Prenatal Intervention with Partial Meal Replacement Improves Micronutrient Intake of Pregnant Women with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
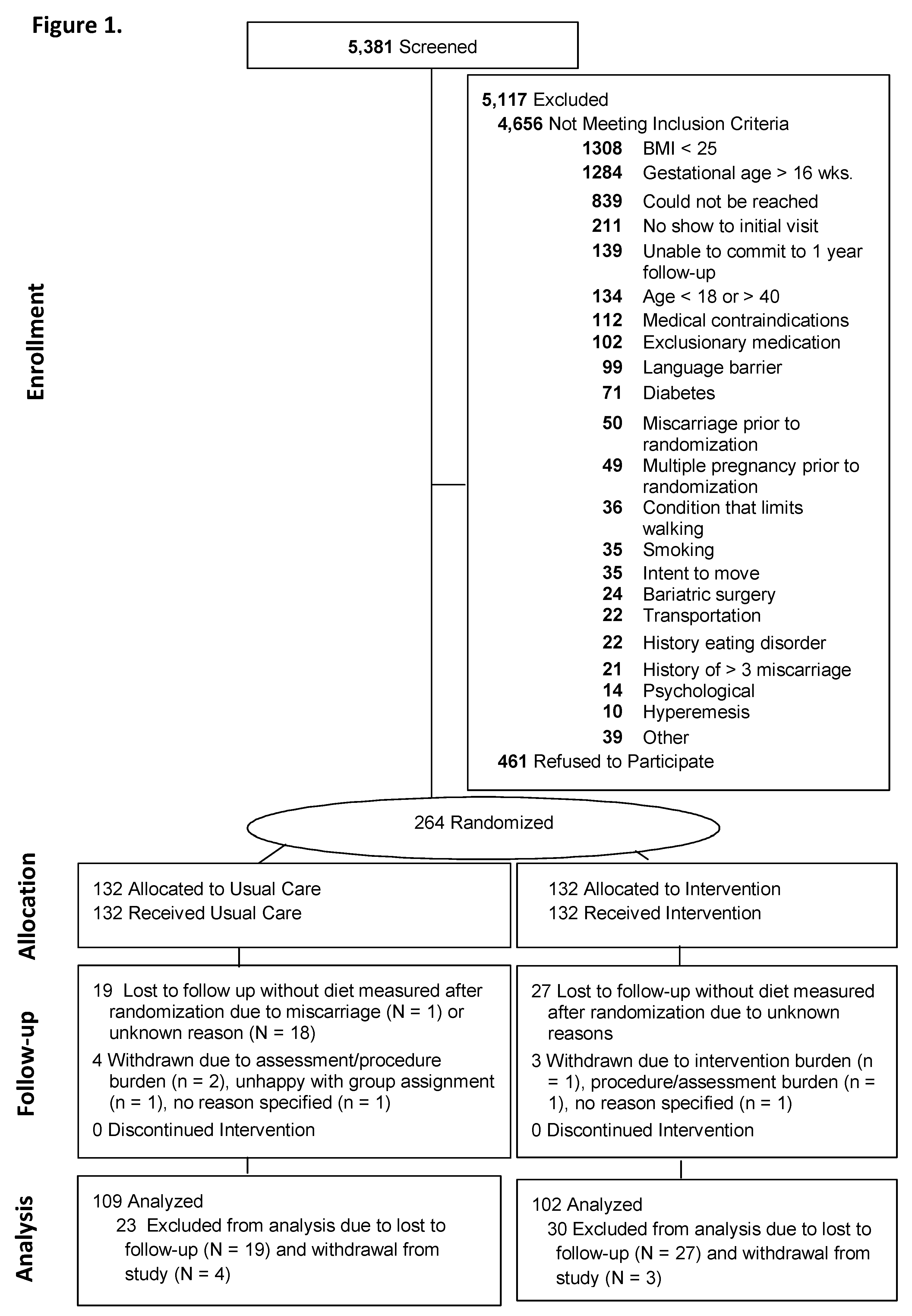
2.2. Participants
2.3. Interventions
2.4. Usual Care
2.5. Behavioral Lifestyle Intervention with Partial Meal Replacement during Pregnancy
2.6. Outcome Assessments
2.7. Statistical Methods
Analysis Plan
3. Results
3.1. Intervention Effects on Micronutrient Intake
3.2. Intervention Effects on Micronutrient Adequacy Based on the RDAs
3.3. Intervention Adherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition during pregnancy, lactation and early childhood and its implications for maternal and long-term child health: The early nutrition project recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Nesby-O’Dell, S.; Scanlon, K.S.; Cogswell, M.E.; Gillespie, C.; Hollis, B.W.; Looker, A.C.; Allen, C.; Doughertly, C.; Gunter, E.W.; Bowman, B.A. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2002, 76, 187–192. [Google Scholar] [CrossRef]
- Agarwal, S.; Reider, C.; Brooks, J.R.; Fulgoni, V.L. Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: An analysis of NHANES 2001–2008. J. Am. Coll. Nutr. 2015, 34, 126–134. [Google Scholar] [CrossRef]
- Astrup, A.; Bugel, S. Overfed but undernourished: recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2018. [Google Scholar] [CrossRef] [PubMed]
- Procter, S.B.; Campbell, C.G. Position of the Academy of Nutrition and Dietetics: Nutrition and lifestyle for a healthy pregnancy outcome. J. Acad. Nutr. Diet. 2014, 114, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Shapira, N. Prenatal nutrition: A critical window of opportunity for mother and child. Womens Health 2008, 4, 639–656. [Google Scholar] [CrossRef]
- Peaceman, A.M.; Clifton, R.G.; Phelan, S.; Gallagher, D.; Evans, M.; Redman, L.M.; Knowler, W.C.; Joshipura, K.; Haire-Joshu, D.; Yanovski, S.Z.; et al. Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE-Moms Prospective Meta-Analysis. Obesity (Silver Spring) 2018, 26, 1396–1404. [Google Scholar] [CrossRef]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An italian consensus document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef]
- Milman, N.; Bergholt, T.; Byg, K.E.; Eriksen, L.; Graudal, N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplementation. Acta. Obstet. Gynecol. Scand. 1999, 78, 749–757. [Google Scholar] [CrossRef]
