Cytomegalovirus Infection and Inflammation in Developing Brain
Abstract
:1. Introduction
1.1. Congenital HCMV Infection
1.2. Mouse Model of Congenital HCMV Infection
2. CMV Infection of Brain-Resident Cells
2.1. Cytomegalovirus Tropism
2.2. Cytomegalovirus Infection of Neurons and Glial Cells
2.3. Cytomegalovirus Latency in Brain
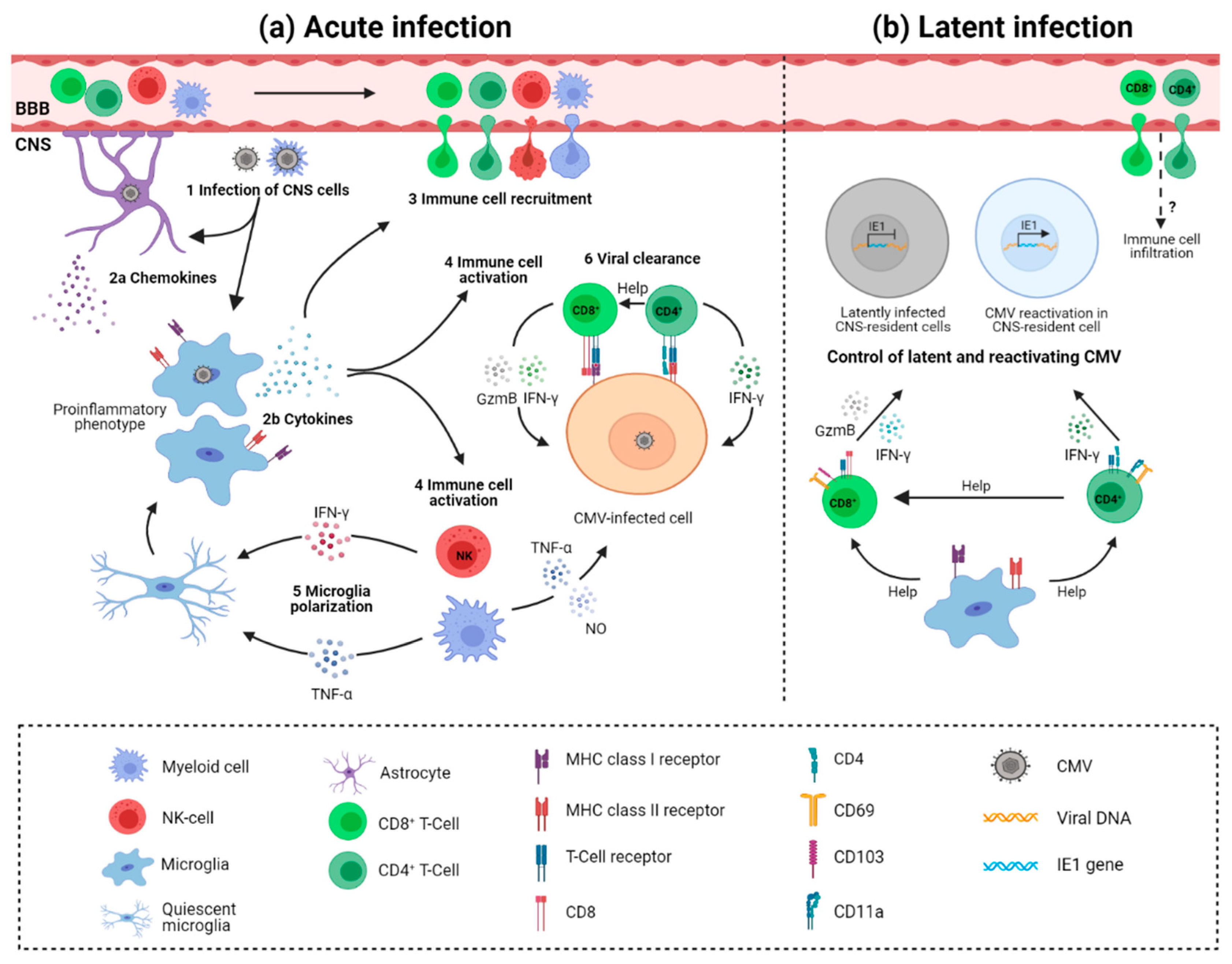
3. Immune Response to Cytomegalovirus Infection in Developing Brain
Pathogenesis of Congenital CMV Infection in the Brain
4. Closing Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cannon, M.J.; Grosse, S.D.; Fowler, K.B. The Epidemiology and Public Health Impact of Congenital Cytomegalovirus Infec-tion. In Cytomegaloviruses from Molecular Pathogenesis to Intervention; Caister Academic Press: London, UK, 2013; pp. 26–43. [Google Scholar]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Boppana, S.B.; Britt, W.J. Synopsis of Clinical Aspects of Human Cytomegalovirus Disease. In Cytomegaloviruses from Molec-ular Pathogenesis to Intervention; Caister Academic Press: London, UK, 2013; pp. 1–26. [Google Scholar]
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, M.E. Severe Cytomegalovirus Infection in Apparently Immunocompetent Patients: A Systematic Review. Virol. J. 2008, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Schleiss, M.R. Cytomegalovirus Vaccine Development and Target Population Congenital HCMV Infection: A Major Public Health Problem The Problem of Congenital HCMV Infection Is Unquestionably the Major Driving. In Current Topics in Microbiology and Immunology; Springer Press: Heidelberg/Berlin, Germany, 2008; pp. 361–382. [Google Scholar]
- Adler, S.P.; Nigro, G. Human Cytomegalovirus. In Cytomegaloviruses from Molecular Pathogenesis to Intervention; Caister Academic Press: London, UK, 2013; pp. 55–73. [Google Scholar]
- Cannon, M.J. Congenital cytomegalovirus (CMV) epidemiology and awareness. J. Clin. Virol. 2009, 46, S6–S10. [Google Scholar] [CrossRef]
- Cannon, M.J.; Hyde, T.B.; Schmid, D.S. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med Virol. 2011, 21, 240–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britt, W.J. Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection. Viruses 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Boppana, S.B.; Fowler, K.B.; Pass, R.F.; Rivera, L.B.; Bradford, R.; Lakeman, F.D.; Britt, W.J. Congenital Cytomegalovirus Infection: Association between Virus Burden in Infancy and Hearing Loss. J. Pediatr. 2005, 146, 817–823. [Google Scholar] [CrossRef]
- Britt, W. Manifestations of Human Cytomegalovirus Infection: Proposed Mechanisms of Acute and Chronic Disease. In Current Topics in Microbiology and Immunology; Springer Press: Heidelberg/Berlin, Germany, 2008; pp. 417–470. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef] [PubMed]
- Zavattoni, M.; Rustico, M.; Tassis, B.; Lombardi, G.; Furione, M.; Piralla, A.; Baldanti, F. Risk of congenital disease in 46 infected fetuses according to gestational age of primary human cytomegalovirus infection in the mother. J. Med. Virol. 2015, 88, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, E.; Somech, R. Maturation of the immune system in the fetus and the implications for congenital CMV. Best Pr. Res. Clin. Obstet. Gynaecol. 2019, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Bialek, S.R.; Boppana, S.B.; Griffiths, P.D.; Laughlin, C.A.; Ljungman, P.; Mocarski, E.S.; Pass, R.F.; Read, J.S.; Schleiss, M.R.; et al. Priorities for CMV vaccine development. Vaccine. 2013, 32, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, G.; Adler, S.P.; La Torre, R.; Best, A.M. Passive Immunization during Pregnancy for Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2005, 353, 1350–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A Randomized Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef] [Green Version]
- Brizić, I.; Lisnić, B.; Brune, W.; Hengel, H.; Jonjić, S. Cytomegalovirus Infection: Mouse Model. Curr. Protoc. Immunol. 2018, 122, e51. [Google Scholar] [CrossRef]
- Schleiss, M.R.; McVoy, M.A. Guinea pig cytomegalovirus: A model for the prevention and treatment of maternal–fetal cytomegalovirus transmission. Futur. Virol. 2010, 5, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Y.; Barry, P.A. Chapter 5 Rhesus Cytomegalovirus. A Nonhuman Primate Model for the Study of Human Cytomegalovirus. In Advances in Virus Research; Springer press: Cham, Switzerland, 2008; pp. 207–226. [Google Scholar]
