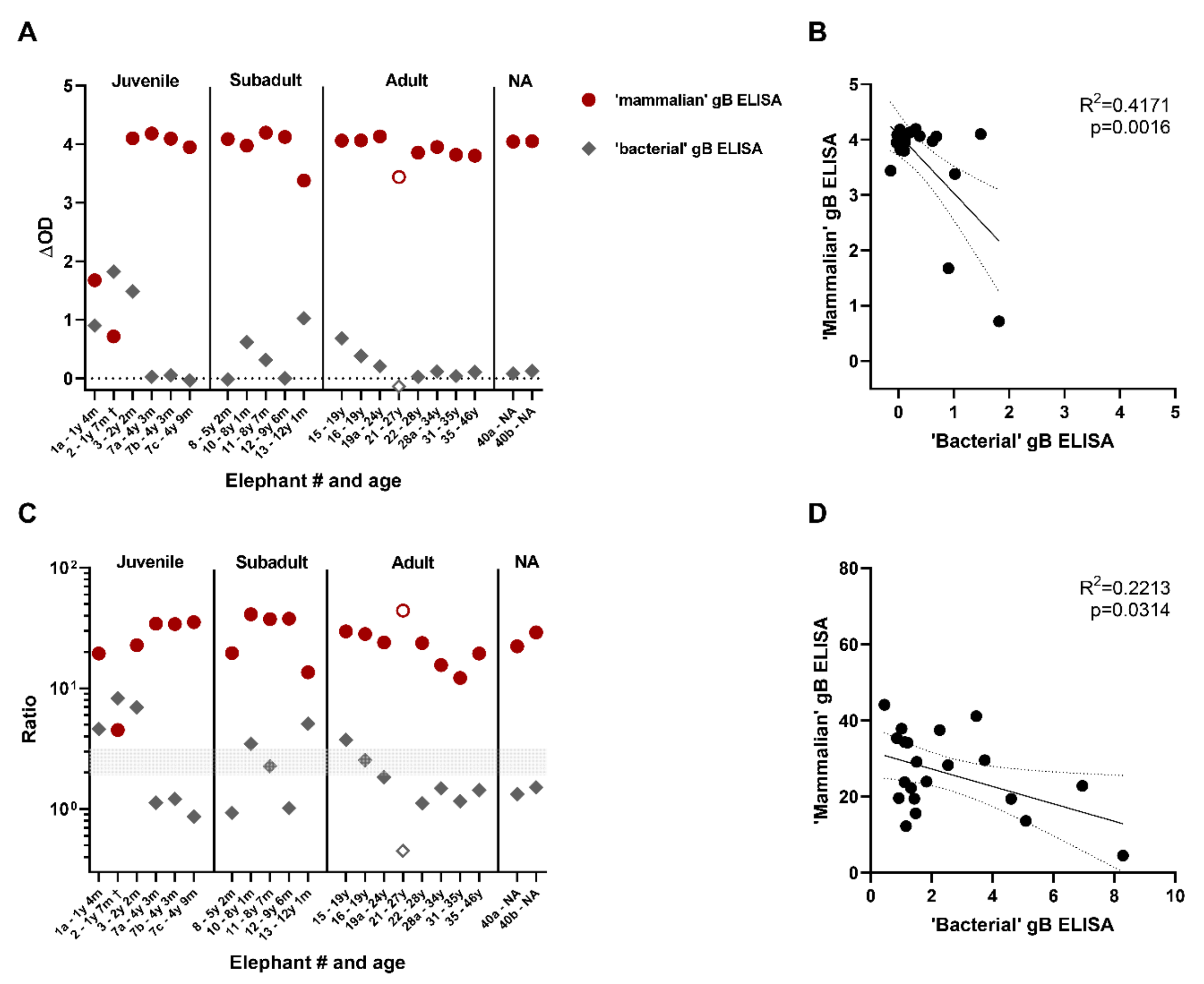
3.4. Comparison of the Novel gB ELISA to the Previously Published EEHV gB ELISA
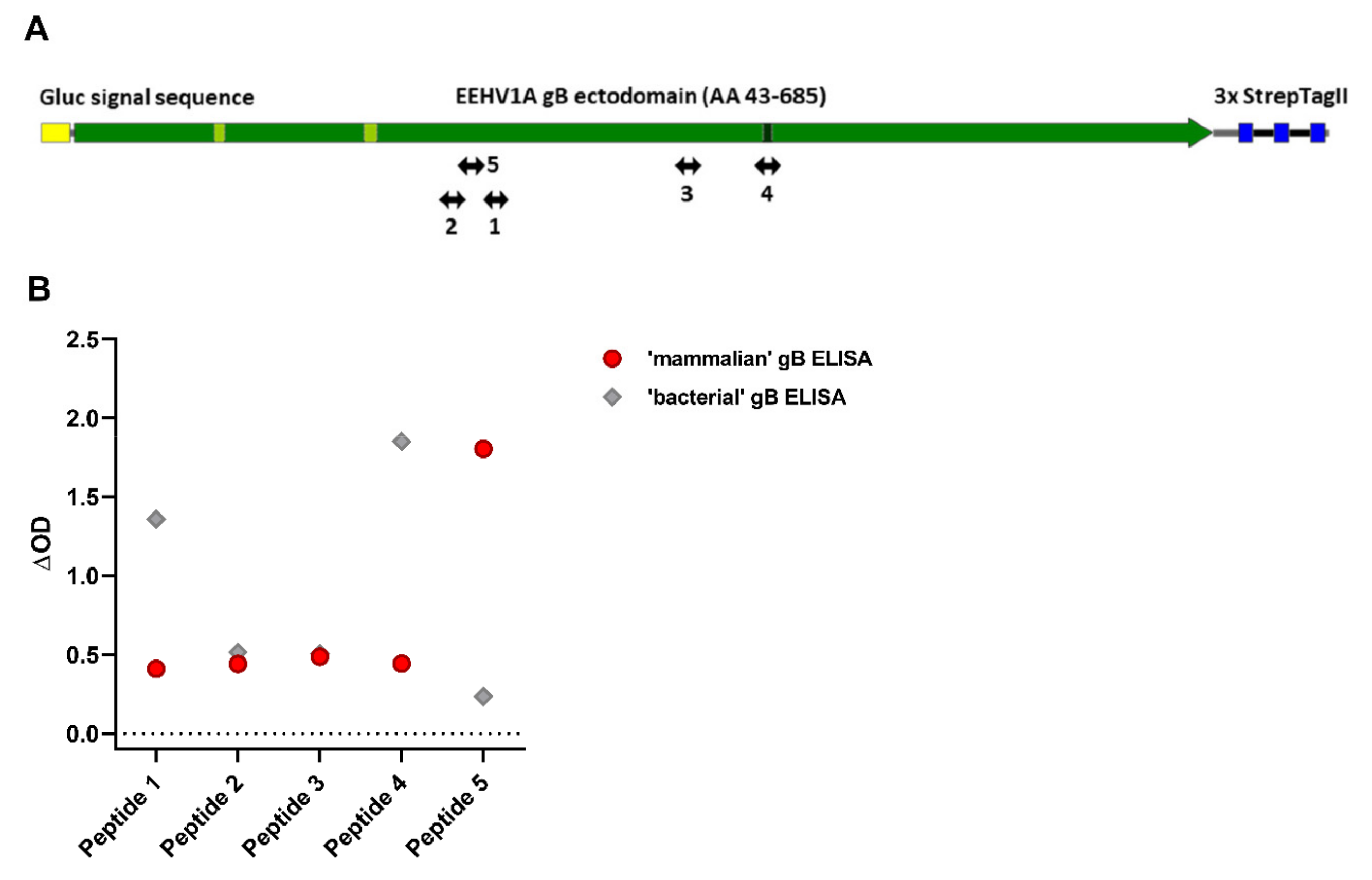
3.5. Antisera Generated against Linear gB Epitopes Suggest Altered Epitope Availability in gB Produced in Bacterial versus Mammalian Cells
3.6. All Tested Laotian Elephants under Human Care Are Seropositive for EEHV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, L.; Schaftenaar, W. Elephant Endotheliotropic Herpesvirus. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Elsevier: Saunders, PA, USA, 2018; Volume 9. [Google Scholar]
- Jeffrey, A.; Evans, T.S.; Molter, C.; Howard, L.L.; Ling, P.; Goldstein, T.; Gilardi, K. Noninvasive sampling for detection of elephant endotheliotropic herpesvirus and genomic DNA in Asian (Elephas maximus) and African (Loxodonta africana) elephants. J. Zoo Wildl. Med. 2020, 51, 433–437. [Google Scholar] [CrossRef]
- Long, S.Y.; Latimer, E.M.; Hayward, G.S. Review of Elephant Endotheliotropic Herpesviruses and Acute Hemorrhagic Disease. ILAR J. 2016, 56, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonprasert, K.; Punyapornwithaya, V.; Tankaew, P.; Angkawanish, T.; Sriphiboon, S.; Titharam, C.; Brown, J.L.; Somgird, C. Survival analysis of confirmed elephant endotheliotropic herpes virus cases in Thailand from 2006–2018. PLoS ONE 2019, 14, e0219288. [Google Scholar] [CrossRef] [PubMed]
- Mahato, G.; Sarma, K.K.; Pathak, D.C.; Barman, N.N.; Gogoi, P.; Dutta, M.; Basumatary, P. Endotheliotropic herpesvirus infection in Asian elephants (Elephas maximus) of Assam, India. Vet. World 2019, 12, 1790–1796. [Google Scholar] [CrossRef] [Green Version]
- Oo, Z.M.; Aung, Y.H.; Aung, T.T.; San, N.; Tun, Z.M.; Hayward, G.S.; Zachariah, A. Elephant Endotheliotropic Herpesvirus Hemorrhagic Disease in Asian Elephant Calves in Logging Camps, Myanmar. Emerg. Infect. Dis. 2020, 26, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardman, K.; Dastjerdi, A.; Gurrala, R.; Routh, A.; Banks, M.; Steinbach, F.; Bouts, T. Detection of elephant endotheliotropic herpesvirus type 1 in asymptomatic elephants using TaqMan real-time PCR. Vet. Rec. 2012, 170, 205. [Google Scholar] [CrossRef]