- Czeizel, A.E. Periconceptional folic acid containing multivitamin supplementation. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 78, 151–161. [Google Scholar] [CrossRef]
- Murphy, M.M.; Scott, J.M.; McPartlin, J.M.; Fernandez-Ballart, J.D. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am. J. Clin. Nutr. 2002, 76, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Zeghoud, F.; Vervel, C.; Guillozo, H.; Walrant-Debray, O.; Boutignon, H.; Garabedian, M. Subclinical vitamin D deficiency in neonates: Definition and response to vitamin D supplements. Am. J. Clin. Nutr. 1997, 65, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Pairaideau, N. Compliance with prenatal vitamins. Patients with morning sickness sometimes find it difficult. Can. Fam. Phys. 2006, 52, 1392–1393. [Google Scholar] [PubMed]
- Dobbing, J.; Rybar Laboratories. Prevention of Spina Bifida and Other Neural Tube Defects; Academic Press: London, UK; New York, NY, USA, 1983; p. xiv. 251p. [Google Scholar]
- Fall, C.H.; Yajnik, C.S.; Rao, S.; Davies, A.A.; Brown, N.; Farrant, H.J. Micronutrients and fetal growth. J. Nutr. 2003, 133, S1747–S1756. [Google Scholar] [CrossRef]
- Long, V.A.; Martin, T.; Janson-Sand, C. The great beginnings program: Impact of a nutrition curriculum on nutrition knowledge, diet quality, and birth outcomes in pregnant and parenting teens. J. Am. Diet. Assoc. 2002, 102, S86–S89. [Google Scholar] [CrossRef]
- Phelan, S.; Wing, R.R.; Brannen, A.; McHugh, A.; Hagobian, T.A.; Schaffner, A.; Jelalian, E.; Hart, C.N.; Scholl, T.O.; Munoz-Christian, K.; et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. Am. J. Clin. Nutr. 2018, 107, 183–194. [Google Scholar] [CrossRef]
- Clifton, R.G.; Evans, M.; Cahill, A.G.; Franks, P.W.; Gallagher, D.; Phelan, S.; Pomeroy, J.; Redman, L.M.; Van Horn, L.; Group, L.I.-M.R. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring) 2016, 24, 305–313. [Google Scholar] [CrossRef]
- Phelan, S.; Wing, R.R.; Brannen, A.; McHugh, A.; Hagobian, T.; Schaffner, A.; Jelalian, E.; Hart, C.N.; Scholl, T.O.; Munoz-Christian, K.; et al. Does Partial Meal Replacement During Pregnancy Reduce 12-Month Postpartum Weight Retention? Obesity (Silver Spring) 2019, 27, 226–236. [Google Scholar] [CrossRef]
- Conner, P.; Bartlett, S.; Mendelson, M.; Condon, K.; Sutcliffe, C. (Eds.) WIC Participant and Program Characteristics 2008, WIC-08-PC; U.S. Department of Agriculture, Food and Nutrition Service, Office of Research and Analysis: Alexandria, VA, USA, 2010.