- Powers, C.; Früh, K. Rhesus CMV: An emerging animal model for human CMV. Med. Microbiol. Immunol. 2008, 197, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleiss, M.R.; Lacayo, J.C.; Belkaid, Y.; McGregor, A.; Stroup, G.; Rayner, J.; Alterson, K.; Chulay, J.D.; Smith, J.F. Preconceptual Administration of an Alphavirus Replicon UL83 (pp65 Homolog) Vaccine Induces Humoral and Cellular Immunity and Improves Pregnancy Outcome in the Guinea Pig Model of Congenital Cytomegalovirus Infection. J. Infect. Dis. 2007, 195, 789–798. [Google Scholar] [CrossRef]
- Griffith, B.P.; Lucia, H.L.; Hsiung, G.D. Brain and Visceral Involvement during Congenital Cytomegalovirus Infection of Guinea Pigs. Pediatr. Res. 1982, 16, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Loh, H.-S.; Mohd-Lila, M.-A.; Abdul-Rahman, S.-O.; Kiew, L.-J. Pathogenesis and vertical transmission of a transplacental rat cytomegalovirus. Virol. J. 2006, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Reddehase, M.J.; Lemmermann, N.A.W. Mouse Model of Cytomegalovirus Disease and Immunotherapy in the Immunocompromised Host: Predictions for Medical Translation that Survived the “Test of Time”. Viruses 2018, 10, 693. [Google Scholar] [CrossRef] [Green Version]
- Koontz, T.; Bralic, M.; Tomac, J.; Pernjak-Pugel, E.; Bantug, G.; Jonjic, S.; Britt, W.J. Altered development of the brain after focal herpesvirus infection of the central nervous system. J. Exp. Med. 2008, 205, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, I.; Kawasaki, H.; Arai, Y.; Tsutsui, Y. Innate Immune Responses to Cytomegalovirus Infection in the Developing Mouse Brain and Their Evasion by Virus-Infected Neurons. Am. J. Pathol. 2002, 161, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.A.; Paveglio, S.A.; Lingenheld, E.G.; Zhu, L.; Lefrançois, L.; Puddington, L. Transmission of Murine Cytomegalovirus in Breast Milk: A Model of Natural Infection in Neonates. J. Virol. 2011, 85, 5115–5124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiwata, M.; Baba, S.; Kawashima, M.; Kosugi, I.; Kawasaki, H.; Kaneta, M.; Tsuchida, T.; Kozuma, S.; Tsutsui, Y. Differential expression of the immediate-early 2 and 3 proteins in developing mouse brains infected with murine cytomegalovirus. Arch. Virol. 2006, 151, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kosugi, I.; Han, G.-P.; Kawasaki, H.; Arai, Y.; Takeshita, T.; Tsutsui, Y. Induction of cytomegalovirus-infected labyrinthitis in newborn mice by lipopolysaccharide: A model for hearing loss in congenital CMV infection. Lab. Investig. 2008, 88, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J.; Cekinović, D.; Jonjić, S. Cytomegaloviruses from Molecular Pathogenesis to Intervention; Caister Academic Press: London, UK, 2013; pp. 119–141. [Google Scholar]
- Treuting, P.M.; Dintzis, S.M. Comparative Anatomy and Histology: A Mouse and Human Atlas; Elsevier Press: Waltham, MA, USA, 2012. [Google Scholar]
- Clancy, B.; Kersh, B.; Hyde, J.; Darlington, R.B.; Anand, K.J.S.; Finlay, B.L. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 2007, 5, 79–94. [Google Scholar] [CrossRef]
- Ornaghi, S.; Hsieh, L.S.; Bordey, A.; Vergani, P.; Paidas, M.J.; van den Pol, A.N. Valnoctamide Inhibits Cytomegalovirus Infection in Developing Brain and Attenuates Neurobehavioral Dysfunctions and Brain Abnormalities. J. Neurosci. 2017, 37, 6877–6893. [Google Scholar] [CrossRef] [Green Version]
- Sung, C.Y.W.; Seleme, M.C.; Payne, S.; Jonjic, S.; Hirose, K.; Britt, W. Virus-induced cochlear inflammation in newborn mice alters auditory function. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef]
- Bradford, R.; Yoo, Y.-G.; Golemac, M.; Pugel, E.P.; Jonjic, S.; Britt, W.J. Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons. PLoS Pathog. 2015, 11, e1004774. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, N.A.; Papadimitriou, J.M.; Shellam, G.R. Cytomegalovirus-Induced Pneumonitis and Myocarditis in Newborn Mice—A Model for Perinatal Human Cytomegalovirus Infection. Arch. Virol. 1990, 115, 75–88. [Google Scholar] [CrossRef]
