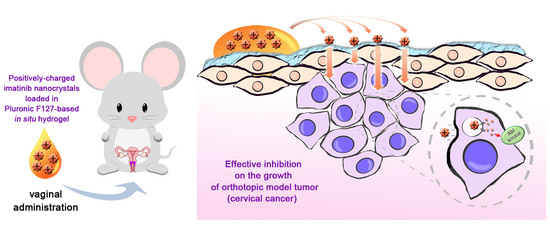
Enhanced Delivery of Imatinib into Vaginal Mucosa via a New Positively Charged Nanocrystal-Loaded in Situ Hydrogel Formulation for Treatment of Cervical Cancer
Abstract
:1. Introduction
2. Methods
2.1. Materials and Animals
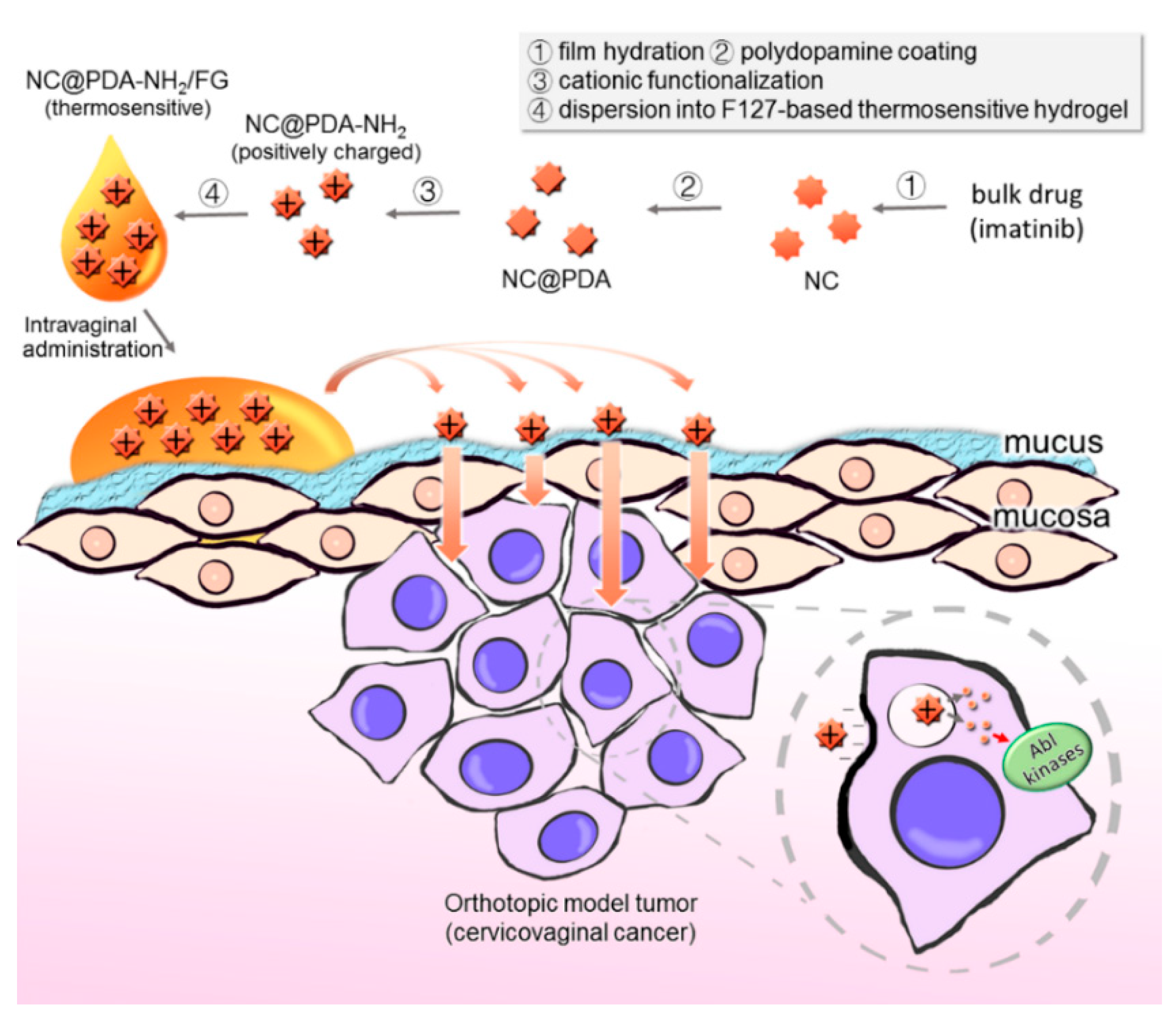
2.2. Preparation of NC, NC@PDA, and NC@PDA-NH2
2.3. Characterization of NC, NC@PDA, and NC@PDA-NH2
2.4. Interaction between Mucin and Nanocrystals
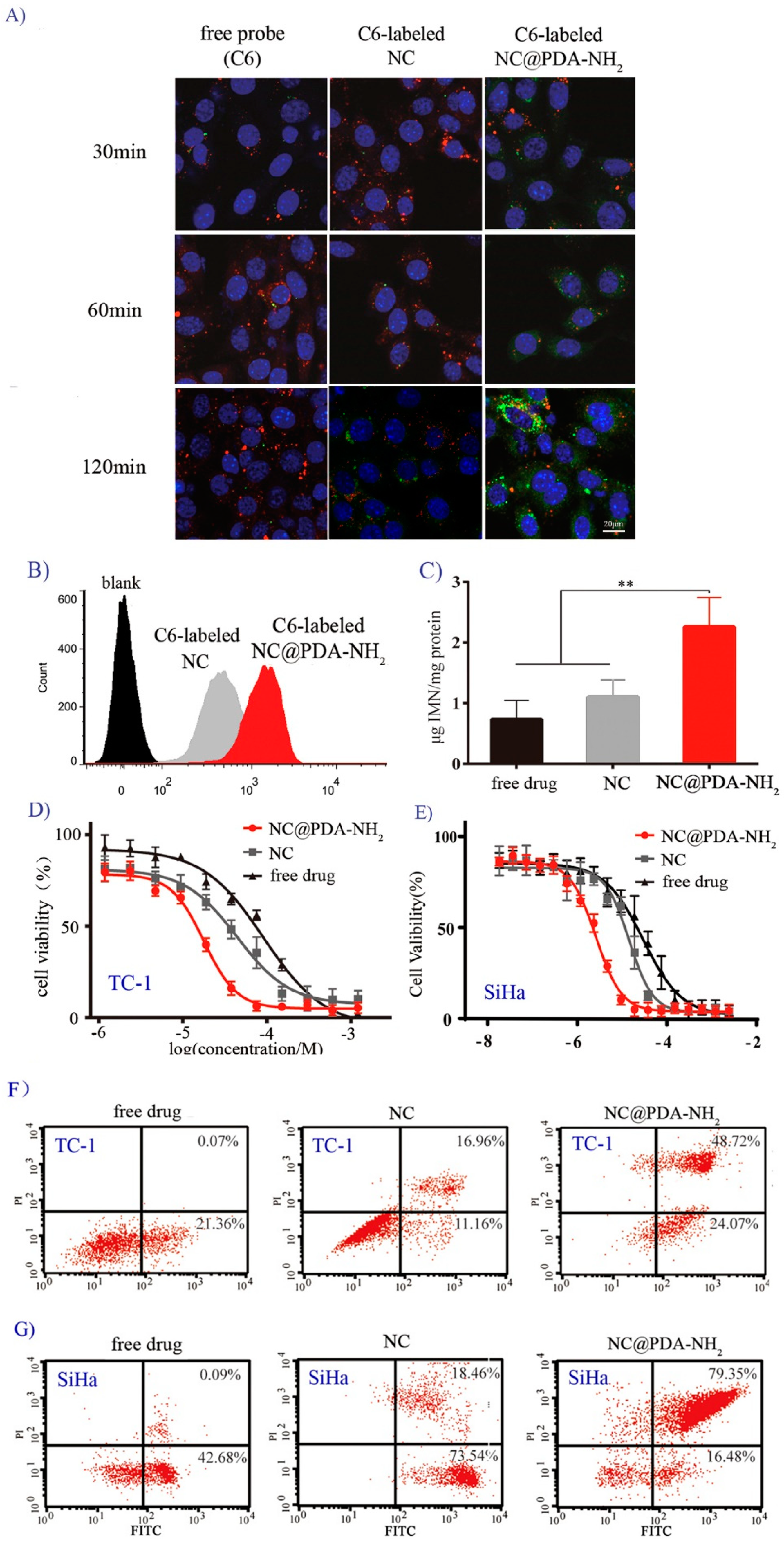
2.5. Interaction of Nanocrystals with Cervicovaginal-Related Cell Lines
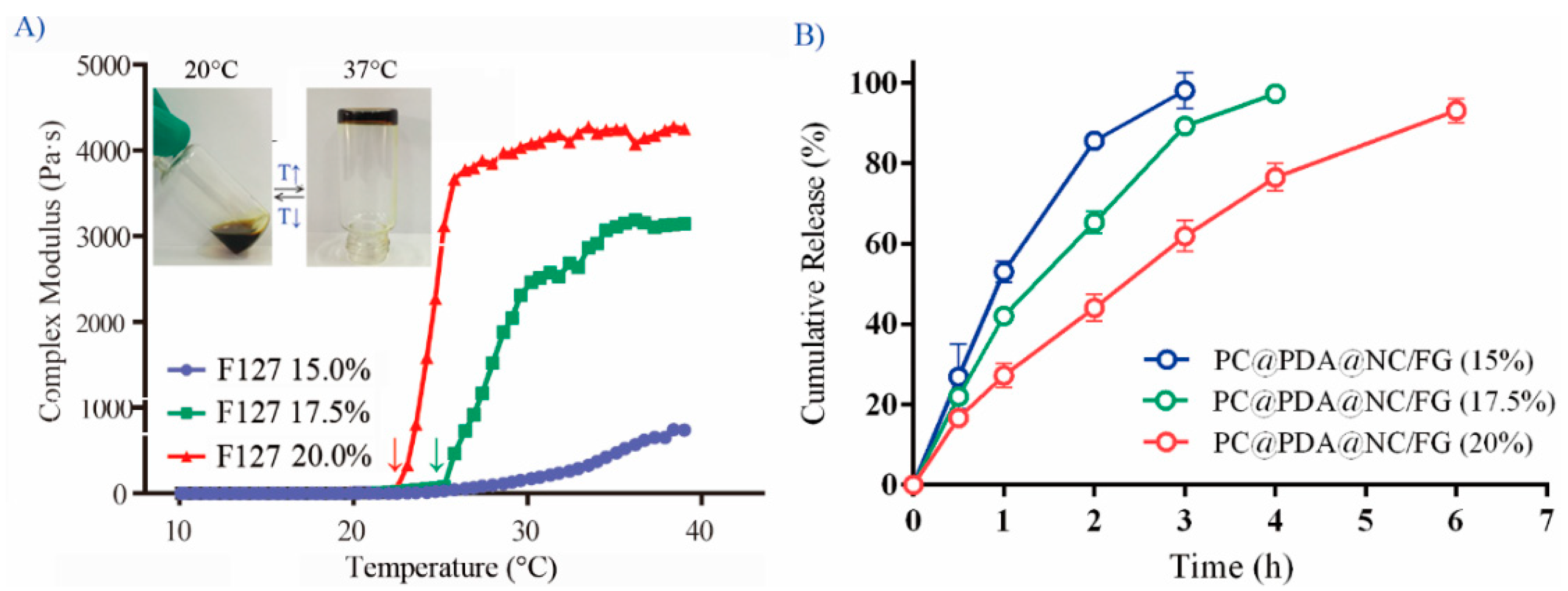
2.6. Preparation and Characterization of NC@PDA-NH2/FG
2.7. Ex Vivo and In Vivo Evaluation of the Mucoadhesiveness of NC@PDA-NH2/FG
2.8. In Vivo Efficacy against the Orthotopic Cervicovaginal Tumor Model in Mice
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of NC@PDA-NH2
3.2. Cationic Functionalization Favored Interaction of Nanocrystals with Mucin
3.3. Cationic Functionalization Favored Interaction of Nanocrystals with Cells
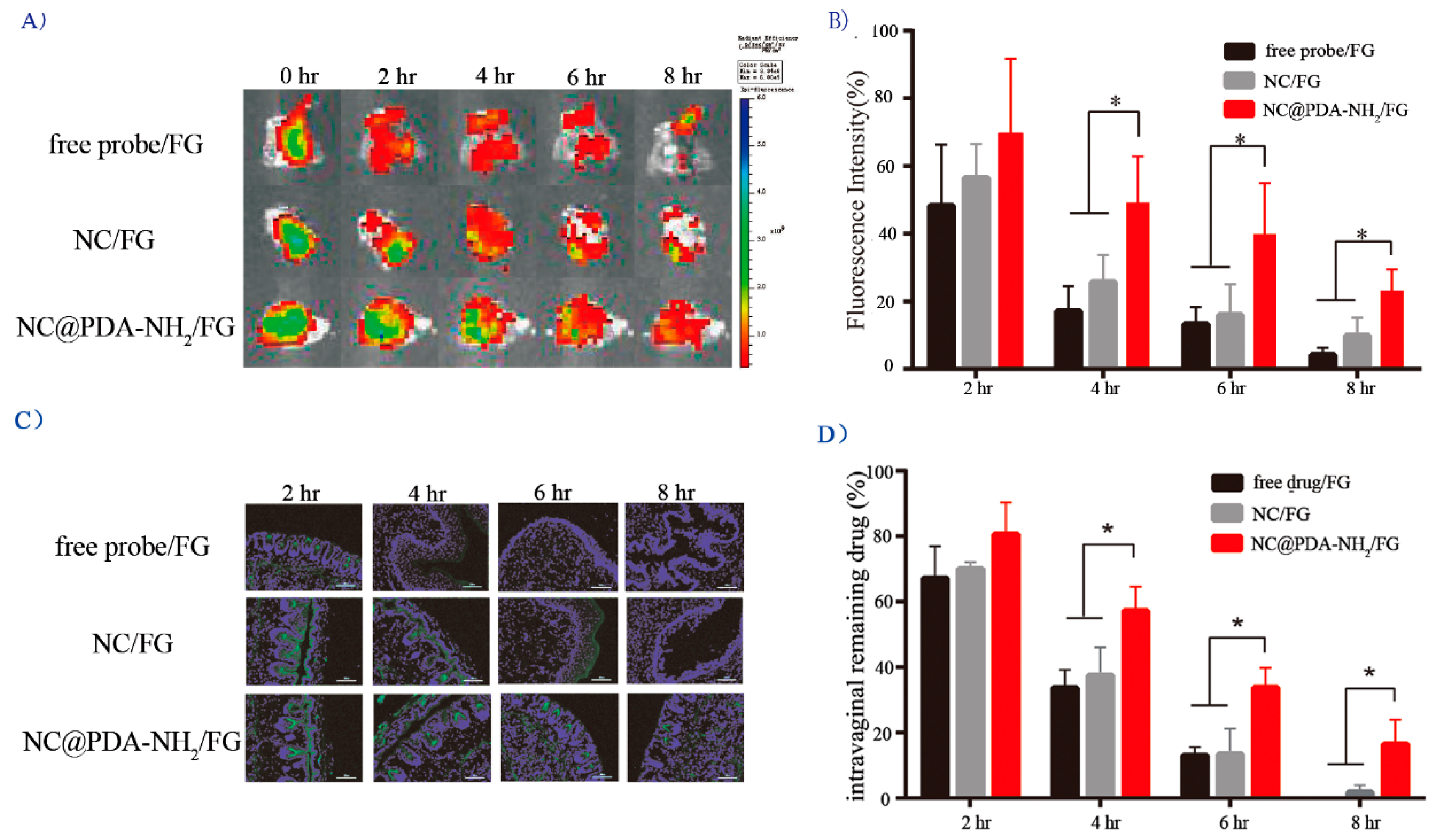
3.4. Cationic Functionalization Prolonged Intravaginal Retention and Enhanced Mucosal Penetration
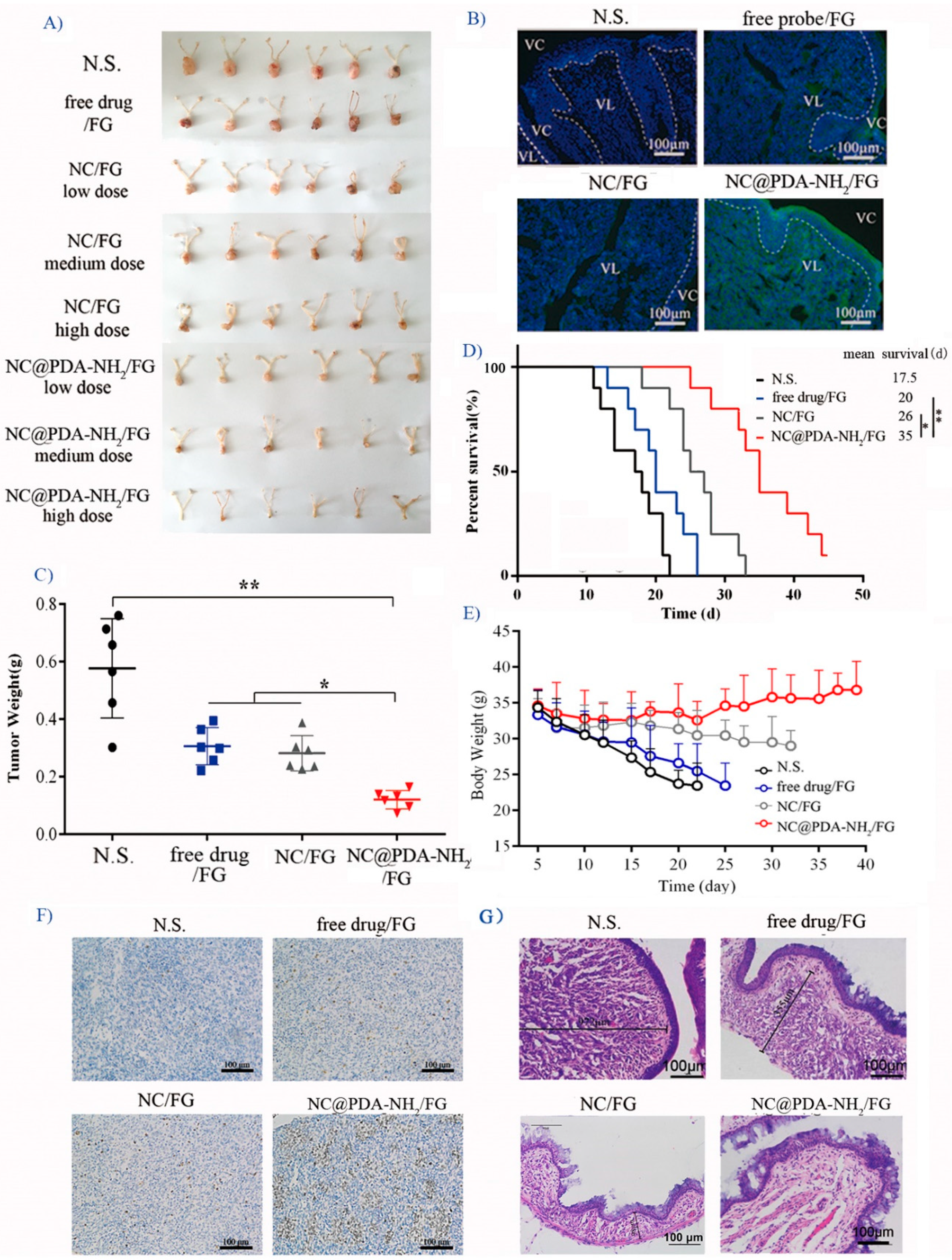
3.5. Cationic Functionalization Improved Tumor Inhibition in the Orthotopic TC-1 Cervical Cancer Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDA | polydopamine |