- Phelan, S.; Phipps, M.G.; Abrams, B.; Darroch, F.; Schaffner, A.; Wing, R.R. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for Delivery Study. Am. J. Clin. Nutr. 2011, 93, 772–779. [Google Scholar] [CrossRef]
- Wing, R.R.; Jeffery, R.W.; Burton, L.R.; Thorson, C.; Nissinoff, K.S.; Baxter, J.E. Food provision vs structured meal plans in the behavioral treatment of obesity. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 56–62. [Google Scholar]
- Artal, R.; Catanzaro, R.B.; Gavard, J.A.; Mostello, D.J.; Friganza, J.C. A lifestyle intervention of weight-gain restriction: Diet and exercise in obese women with gestational diabetes mellitus. Appl. Physiol. Nutr. Metab. 2007, 32, 596–601. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. DRI, Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; p. xiii. 543p. [Google Scholar]
- Kirkpatrick, S.I.; Subar, A.F.; Douglass, D.; Zimmerman, T.P.; Thompson, F.E.; Kahle, L.L.; George, S.M.; Dodd, K.W.; Potischman, N. Performance of the Automated Self-Administered 24-h Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am. J. Clin. Nutr. 2014, 100, 233–240. [Google Scholar] [CrossRef]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-h dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (U.S.). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes: For Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997; p. xv. 432p. [Google Scholar]
- Institute of Medicine (U.S.). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Institute of Medicine (U.S.). Panel on Folate Other B Vitamins and Choline; Institute of Medicine (U.S.). Subcommittee on Upper Reference Levels of Nutrients. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B₆, Folate, Vitamin B₁₂, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998; p. xxii. 564p. [Google Scholar]
- Institute of Medicine (U.S.). Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine; National Academy Press: Washington, DC, USA, 2000; p. xx. 506p. [Google Scholar]
- Institute of Medicine (U.S.). Panel on Micronutrients. DRI: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel on Micronutrients and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine; National Academy Press: Washington, DC, USA, 2001; p. xxii. 773p. [Google Scholar]
- Institute of Medicine (U.S.). Panel on Macronutrients; Institute of Medicine (U.S.). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005; p. xxv. 1331p. [Google Scholar]
- Ross, A.C. Dietary Reference Intakes for Adequacy: Calcium and Vitamin D. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011; pp. 345–402. [Google Scholar]
- Beaton, G.H. Approaches to analysis of dietary data: relationship between planned analyses and choice of methodology. Am. J. Clin. Nutr. 1994, 59, 253S–261S. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (U.S.). Food and Nutrition Board; Suitor, C.W.; Meyers, L.D. Dietary Reference Intakes Research Synthesis Workshop Summary; National Academies Press: Washington, DC, USA, 2007; p. xii. 297p. [Google Scholar]
- Institute of Medicine (U.S.). Panel on Dietary Reference Intakes for Electrolytes and Water. DRI, Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academies Press: Washington, DC, USA, 2005; p. xviii. 617p. [Google Scholar]
- Rasmussen, K.M.; Yaktine, A.L.; Institute of Medicine (U.S.). Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Asayama, K.; Imai, Y. The impact of salt intake during and after pregnancy. Hypertens. Res. 2018, 41, 1–5. [Google Scholar] [CrossRef]
- Luo, T.; Ji, W.J.; Yuan, F.; Guo, Z.Z.; Li, Y.X.; Dong, Y.; Ma, Y.Q.; Zhou, X.; Li, Y.M. Th17/Treg Imbalance Induced by Dietary Salt Variation Indicates Inflammation of Target Organs in Humans. Sci. Rep. 2016, 6, 26767. [Google Scholar] [CrossRef] [Green Version]
- Lenda, D.M.; Boegehold, M.A. Effect of a high salt diet on microvascular antioxidant enzymes. J. Vasc. Res. 2002, 39, 41–50. [Google Scholar] [CrossRef]
- Lenda, D.M.; Boegehold, M.A. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am. J. Physiol. Heart. Circ. Physiol. 2002, 282, H395–H402. [Google Scholar] [CrossRef] [PubMed]
- Dishy, V.; Sofowora, G.G.; Imamura, H.; Nishimi, Y.; Xie, H.G.; Wood, A.J.; Stein, C.M. Nitric oxide production decreases after salt loading but is not related to blood pressure changes or nitric oxide-mediated vascular responses. J. Hypertens. 2003, 21, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Visintin, C.; Mugglestone, M.A.; Almerie, M.Q.; Nherera, L.M.; James, D.; Walkinshaw, S.; Guideline Development Group. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. BMJ 2010. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131.