- Shellam, G.R.; Flexman, J.P. Genetically determined resistance to murine cytomegalovirus and herpes simplex virus in newborn mice. J. Virol. 1986, 58, 152–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bantug, G.R.B.; Cekinovic, D.; Bradford, R.; Koontz, T.; Jonjic, S.; Britt, W.J. CD8+T Lymphocytes Control Murine Cytomegalovirus Replication in the Central Nervous System of Newborn Animals. J. Immunol. 2008, 181, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Brizić, I.; Šušak, B.; Arapović, M.; Huszthy, P.C.; Hiršl, L.; Kveštak, D.; Juranić Lisnić, V.; Golemac, M.; Pernjak Pugel, E.; Tomac, J.; et al. Brain-Resident Memory CD8+ T Cells Induced by Congenital CMV Infection Prevent Brain Pathology and Virus Reactivation. Eur. J. Immunol. 2018, 48, 950–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kveštak, D.; Juranić Lisnić, V.; Lisnić, B.; Tomac, J.; Golemac, M.; Brizić, I.; Indenbirken, D.; Cokarić Brdovčak, M.; Bernardini, G.; Krstanović, F.; et al. NK/ILC1 Cells Mediate Neuroinflammation and Brain Pathology Following Congenital CMV Infection. J. Exp. Med. 2021, 218, e20201503. [Google Scholar] [CrossRef] [PubMed]
- Brizić, I.; Lenac Roviš, T.; Krmpotić, A.; Jonjić, S. MCMV Avoidance of Recognition and Control by NK Cells. Semin. Immunopathol. 2014, 36, 641–650. [Google Scholar] [CrossRef]
- Boeckh, M.; Geballe, A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Investig. 2011, 121, 1673–1680. [Google Scholar] [CrossRef]
- Sinzger, C.; Digel, M.; Jahn, G. Cytomegalovirus Cell Tropism. In Current Topics in Microbiology and Immunology; Springer Press: Heidelberg/Berlin, Germany, 2008; pp. 63–83. [Google Scholar] [CrossRef]
- Jackson, J.W.; Sparer, T. There Is Always Another Way! Cytomegalovirus’ Multifaceted Dissemination Schemes. Viruses 2018, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Sacher, T.; Podlech, J.; Mohr, C.A.; Jordan, S.; Ruzsics, Z.; Reddehase, M.J.; Koszinowski, U.H. The Major Virus-Producing Cell Type during Murine Cytomegalovirus Infection, the Hepatocyte, Is Not the Source of Virus Dissemination in the Host. Cell Host Microbe. 2008, 3, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.C.; Kamil, J.P. Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism. Viruses 2018, 10, 704. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Genet. 2021, 19, 110–121. [Google Scholar] [CrossRef]
- Zhou, M.; Lanchy, J.-M.; Ryckman, B.J. Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism. J. Virol. 2015, 89, 8999–9009. [Google Scholar] [CrossRef] [Green Version]
- Hahn, G.; Revello, M.G.; Patrone, M.; Percivalle, E.; Campanini, G.; Sarasini, A.; Wagner, M.; Gallina, A.; Milanesi, G.; Koszinowski, U.; et al. Human Cytomegalovirus UL131-128 Genes Are Indispensable for Virus Growth in Endothelial Cells and Virus Transfer to Leukocytes. J. Virol. 2004, 78, 10023–10033. [Google Scholar] [CrossRef] [Green Version]
- Gerna, G.; Percivalle, E.; Lilleri, D.; Lozza, L.; Fornara, C.; Hahn, G.; Baldanti, F.; Revello, M.G. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131–128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 2005, 86, 275–284. [Google Scholar] [CrossRef]
- Wille, P.T.; Knoche, A.J.; Nelson, J.A.; Jarvis, M.A.; Johnson, D.C. A Human Cytomegalovirus GO-Null Mutant Fails to Incorporate GH/GL into the Virion Envelope and Is Unable To Enter Fibroblasts and Epithelial and Endothelial Cells. J. Virol. 2010, 84, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Ryckman, B.J.; Jarvis, M.A.; Drummond, D.D.; Nelson, J.A.; Johnson, D.C. Human Cytomegalovirus Entry into Epithelial and Endothelial Cells Depends on Genes UL128 to UL150 and Occurs by Endocytosis and Low-PH Fusion. J. Virol. 2006, 80, 710–722. [Google Scholar] [CrossRef] [Green Version]
- Kabanova, A.; Marcandalli, J.; Zhou, T.; Bianchi, S.; Baxa, U.; Tsybovsky, Y.; Lilleri, D.; Silacci-Fregni, C.; Foglierini, M.; Fernandez-Rodriguez, B.M.; et al. Platelet-derived growth factor-α receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Wu, Y.; Prager, A.; Boos, S.; Resch, M.; Brizic, I.; Mach, M.; Wildner, S.; Scrivano, L.; Adler, B. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry. PLoS Pathog. 2017, 13, e1006281. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, N.; Marcandalli, J.; Huang, C.S.; Arthur, C.P.; Perotti, M.; Foglierini, M.; Ho, H.; Dosey, A.M.; Shriver, S.; Payandeh, J.; et al. An Unbiased Screen for Human Cytomegalovirus Identifies Neuropilin-2 as a Central Viral Receptor. Cell 2018, 174, 1158–1171.e19. [Google Scholar] [CrossRef] [Green Version]