| NC | nanocrystals |
| NC@PDA-NH2 | cationic functionalized polydopamine-coated imatinib nanocrystals |
| NC@PDA | polydopamine-coated imatinib nanocrystals |
| F127 | Pluronic F127 |
| FG | Pluronic F127-based in situ hydrogel |
| TKI | tyrosine kinase inhibitors |
| IMN | imatinib |
| C6 | 6-coumarin |
| DiR | 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide |
| OCT | optical coherence tomography |
| DAPI | 2-(4-amidinophenyl)-6-indolecarbamindine dihydrochloride |
| PGM | porcine gastric mucin |
| DL | drug loading |
| EE | encapsulation efficiency |
| TEM | transmission electron microscope |
| DLS | dynamic light scattering |
| XRPD | X-ray powder diffraction |
| DSC | differential scanning calorimetry |
| VFS | vaginal fluid stimulant |
References
- Bernard, W.S.; Christopher, P.W. World Cancer Report 2014; World Health Organization: Switzerland, Geneva, 2014. [Google Scholar]
- Major, I.; McConville, C. Vaginal drug delivery for the localised treatment of cervical cancer. Drug Deliv. Transl. Res. 2017, 7, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Taja-Chayeb, L.; Chavez-Blanco, A.; Martínez-Tlahuel, J.; González-Fierro, A.; Candelaria, M.; Chanona-Vilchis, J.; Robles, E.; Dueñas-Gonzalez, A. Expression of platelet derived growth factor family members and the potential role of imatinib mesylate for cervical cancer. Cancer Cell Int. 2006, 6, 22. [Google Scholar] [CrossRef]