- Fuery, A.; Pursell, T.; Tan, J.; Peng, R.; Burbelo, P.D.; Hayward, G.S.; Ling, P.D. Lethal Hemorrhagic Disease and Clinical Illness Associated with Elephant Endotheliotropic Herpesvirus 1 Are Caused by Primary Infection: Implications for the Detection of Diagnostic Proteins. J. Virol. 2020, 94, e01528-19. [Google Scholar] [CrossRef] [Green Version]
- Sripiboon, S.; Ditcham, W.; Vaughan-Higgins, R.; Jackson, B.; Robertson, I.; Thitaram, C.; Angkawanish, T.; Phatthanakunanan, S.; Lertwatcharasarakul, P.; Warren, K. Subclinical infection of captive Asian elephants (Elephas maximus) in Thailand with elephant endotheliotropic herpesvirus. Arch. Virol. 2020, 165, 397–401. [Google Scholar] [CrossRef]
- AsERSM. Asian elephant range states meeting (AsERSM) final report, 2017. In Proceedings of the AsERSM, Jakarta, Indonesia, 18–20 April 2017. [Google Scholar]
- Wald, A.; Corey, L. Persistence in the population: Epidemiology, transmission. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- van den Doel, P.B.; Prieto, V.R.; van Rossum-Fikkert, S.E.; Schaftenaar, W.; Latimer, E.; Howard, L.; Chapman, S.; Masters, N.; Osterhaus, A.D.; Ling, P.D.; et al. A novel antigen capture ELISA for the specific detection of IgG antibodies to elephant endotheliotropic herpes virus. BMC Vet. Res. 2015, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- Angkawanish, T.; Nielen, M.; Vernooij, H.; Brown, J.L.; Van Kooten, P.J.S.; Doel, P.B.V.D.; Schaftenaar, W.; Na Lampang, K.; Rutten, V.P.M.G. Evidence of high EEHV antibody seroprevalence and spatial variation among captive Asian elephants (Elephas maximus) in Thailand. Virol. J. 2019, 16, 33. [Google Scholar] [CrossRef]
- Heldwein, E.E.; Lou, H.; Bender, F.C.; Cohen, G.H.; Eisenberg, R.J.; Harrison, S.C. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 2006, 313, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, R.J.; Atanasiu, D.; Cairns, T.M.; Gallagher, J.R.; Krummenacher, C.; Cohen, G.H. Herpes Virus Fusion and Entry: A Story with Many Characters. Viruses 2012, 4, 800–832. [Google Scholar] [CrossRef]