- Scrivano, L.; Esterlechner, J.; Mühlbach, H.; Ettischer, N.; Hagen, C.; Grünewald, K.; Mohr, C.A.; Ruzsics, Z.; Koszinowski, U.; Adler, B. The m74 Gene Product of Murine Cytomegalovirus (MCMV) Is a Functional Homolog of Human CMV gO and Determines the Entry Pathway of MCMV. J. Virol. 2010, 84, 4469–4480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, F.M.; Brizić, I.; Prager, A.; Tršan, T.; Arapović, M.; Lemmermann, N.A.W.; Podlech, J.; Reddehase, M.J.; Lemnitzer, F.; Bosse, J.B.; et al. The Viral Chemokine MCK-2 of Murine Cytomegalovirus Promotes Infection as Part of a gH/gL/MCK-2 Complex. PLoS Pathog. 2013, 9, e1003493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, S.; Aguirre, S.A.; Bitmansour, A.; Brown, J.M.; Sparer, T.E.; Huang, J.; Mocarski, E.S. Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination. Blood 2006, 107, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmermann, N.A.W.; Krmpotic, A.; Podlech, J.; Brizic, I.; Prager, A.; Adler, H.; Karbach, A.; Wu, Y.; Jonjic, S.; Reddehase, M.J.; et al. Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread. PLoS Pathog. 2015, 11, e1004640. [Google Scholar] [CrossRef]
- Pereira, L.; Tabata, T.; Petitt, M.; Fang-Hoover, J. Cytomegalovirus Replication in the Developing Human Placenta. In Cytomegaloviruses from Molec-Ular Pathogenesis to Intervention; Caister Academic Press: London, UK, 2013; pp. 74–87. [Google Scholar]
- León-Juárez, M.; Martínez–Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Pliego, A.F.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielli, L.; Bonasoni, M.; Santini, D.; Piccirilli, G.; Chiereghin, A.; Petrisli, E.; Dolcetti, R.; Guerra, B.; Piccioli, M.; Lanari, M.; et al. Congenital cytomegalovirus infection: Patterns of fetal brain damage. Clin. Microbiol. Infect. 2012, 18, E419–E427. [Google Scholar] [CrossRef] [Green Version]
- van den Pol, A.N.; Reuter, J.D.; Santarelli, J.G. Enhanced Cytomegalovirus Infection of Developing Brain Independent of the Adaptive Immune System. J. Virol. 2002, 76, 8842–8854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, J.D.; Gomez, D.L.; Wilson, J.H.; van den Pol, A.N. Systemic Immune Deficiency Necessary for Cytomegalovirus Invasion of the Mature Brain. J. Virol. 2004, 78, 1473–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavuljica, I.; Kvestak, D.; Huszthy, P.C.; Kosmac, K.; Britt, W.J.; Jonjić, S. Immunobiology of congenital cytomegalovirus infection of the central nervous system—The murine cytomegalovirus model. Cell. Mol. Immunol. 2014, 12, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeran, M.C.-J.; Lokensgard, J.R.; Schleiss, M.R. Neuropathogenesis of Congenital Cytomegalovirus Infection: Disease Mechanisms and Prospects for Intervention. Clin. Microbiol. Rev. 2009, 22, 99–126. [Google Scholar] [CrossRef] [Green Version]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Commun. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Teissier, N.; Fallet-Bianco, C.; Delezoide, A.-L.; Laquerrière, A.; Marcorelles, P.; Khung-Savatovsky, S.; Nardelli, J.; Cipriani, S.; Csaba, Z.; Picone, O.; et al. Cytomegalovirus-Induced Brain Malformations in Fetuses. J. Neuropathol. Exp. Neurol. 2014, 73, 143–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokensgard, J.R.; Cheeran, M.C.; Gekker, G.; Hu, S.; Chao, C.C.; Peterson, P.K. Human cytomegalovirus replication and modulation of apoptosis in astrocytes. J. Hum. Virol. 1999, 2, 91–101. [Google Scholar] [PubMed]
- Ho, W.S.C.; van den Pol, A.N. Bystander Attenuation of Neuronal and Astrocyte Intercellular Communication by Murine Cytomegalovirus Infection of Glia. J. Virol. 2007, 81, 7286–7292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecointe, D.; Héry, C.; Janabi, N.; Dussaix, E.; Tardieu, M. Differences in kinetics of human cytomegalovirus cell-free viral release after in vitro infection of human microglial cells, astrocytes and monocyte-derived macrophages. J. NeuroVirol. 1999, 5, 308–313. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N.; Mocarski, E.; Saederup, N.; Vieira, J.; Meier, T.J. Cytomegalovirus Cell Tropism, Replication, and Gene Transfer in Brain. J. Neurosci. 1999, 19, 10948–10965. [Google Scholar] [CrossRef] [Green Version]