- Kummel, S.; Heidecke, H.; Brock, B.; Denkert, C.; Hecktor, J.; Koninger, A.; Becker, I.; Sehouli, J.; Thomas, A.; Blohmer, J.U.; et al. Imatinib—A possible therapeutic option for cervical carcinoma: Results of a preclinical phase I study. Gynakol. Geburtshilfliche Rundsch. 2008, 48, 94–100. [Google Scholar]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals: Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Wu, W. Injected nanocrystals for targeted drug delivery. Acta Pharm. Sin. B 2016, 6, 106–113. [Google Scholar] [CrossRef]
- Guo, H.; Xu, W.; Chen, J.; Yan, L.; Ding, J.; Hou, Y.; Chen, X. Positively charged polypeptide nanogel enhances mucoadhesion and penetrability of 10-hydroxycamptothecin in orthotopic bladder carcinoma. J. Control. Release 2017, 259, 136–148. [Google Scholar] [CrossRef]
- Ci, L.Q.; Huang, Z.G.; Liu, Y.; Liu, Z.P.; Wei, G.; Lu, W.Y. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: Preparation, characterization and application in vaginal drug delivery system. Acta Pharm. Sin. B 2017, 7, 593–602. [Google Scholar] [CrossRef]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, H.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Effects of PEGylated paclitaxel nanocrystals on breast cancer and its lung metastasis. Nanoscale 2015, 7, 10790–10800. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Sun, B.; Yeo, Y. Albumin-coated nanocrystals for carrier-free delivery of paclitaxel. J. Control. Release 2017, 263, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W.; Ma, G. HSA induced HCPT nanocrystal for high-performance antitumor therapy. J. Control. Release 2017, 259, e82. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Thompson, D.H.; Park, K.; Li, T. Impact of surfactant treatment of paclitaxel nanocrystals on biodistribution and tumor accumulation in tumor-bearing mice. J. Control. Release 2016, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lee, D.; Meng, Z.; Li, T. Exploring intracellular fate of drug nanocrystals with crystal-integrated and environment-sensitive fluorophores. J. Control. Release 2017, 267, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, W.; Zhang, X.; Dong, Y.; Hua, Y.; Zhang, H.; Gao, J.; Zhao, L.; Li, Y.; Zheng, A. In vitro and in vivo evaluation of SN-38 nanocrystals with different particle sizes. Int. J. Nanomed. 2017, 12, 5487–5500. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.; Zeng, H.; Xu, Z. Deposition and Adhesion of Polydopamine on the Surfaces of Varying Wettability. ACS Appl. Mater. Interfaces 2017, 9, 30943–30950. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 5, 1353–1359. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef]
- Zeng, X.; Tao, W.; Liu, G.; Mei, L. Polydopamine-based surface modification of copolymeric nanoparticles as a targeted drug delivery system for cancer therapy. J. Control. Release 2017, 259, e150–e151. [Google Scholar] [CrossRef]
- Xue, P.; Sun, L.; Li, Q.; Zhang, L.; Guo, J.; Xu, Z.; Kan, Y. PEGylated polydopamine-coated magnetic nanoparticles for combined targeted chemotherapy and photothermal ablation of tumour cells. Colloids Surf. B Biointerfaces 2017, 160, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Jagtiani, T.; Liang, J.F. A new targeted delivery approach by functionalizing drug nanocrystals through polydopamine coating. Eur. J. Pharm. Biopharm. 2017, 114, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Yang, M.; Yu, T.; Wang, Y.Y.; Lai, S.K.; Zeng, Q.; Miao, B.; Tang, B.C.; Simons, B.W.; Ensign, L.M.; Liu, G.; et al. Vaginal delivery of paclitaxel via nanoparticles with nonmucoadhesive surfaces suppresses cervical tumor growth. Adv. Healthc. Mater. 2014, 3, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Novartis, A.G. Crystalline F, G, H, I and K type of Imatinib Mesylate. CN Patent 2006800440007.7, 26 November 2008. [Google Scholar]
- Prosperi-Porta, G.; Kedzior, S.; Muirhead, B.; Sheardown, H. Phenylboronic-acid-based polymeric micelles for mucoadhesive anterior segment ocular drug delivery. Biomacromolecules 2016, 17, 1449–1457. [Google Scholar] [CrossRef]