- Zeisel, S.H. Nutrition in pregnancy: The argument for including a source of choline. Int. J. Womens Health 2013, 5, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Garriguet, D. Impact of identifying plausible respondents on the under-reporting of energy intake in the Canadian Community Health Survey. Health Rep. 2008, 19, 47–55. [Google Scholar] [PubMed]
- Institute of Medicine (U.S.). DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]

| Nutrient | Recommended Amount |
|---|---|
| Total Water (L/d) ** | 3.0 ** |
| Calcium (mg/d) | 800 |
| CHO (g/d) | 135 |
| Fiber (g/d) ** | 28 ** |
| Added sugars | ≤25% of TE |
| Protein (g/kg/d) | 0.88 |
| Vit A μg, RAE/d | 550 |
| Vit C (mg/d) | 70 |
| Vit D (μg/d) | 10 |
| Vit E (mg/d) | 12 |
| Vit K μg/d ** | 90 ** |
| Thiamin (mg/d) | 1.2 |
| Riboflavin (mg/d) | 1.2 |
| Niacin (mg/d) | 14 |
| Vit B6 (mg/d) | 1.6 |
| Folate (μg/d) | 520 |
| Vit B12 (μg/d) | 2.2 |
| Choline mg/d ** | 450 ** |
| Copper (μg/d) | 800 |
| Iron (mg/d) | 22 |
| Magnesium (mg/d) | 290 |
| Phosphorus (mg/d) | 580 |
| Selenium (μg/d) | 49 |
| Zinc (mg/d) | 9.5 |
| Potassium mg/d ** | 4.7 ** |
| Sodium g/d ** | 1.5 ** |
| Characteristic | Total n = 211 | Usual Care n = 109 | Intervention n = 102 |
|---|---|---|---|
| Age, years, Mean (SD) | 30.5 (5.3) | 30.0 (5.6) | 31.0 (4.9) |
| Hispanic/Latino, No. (%) | |||
| Yes | 85 (40.3) | 43 (39.4) | 42 (41.2) |
| No | 126 (59.7) | 66 (60.6) | 60 (58.8) |
| Heritage, No. (%) (participants could select multiple) | |||
| American Indian or Alaskan Native | 7 (3.3) | 3 (2.8) | 4 (3.9) |
| Asian | 2 (0.9) | 0 (0) | 2 (2.0) |
| Black or African American | 15 (7.1) | 7 (6.4) | 8 (7.8) |
| Native Hawaiian or Pacific Islander | 4 (1.9) | 3 (2.8) | 1 (1.0) |
| White | 131 (62.1) | 67 (61.5) | 64 (62.7) |
| Other | 60 (28.4) | 30 (27.5) | 30 (29.4) |
| Marital Status, No. (%) | |||
| Married or living with significant other | 148 (70.1) | 97 (89.0) | 51 (50.0) |
| Never married/divorced/widowed | 63 (29.9) | 12 (11.0) | 51 (50.0) |
| Annual household Income $, No. (%) | |||
| <$24,999 | 49 (23.2) | 28 (25.7) | 21 (20.6) |
| $25,000–49,999 | 62 (29.4) | 30 (27.5) | 32 (31.4) |
| $50,000–99,999 | 60 (28.4) | 30 (27.5) | 30 (29.4) |
| ≥$100,000 | 40 (19.0) | 21 (19.3) | 19 (18.6) |
| Education, No. (%) | |||
| High school or less | 48 (22.7) | 29 (26.6) | 19 (18.6) |
| Some college/College | 130 (61.6) | 62 (56.9) | 68 (66.7) |
| Post-graduate work | 33 (15.6) | 18 (16.5) | 15 (14.7) |
| Employment, No. (%) | |||
| Employed Full Time (at least 35 hours/week) | 121 (57.3) | 62 (56.9) | 59 (57.8) |
| Employed Part-Time (less than 35 hours/week) | 38 (18.0) | 22 (20.2) | 16 (15.7) |
| Unemployed | 52 (24.6) | 25 (22.9) | 27 (26.5) |
| Childbearing history, No. (%) | |||