- Cheeran, M.C.-J.; Hu, S.; Gekker, G.; Lokensgard, J.R. Decreased Cytomegalovirus Expression Following Proinflammatory Cytokine Treatment of Primary Human Astrocytes. J. Immunol. 2000, 164, 926–933. [Google Scholar] [CrossRef]
- Mutnal, M.B.; Cheeran, M.C.-J.; Hu, S.; Lokensgard, J.R. Murine Cytomegalovirus Infection of Neural Stem Cells Alters Neurogenesis in the Developing Brain. PLoS ONE 2011, 6, e16211. [Google Scholar] [CrossRef] [Green Version]
- Spiller, O.B.; Morgan, B.P.; Borysiewicz, L.K. Development of a model for cytomegalovirus infection of oligodendrocytes. J. Gen. Virol. 1997, 78, 3349–3356. [Google Scholar] [CrossRef] [Green Version]
- Gabrielli, L.; Bonasoni, M.P.; Lazzarotto, T.; Lega, S.; Santini, D.; Foschini, M.P.; Guerra, B.; Baccolini, F.; Piccirilli, G.; Chiereghin, A.; et al. Histological findings in foetuses congenitally infected by cytomegalovirus. J. Clin. Virol. 2009, 46, S16–S21. [Google Scholar] [CrossRef]
- Miron, V.E.; Priller, J. Investigating Microglia in Health and Disease: Challenges and Opportunities. Trends Immunol. 2020, 41, 785–793. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Chhatbar, C.; Prinz, M. The roles of microglia in viral encephalitis: From sensome to therapeutic targeting. Cell. Mol. Immunol. 2021, 18, 250–258. [Google Scholar] [CrossRef]
- Pulliam, L. Cytomegalovirus Preferentially Infects a Monocyte Derived Macrophage/Microglial Cell in Human Brain Cultures: Neuropathology Differs between Strains. J. Neuropathol. Exp. Neurol. 1991, 50, 432–440. [Google Scholar] [CrossRef]
- Cheeran, M.C.-J.; Hu, S.; Yager, S.L.; Gekker, G.; Peterson, P.K.; Lokensgard, J.R. Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: Antiviral implications. J. NeuroVirology 2001, 7, 135–147. [Google Scholar] [CrossRef]
- Cloarec, R.; Bauer, S.; Luche, H.; Buhler, E.; Pallesi-Pocachard, E.; Salmi, M.; Courtens, S.; Massacrier, A.; Grenot, P.; Teissier, N.; et al. Cytomegalovirus Infection of the Rat Developing Brain In Utero Prominently Targets Immune Cells and Promotes Early Microglial Activation. PLoS ONE 2016, 11, e0160176. [Google Scholar] [CrossRef] [Green Version]
- Schut, R.L.; Gekker, G.; Chao, C.C.; Jordan, M.C.; Peterson, P.K.; Hu, S.; Pomeroy, C. Cytomegalovirus Replication in Murine Microglial Cell Cultures: Suppression of Permissive Infection by Interferon-γ. J. Infect. Dis. 1994, 169, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Kučić, N.; Rački, V.; Jurdana, K.; Marcelić, M.; Grabušić, K. Immunometabolic Phenotype of BV-2 Microglia Cells upon Murine Cytomegalovirus Infection. J. Neurovirol. 2019, 25, 496–507. [Google Scholar] [CrossRef]
- Holt, C.E.; Martin, K.C.; Schuman, E.M. Local translation in neurons: Visualization and function. Nat. Struct. Mol. Biol. 2019, 26, 557–566. [Google Scholar] [CrossRef]
- Steward, M.M.; Sridhar, A.; Meyer, J.S. Neural Regeneration. In Current Topics in Microbiology and Immunology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; Volume 71, pp. 163–191. [Google Scholar]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus Infections in the Nervous System. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [Green Version]
- DeBiasi, R.L.; Kleinschmidt-DeMasters, B.K.; Richardson-Burns, S.; Tyler, K.L. Central Nervous System Apoptosis in Human Herpes Simplex Virus and Cytomegalovirus Encephalitis. J. Infect. Dis. 2002, 186, 1547–1557. [Google Scholar] [CrossRef]
- Luo, M.H.; Schwartz, P.H.; Fortunato, E.A. Neonatal Neural Progenitor Cells and Their Neuronal and Glial Cell Derivatives Are Fully Permissive for Human Cytomegalovirus Infection. J. Virol. 2008, 82, 9994–10007. [Google Scholar] [CrossRef] [Green Version]
- Cheeran, M.C.-J.; Hu, S.; Ni, H.T.; Sheng, W.; Palmquist, J.M.; Peterson, P.K.; Lokensgard, J.R. Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J. Neurosci. Res. 2005, 82, 839–850. [Google Scholar] [CrossRef]
- Poland, S.D.; Bambrick, L.L.; Dekaban, G.A.; Rice, G.P.A. The Extent of Human Cytomegalovirus Replication in Primary Neurons Is Dependent On Host Cell Differentiation. J. Infect. Dis. 1994, 170, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.G.; Cooper, E. Depolarization Strongly Induces Human Cytomegalovirus Major Immediate-Early Promoter/Enhancer Activity in Neurons. J. Biol. Chem. 2001, 276, 31978–31985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Rauvala, H.; Gahmberg, C. Neuronal regulation of immune responses in the central nervous system. Trends Immunol. 2009, 30, 91–99. [Google Scholar] [CrossRef]