- Nikogeorgos, N.; Efler, P.; Kayitmazer, A.B.; Lee, S. “Bio-glues” to enhance slipperiness of mucins: Improved lubricity and wear resistance of porcine gastric mucin (PGM) layers assisted by mucoadhesion with chitosan. Soft Matter 2015, 11, 489–498. [Google Scholar] [CrossRef]
- Adamczak, M.I.; Hagesaether, E.; Smistad, G.; Hiorth, M. An in vitro study of mucoadhesion and biocompatibility of polymer coated liposomes on HT29-MTX mucus-producing cells. Int. J. Pharm. 2016, 498, 225–233. [Google Scholar] [CrossRef]
- Büyükköroğlu, G.; Şenel, B.; Başaran, E.; Yenilmez, E.; Yazan, Y. Preparation and in vitro evaluation of vaginal formulations including siRNA and paclitaxel-loaded SLNs for cervical cancer. Eur. J. Pharm. Biopharm. 2016, 109, 174–183. [Google Scholar] [CrossRef]
- Ci, T.; Yuan, L.; Bao, X.; Hou, Y.; Wu, H.; Sun, H.; Cao, D.; Ke, X. Development and anti-Candida evaluation of the vaginal delivery system of amphotericin B nanosuspension-loaded thermogel. J. Drug Target. 2018, 26, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, F.J.; Feng, L.L.; Yang, L.; Chen, L.Y.; Wei, G.; Lu, W.Y. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Guarnieri, F.G.; Staveley-O’Carroll, K.F.; Levitsky, H.I.; August, J.T.; Pardoll, D.M.; Wu, T.C. Treatment of Established Tumors with a Novel Vaccine That Enhances Major Histocompatibility Class II Presentation of Tumor Antigen. Cancer Res. 1996, 56, 21–26. [Google Scholar] [PubMed]