- Cooper, R.S.; Heldwein, E.E. Herpesvirus gB: A Finely Tuned Fusion Machine. Viruses 2015, 7, 6552–6569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, T.M.; Huang, Z.-Y.; Whitbeck, J.C.; De Leon, M.P.; Lou, H.; Wald, A.; Krummenacher, C.; Eisenberg, R.J.; Cohen, G.H. Dissection of the Antibody Response against Herpes Simplex Virus Glycoproteins in Naturally Infected Humans. J. Virol. 2014, 88, 12612–12622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoppel, K.; Kropff, B.; Schmidt, C.; Vornhagen, R.; Mach, M. The humoral immune response against human cytomegalovirus is characterized by a delayed synthesis of glycoprotein-specific antibodies. J. Infect. Dis. 1997, 175, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Klein, M.; Britt, W.J.; Hassfurther, E.; Mach, M. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J. Gen. Virol. 1996, 77 Pt 7, 1537–1547. [Google Scholar] [CrossRef]
- Bu, W.; Joyce, M.G.; Nguyen, H.; Banh, D.V.; Aguilar, F.; Tariq, Z.; Yap, M.L.; Tsujimura, Y.; Gillespie, R.A.; Tsybovsky, Y.; et al. Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells. Immunity 2019, 50, 1305–1316.e6. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.P.; de Vries, E.; Bosch, B.J.; de Groot, R.J.; Rottier, P.J.; de Haan, C.A. The influenza A virus hemagglutinin glycosylation state affects receptor-binding specificity. Virology 2010, 403, 17–25. [Google Scholar] [CrossRef] [Green Version]
- de Vries, R.P.; de Vries, E.; Moore, K.S.; Rigter, A.; Rottier, P.J.; de Haan, C.A. Only two residues are responsible for the dramatic difference in receptor binding between swine and new pandemic H1 hemagglutinin. J. Biol. Chem. 2011, 286, 5868–5875. [Google Scholar] [CrossRef] [Green Version]
- Lassausaie, J.; Bret, A.; Bouapao, X.; Chanthavong, V.; Castonguay-Vanier, J.; Quet, F.; Mikota, S.K.; Théorêt, C.; Buisson, Y.; Bouchard, B. Tuberculosis in Laos, who is at risk: The mahouts or their elephants? Epidemiology Infect. 2014, 143, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, C.; Sukumar, R. Constructing age structures of Asian elephant populations: A comparison of two field methods of age estimation. Gajah 2008, 29, 11–16. [Google Scholar]