- Arai, Y.; Ishiwata, M.; Baba, S.; Kawasaki, H.; Kosugi, I.; Li, R.-Y.; Tsuchida, T.; Miura, K.; Tsutsui, Y. Neuron-Specific Activation of Murine Cytomegalovirus Early Gene e1 Promoter in Transgenic Mice. Am. J. Pathol. 2003, 163, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Ladran, I.; Tran, N.; Topol, A.; Brennand, K.J. Neural stem and progenitor cells in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 701–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12. [Google Scholar] [CrossRef]
- Adle-Biassette, H.; Teissier, N. Cytomegalovirus Infections of the CNS. In Infections of the Central Nervous System; Wiley: Hoboken, NJ, USA, 2020; Volume 1, pp. 65–76. [Google Scholar]
- Odeberg, J.; Wolmer, N.; Falci, S.; Westgren, M.; Sundtröm, E.; Seiger, Å.; Söderberg-Nauclér, C. Late human cytomegalovirus (HCMV) proteins inhibit differentiation of human neural precursor cells into astrocytes. J. Neurosci. Res. 2007, 85, 583–593. [Google Scholar] [CrossRef]
- Odeberg, J.; Wolmer, N.; Falci, S.; Westgren, M.; Seiger, A.; Söderberg-Nauclér, C. Human Cytomegalovirus Inhibits Neuronal Differentiation and Induces Apoptosis in Human Neural Precursor Cells. J. Virol. 2006, 80, 8929–8939. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, I.; Shinmura, Y.; Kawasaki, H.; Arai, Y.; Li, R.-Y.; Baba, S.; Tsutsui, Y. Cytomegalovirus Infection of the Central Nervous System Stem Cells from Mouse Embryo: A Model for Developmental Brain Disorders Induced by Cytomegalovirus. Lab. Investig. 2000, 80, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Cheeran, M.C.-J.; Jiang, Z.; Hu, S.; Ni, H.T.; Palmquist, J.M.; Lokensgard, J.R. Cytomegalovirus infection and interferon-γ modulate major histocompatibility complex class I expression on neural stem cells. J. NeuroVirology 2008, 14, 437–447. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Di Maio, R.; Heath, B.; Raimondi, G.; Milosevic, J.; Watson, A.M.; Bamne, M.; Parks, W.T.; Yang, L.; Lin, B.; et al. Human Induced Pluripotent Stem Cell-Derived Models to Investigate Human Cytomegalovirus Infection in Neural Cells. PLoS ONE 2012, 7, e49700. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Kosugi, I.; Arai, Y.; Tsutsui, Y. The Amount of Immature Glial Cells in Organotypic Brain Slices Determines the Susceptibility to Murine Cytomegalovirus Infection. Lab. Investig. 2002, 82, 1347–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, J.-P.; Kothary, R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Del Bigio, M.R. Ependymal cells: Biology and pathology. Acta Neuropathol. 2010, 119, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Abdi, K.; Lai, C.-H.; Paez-Gonzalez, P.; Lay, M.; Pyun, J.; Kuo, C.T. Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, F. Human Cytomegalovirus Latency: Approaching the Gordian Knot. Annu. Rev. Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef] [Green Version]
- Dupont, L.; Reeves, M.B. Cytomegalovirus Latency and Reactivation: Recent Insights into an Age Old Problem Introduction: The Opportunistic Pathogen. Rev. Med. Virol. 2017, 26, 75–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay with the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef]
- Collins-McMillen, D.; Buehler, J.; Peppenelli, M.; Goodrum, F. Molecular Determinants and the Regulation of Human Cytomegalovirus Latency and Reactivation. Viruses 2018, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Bloom, D.C.; Giordani, N.V.; Kwiatkowski, D.L. Epigenetic regulation of latent HSV-1 gene expression. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1799, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Umbach, J.L.; Kramer, M.F.; Jurak, I.; Karnowski, H.W.; Coen, D.M.; Cullen, B.R. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nat. Cell Biol. 2008, 454, 780–783. [Google Scholar] [CrossRef] [Green Version]