| Drug Loading (DL) (%) | Entrapment Efficiency (EE) (%) | |
|---|---|---|
| NC | 94.47 ± 2.85 | 94.45 ± 4.81 |
| NC@PDA | 82.72 ± 3.66 | 83.55 ± 12.01 |
| NC@PDA-NH2 | 76.84 ± 1.78 | 87.11 ± 11.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ci, L.-q.; Huang, Z.-g.; Lv, F.-m.; Wang, J.; Feng, L.-l.; Sun, F.; Cao, S.-j.; Liu, Z.-p.; Liu, Y.; Wei, G.; et al. Enhanced Delivery of Imatinib into Vaginal Mucosa via a New Positively Charged Nanocrystal-Loaded in Situ Hydrogel Formulation for Treatment of Cervical Cancer. Pharmaceutics 2019, 11, 15. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010015
Ci L-q, Huang Z-g, Lv F-m, Wang J, Feng L-l, Sun F, Cao S-j, Liu Z-p, Liu Y, Wei G, et al. Enhanced Delivery of Imatinib into Vaginal Mucosa via a New Positively Charged Nanocrystal-Loaded in Situ Hydrogel Formulation for Treatment of Cervical Cancer. Pharmaceutics. 2019; 11(1):15. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010015
Chicago/Turabian StyleCi, Li-qian, Zhi-gang Huang, Feng-mei Lv, Jun Wang, Ling-lin Feng, Feng Sun, Shui-juan Cao, Zhe-peng Liu, Yu Liu, Gang Wei, and et al. 2019. "Enhanced Delivery of Imatinib into Vaginal Mucosa via a New Positively Charged Nanocrystal-Loaded in Situ Hydrogel Formulation for Treatment of Cervical Cancer" Pharmaceutics 11, no. 1: 15. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11010015