- Humphreys, A.F.; Tan, J.; Peng, R.; Benton, S.M.; Qin, X.; Worley, K.C.; Mikulski, R.L.; Chow, D.C.; Palzkill, T.G.; Ling, P.D. Generation and characterization of antibodies against Asian elephant (Elephas maximus) IgG, IgM, and IgA. PLoS ONE 2015, 10, e0116318. [Google Scholar] [CrossRef]
- Paungpin, W.; Wiriyarat, W.; Chaichoun, K.; Tiyanun, E.; Sangkachai, N.; Changsom, D.; Poltep, K.; Ratanakorn, P.; Puthavathana, P. Serosurveillance for pandemic influenza A (H1N1) 2009 virus infection in domestic elephants, Thailand. PLoS ONE 2017, 12, e0186962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, L.; Browne, H.; Wargent, V.; Davis-Poynter, N.; Primorac, S.; Goldsmith, K.; Minson, A.C.; Johnson, D.C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 1992, 66, 2240–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Ponce-de-Leon, M.; Jiang, H.; Dubin, G.; Lubinski, J.M.; Eisenberg, R.J.; Cohen, G.H. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 1998, 72, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Klyachkin, Y.M.; Stoops, K.D.; Geraghty, R.J. Herpes simplex virus type 1 glycoprotein L mutants that fail to promote trafficking of glycoprotein H and fail to function in fusion can induce binding of glycoprotein L-dependent anti-glycoprotein H antibodies. J. Gen. Virol. 2006, 87, 759–767. [Google Scholar] [CrossRef]
- Gardner, T.J.; Hernandez, R.E.; Noriega, V.M.; Tortorella, D. Human cytomegalovirus gH stability and trafficking are regulated by ER-associated degradation and transmembrane architecture. Sci. Rep. 2016, 6, 23692. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784-3788(2003). Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Jung, E.; Brunak, S. NetNGlyc 1.0 Server—Prediction of N-glycosylation Sites in Human Proteins. Available online: http://www.cbs.dtudk/services/NetNGlyc/.