- Shnayder, M.; Nachshon, A.; Krishna, B.; Poole, E.; Boshkov, A.; Binyamin, A.; Maza, I.; Sinclair, J.; Schwartz, M.; Stern-Ginossar, N. Defining the Transcriptional Landscape during Cytomegalovirus Latency with Single-Cell RNA Sequencing. MBio 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.; Stern-Ginossar, N. The Transcriptome of Latent Human Cytomegalovirus. J. Virol. 2019, 93, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Caviness, K.; Buehler, J.; Smithey, M.; Nikolich-Žugich, J.; Goodrum, F. Transcriptome-wide characterization of human cytomegalovirus in natural infection and experimental latency. Proc. Natl. Acad. Sci. USA 2017, 114, E10586–E10595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belzile, J.-P.; Stark, T.J.; Yeo, E.; Spector, D.H. Human Cytomegalovirus Infection of Human Embryonic Stem Cell-Derived Primitive Neural Stem Cells Is Restricted at Several Steps but Leads to the Persistence of Viral DNA. J. Virol. 2014, 88, 4021–4039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, M.J.; Wheeler, D.G.; Cooper, E.; Meier, J.L. Role of the Human Cytomegalovirus Major Immediate-Early Promoter’s 19-Base-Pair-Repeat Cyclic AMP-Response Element in Acutely Infected Cells. J. Virol. 2003, 77, 6666–6675. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.J.; Wu, A.W.; Andrews, J.I.; McGonagill, P.W.; Tibesar, E.E.; Meier, J.L. Reversal of Human Cytomegalovirus Major Immediate-Early Enhancer/Promoter Silencing in Quiescently Infected Cells via the Cyclic AMP Signaling Pathway. J. Virol. 2007, 81, 6669–6681. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, Y.; Kawasaki, H.; Kosugi, I. Reactivation of Latent Cytomegalovirus Infection in Mouse Brain Cells Detected after Transfer to Brain Slice Cultures. J. Virol. 2002, 76, 7247–7254. [Google Scholar] [CrossRef] [Green Version]
- Brizić, I.; Hiršl, L.; Šustić, M.; Golemac, M.; Britt, W.J.; Krmpotić, A.; Jonjić, S. CD4 T Cells Are Required for Maintenance of CD8 TRM Cells and Virus Control in the Brain of MCMV-Infected Newborn Mice. Med. Microbiol. Immunol. 2019, 208, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Krenzlin, H.; Behera, P.; Lorenz, V.; Passaro, C.; Zdioruk, M.; Nowicki, M.O.; Grauwet, K.; Zhang, H.; Skubal, M.; Ito, H.; et al. Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J. Clin. Investig. 2019, 129, 1671–1683. [Google Scholar] [CrossRef] [Green Version]
- Joseph, G.P.; McDermott, R.; Baryshnikova, M.A.; Cobbs, C.S.; Ulasov, I.V. Cytomegalovirus as an oncomodulatory agent in the progression of glioma. Cancer Lett. 2017, 384, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Brizić, I.; Hiršl, L.; Britt, W.J.; Krmpotić, A.; Jonjić, S. Immune Responses to Congenital Cytomegalovirus Infection. Microbes Infect. 2018, 20, 543–551. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Seleme, M.C.; Kosmac, K.; Jonjic, S.; Britt, W.J. Tumor Necrosis Factor Alpha-Induced Recruitment of Inflammatory Mononuclear Cells Leads to Inflammation and Altered Brain Development in Murine Cytomegalovirus-Infected Newborn Mice. J. Virol. 2017, 91, e01983-16. [Google Scholar] [CrossRef] [Green Version]
- Kosmac, K.; Bantug, G.R.; Pugel, E.P.; Cekinovic, D.; Jonjic, S.; Britt, W.J. Glucocortiocoid Treatment of MCMV Infected Newborn Mice Attenuates CNS Inflammation and Limits Deficits in Cerebellar Development. PLoS Pathog. 2013, 9, e1003200. [Google Scholar] [CrossRef]
- Podlech, J.; Reddehase, M.J.; Holtappels, R.; Steffens, H.P.; Wirtz, N. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J. Gen. Virol. 1998, 79, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Stagno, S.; Pass, R. Maternal Immunity and Prevention of Congenital Cytomegalovirus Infection. JAMA 2003, 289, 1008–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiršl, L.; Brizić, I.; Jenuš, T.; Lisnić, V.J.; Reichel, J.J.; Jurković, S.; Krmpotić, A.; Jonjić, S. Murine CMV Expressing the High Affinity NKG2D Ligand MULT-1: A Model for the Development of Cytomegalovirus-Based Vaccines. Front. Immunol. 2018, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Slavuljica, I.; Busche, A.; Babić, M.; Mitrović, M.; Gašparović, I.; Cekinović, D.; Car, E.M.; Pugel, E.P.; Ciković, A.; Lisnić, V.J.; et al. Recombinant Mouse Cytomegalovirus Expressing a Ligand for the NKG2D Receptor Is Attenuated and Has Improved Vaccine Properties. J. Clin. Investig. 2010, 120, 4532–4545. [Google Scholar] [CrossRef]
- Cekinović, Đ.; Golemac, M.; Pugel, E.P.; Tomac, J.; Čičin-Šain, L.; Slavuljica, I.; Bradford, R.; Misch, S.; Winkler, T.H.; Mach, M.; et al. Passive Immunization Reduces Murine Cytomegalovirus-Induced Brain Pathology in Newborn Mice. J. Virol. 2008, 82, 12172–12180. [Google Scholar] [CrossRef] [Green Version]
- Butts, T.; Green, M.J.; Wingate, R.J.T. Development: For Advances in Developmental Biology and Stem Cells; The Company of Biologists Ltd. Press: Cambridge, UK, 2014; pp. 4031–4041. [Google Scholar]
- De Vries, L.S.; Gunardi, H.; Barth, P.G.; Bok, L.A.; Verboon-Maciolek, M.A.; Groenendaal, F. The Spectrum of Cranial Ultrasound and Magnetic Resonance Imaging Abnormalities in Congenital Cytomegalovirus Infection. Neuropediatrics 2004, 35, 113–119. [Google Scholar] [CrossRef]
- Penisson, M.; Ladewig, J.; Belvindrah, R.; Francis, F. Genes and Mechanisms Involved in the Generation and Amplification of Basal Radial Glial Cells. Front. Cell. Neurosci. 2019, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Jiang, X.; Wang, X.-Z.; Liu, X.-J.; Li, X.-J.; Yang, B.; Ye, H.-Q.; Harwardt, T.; Jiang, M.; Xia, H.-M.; et al. Human Cytomegalovirus Immediate Early 1 Protein Causes Loss of SOX2 from Neural Progenitor Cells by Trapping Unphosphorylated STAT3 in the Nucleus. J. Virol. 2018, 92, e00340-18. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-J.; Yang, B.; Huang, S.-N.; Wu, C.-C.; Li, X.-J.; Cheng, S.; Jiang, X.; Hu, F.; Ming, Y.-Z.; Nevels, M.; et al. Human cytomegalovirus IE1 downregulates Hes1 in neural progenitor cells as a potential E3 ubiquitin ligase. PLoS Pathog. 2017, 13, e1006542. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.H.; Hannemann, H.; Kulkarni, A.S.; Schwartz, P.H.; O’Dowd, J.M.; Fortunato, E.A. Human Cytomegalovirus Infection Causes Premature and Abnormal Differentiation of Human Neural Progenitor Cells. J. Virol. 2010, 84, 3528–3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Liu, Y. Signaling pathways in cerebellar granule cells development. Am. J. Stem. Cells 2019, 8, 1–6. [Google Scholar] [PubMed]
- Carter, A.R.; Chen, C.; Schwartz, P.M.; Segal, R.A. Brain-Derived Neurotrophic Factor Modulates Cerebellar Plasticity and Synaptic Ultrastructure. J. Neurosci. 2002, 22, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N.; Robek, M.D.; Ghosh, P.K.; Ozduman, K.; Bandi, P.; Whim, M.D.; Wollmann, G. Cytomegalovirus Induces Interferon-Stimulated Gene Expression and Is Attenuated by Interferon in the Developing Brain. J. Virol. 2007, 81, 332–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, T.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium Waves Propagate through Radial Glial Cells and Modulate Proliferation in the Developing Neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. In GLIA; Wiley: Hoboken, NJ, USA, 2018; pp. 221–238. [Google Scholar]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay, P.A.; McAllister, A.K. Novel Roles for Immune Molecules in Neural Development: Implications for Neurodevelopmental Disorders. Front. Syn. Neurosci. 2010, 2, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cell Type | Acute Infection | Latent Infection | Selected References | |
|---|---|---|---|---|
| In Vivo | In Vitro | |||
| Astrocytes | HCMV (+) MCMV (?) | HCMV (+) MCMV (+) | No information | [71] [72] |
| Microglia | HCMV (+) MCMV (+) | HCMV (+/−) MCMV (+) | No information | [71] [41] |
| Neurons | HCMV (+) MCMV (+) | HCMV (+/−) MCMV (?) | Possible site of latency | [71] [34] |
| NSPCs | HCMV (+) MCMV (+) | HCMV (+) MCMV (+) | Possible site of latency | [71] [77] |
| Oligodendrocytes | HCMV (?) MCMV (?) | HCMV (+) MCMV (?) | No information | [78] |
| Ependymal cells | HCMV (+) MCMV (+) | HCMV (+) MCMV (?) | No information | [79] [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstanović, F.; Britt, W.J.; Jonjić, S.; Brizić, I. Cytomegalovirus Infection and Inflammation in Developing Brain. Viruses 2021, 13, 1078. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061078
Krstanović F, Britt WJ, Jonjić S, Brizić I. Cytomegalovirus Infection and Inflammation in Developing Brain. Viruses. 2021; 13(6):1078. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061078
Chicago/Turabian StyleKrstanović, Fran, William J. Britt, Stipan Jonjić, and Ilija Brizić. 2021. "Cytomegalovirus Infection and Inflammation in Developing Brain" Viruses 13, no. 6: 1078. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061078