- Ciferri, C.; Chandramouli, S.; Donnarumma, D.; Nikitin, P.A.; Cianfrocco, M.A.; Gerrein, R.; Feire, A.L.; Barnett, S.W.; Lilja, A.E.; Rappuoli, R.; et al. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc. Natl. Acad. Sci. USA 2015, 112, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Ng, D.T.W. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 2015, 16, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Kochagul, V.; Srivorakul, S.; Boonsri, K.; Somgird, C.; Sthitmatee, N.; Thitaram, C.; Pringproa, K. Production of antibody against elephant endotheliotropic herpesvirus (EEHV) unveils tissue tropisms and routes of viral transmission in EEHV-infected Asian elephants. Sci. Rep. 2018, 8, 4675. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Kok, E.; Adolfsson, R.; Lövheim, H.; Elgh, F. Herpes virus seroepidemiology in the adult Swedish population. Immun. Ageing 2017, 14, 10. [Google Scholar] [CrossRef]
- Ackermann, M.; Hatt, J.-M.; Schetle, N.; Steinmetz, H. Identification of shedders of elephant endotheliotropic herpesviruses among Asian elephants (Elephas maximus) in Switzerland. PLoS ONE 2017, 12, e0176891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachariah, A.; Sajesh, P.K.; Santhosh, S.; Bathrachalam, C.; Megha, M.; Pandiyan, J.; Jishnu, M.; Kobragade, R.S.; Long, S.Y.; Zong, J.-C.; et al. Extended genotypic evaluation and comparison of twenty-two cases of lethal EEHV1 hemorrhagic disease in wild and captive Asian elephants in India. PLoS ONE 2018, 13, e0202438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lundkvist, Å.; Vapalahti, O.; Henttonen, H.; Vaheri, A.; Plyusnin, A. Hantavirus infections among mammalogists studied by focus reduction neutralisation test. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 802–803. [Google Scholar] [CrossRef] [PubMed]
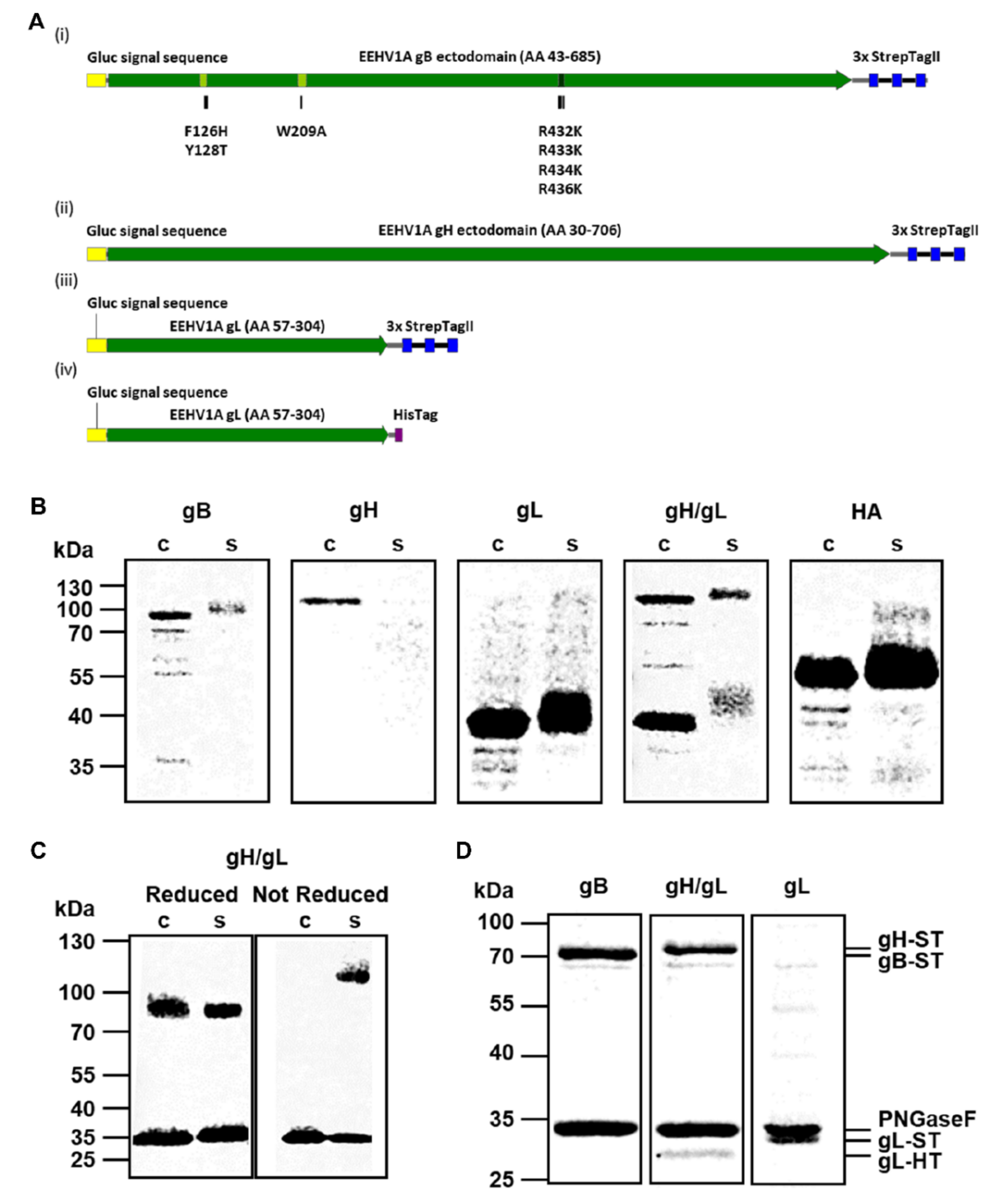

| Construct | Predicted MW (kDa) a | Predicted N-Linked Glycosylation Sites b | Predicted MW, N-Linked Glycosylation Included (kDa) c |
|---|---|---|---|
| gB-ST | 80.4 | 6 | 95.4 |
| gH-ST | 84.6 | 8 | 104.6 |
| gL-ST | 34.8 | 4 | 44.8 |
| gL-HT | 29.9 | 4 | 39.9 |
| gH-ST/gL-ST | 119.4 | 8/4 | 149.4 |
| gH-ST/gL-HT | 114.5 | 8/4 | 144.5 |
| IAV HA-ST | 42.5 | 4 | 52.5 |
| Subtype | Associated Elephant Species | EEHV1A gB (AA 43-685) | EEHV1A gH (AA 30-706) | EEHV1A gL (AA 57-304) | |||
|---|---|---|---|---|---|---|---|
| AA Identity (Min–Max) a | n | AA Identity (Min–Max) a | n | AA Identity (Min–Max) a | n | ||
| EEHV1A | EM | 97.52–98.91% | 19 | 78.29–100.00% | 14 | 94.35–100.00% | 19 |
| EEHV6 | LA | 88.84–88.99% | 2 | 84.07% | 1 | 77.96–79.18% | 2 |
| EEHV1B | EM | 85.71–85.96% | 5 | 67.6–67.89% | 2 | 71.02% | 3 |
| EEHV2 | LA | 81.71% | 1 | 59.74% | 1 | 58.06% | 2 |
| EEHV5 | EM | 81.24–81.86% | 4 | 58.69% | 1 | 57.66–58.06% | 3 |
| EEHV4 | EM | 67.03% | 1 | 46.04–46.27% | 2 | 37.34% | 1 |
| EEHV3 | LA | NA | 0 | NA | 0 | NA | 0 |
| EEHV7 | LA | NA | 0 | NA | 0 | NA | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoornweg, T.E.; Schaftenaar, W.; Maurer, G.; van den Doel, P.B.; Molenaar, F.M.; Chamouard-Galante, A.; Vercammen, F.; Rutten, V.P.M.G.; de Haan, C.A.M. Elephant Endotheliotropic Herpesvirus Is Omnipresent in Elephants in European Zoos and an Asian Elephant Range Country. Viruses 2021, 13, 283. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020283
Hoornweg TE, Schaftenaar W, Maurer G, van den Doel PB, Molenaar FM, Chamouard-Galante A, Vercammen F, Rutten VPMG, de Haan CAM. Elephant Endotheliotropic Herpesvirus Is Omnipresent in Elephants in European Zoos and an Asian Elephant Range Country. Viruses. 2021; 13(2):283. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020283
Chicago/Turabian StyleHoornweg, Tabitha E., Willem Schaftenaar, Gilles Maurer, Petra B. van den Doel, Fieke M. Molenaar, Alexandre Chamouard-Galante, Francis Vercammen, Victor P. M. G. Rutten, and Cornelis A. M. de Haan. 2021. "Elephant Endotheliotropic Herpesvirus Is Omnipresent in Elephants in European Zoos and an Asian Elephant Range Country" Viruses 13, no. 2: 283. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020283