| Primiparous | 53 (25.1) | 25 (22.9) | 28 (27.5) |
| Multiparous | 154 (73.0) | 82 (75.2) | 72 (70.6) |
| Weeks of gestation at study entry, Mean (SD) | 13.6 (1.7) | 13.5 (1.9) | 13.8 (1.5) |
| Weight, kg, at study entry, Mean (SD) | 84.9 (16.5) | 86.1 (17.9) | 83.6 (14.8) |
| BMI, kg/m2, at study entry, Mean (SD) | 32. (5.3) | 32.5 (5.4) | 32.1 (5.3) |
| Weight status | |||
| Overweight, No. (%) | 86 (40.8) | 42 (38.5) | 44 (43.1) |
| Obese, No. (%) | 125 (59.2) | 67 (61.5) | 58 (56.9) |
| Preconception weight, Mean (SD) | 83.0 (16.5) | 81.9 (14.8) | 84.1 (17.9) |
| Preconception weight status | |||
| Overweight, No. (%) | 94 (44.5) | 44 (40.4) | 50 (49.0) |
| Obese, No. (%) | 114 (54.0) | 63 (57.8) | 51 (50.0) |
| Weight gain from preconception to study entry, kg, Mean (SD) | 1.9 (4.4) | 1.8 (3.3) | 19 (5.0) |
| Daily prenatal vitamin intake, No. (%) | 105 (96.7%) | 105 (96.3) | 99 (97.0) |
| EAR | Usual Care; n = 109 Mean (SD) | Intervention; n = 102 Mean (SD) | Statistical Results 1 | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 35 Weeks | Baseline | 35 Weeks | T | G × T | ||
| Total Water, L/d | 3.0 ** | 2.6 (0.9) | 2.8 (1.1) | 2.5 (0.8) | 2.8 (0.9) | 0.20 | 0.72 |
| Calcium, mg/d | 800 | 973.5 (355.8) | 1026.0 (387.8) | 928.9 (352.1) | 1097.2 (378.0) | 0.06 | 0.14 |
| CHO, g/d | 135 | 219.6 (68.3) | 225.1 (71.9) | 219.7 (75.6) | 217.7 (63.3) | 0.001 | 0.783 |
| Fiber, g/d | 28 ** | 16.5 (6.3) | 15.6 (6.6) | 16.7 (6.9) | 14.2 (6.4) | 0.02 | 0.07 |
| Added sugars, % TE | <25% of TE | 9.6 (4.6) | 10.3 (7.0) | 10.5 (6.1) | 12.3 (10.3) | 0.28 | 0.35 |
| Protein, g/kg/d, | 0.88 | 0.9 (0.3) | 0.8 (0.3) | 0.9 (.31665) | 0.8 (0.3) | 0.92 | 0.76 |
| Vit A μg, RAE/d· | 550 | 631.2 (336.6) | 667.6 (372.3) | 720.0 (378.9) | 898.0 (402.9) | 0.10 | 0.0001 |
| Vit C, mg/d | 70 | 90.1 (62.4) | 78.0 (57.3) | 103.3 (70.5) | 94.6 (64.8) | 0.03 | 0.09 |
| Vit D, μg/d | 10 | 4.1 (2.6) | 4.6 (2.9) | 4.0 (2.5) | 5.5 (3.3) | 0.55 | 0.045 |
| Vit E, mg/d | 12 | 7.2 (4.4) | 6.9 (3.1) | 7.3 (3.7) | 9.2 (5.0) | 0.03 | 0.0001 |
| Vit K, μg/d | 90 * | 111.8 (108.6) | 100.0 (105.0) | 116.1 (114.3) | 140.0 (175.3) | 0.07 | 0.04 |
| Thiamin, mg/d | 1.2 | 1.5 (0.5) | 1.6 (0.6) | 1.5 (0.6) | 1.5 (0.62) | 0.04 | 0.91 |
| Riboflavin, mg/d | 1.2 | 1.9 (0.6) | 2.1 (0.7) | 1.9 (0.8) | 2.0 (0.8) | 0.63 | 0.77 |
| Niacin, mg/d | 14 | 22.2 (7.6) | 22.3 (8.6) | 22.2 (7.8) | 22.4 (7.4) | 0.01 | 0.95 |
| Vit B6, mg/d | 1.6 | 1.9 (0.9) | 2.0 (0.9) | 1.9 (0.8) | 2.0 (2.0) | 0.02 | 0.72 |
| Folate, μg/d | 520 | 519.4 (218.2) | 554.1 (248.7) | 557.9 (267.5) | 550.3 (287.3) | 0.01 | 0.71 |
| Vit B12, μg/d | 2.2 | 4.8 (3.1) | 5.3 (2.8) | 5.4 (4.2) | 5.2 (2.9) | 0.9 | 0.70 |
| Choline, mg/d ** | 450 ** | 285.1 (160.4) | 294.0 (150.3) | 291.5 (208.6) | 230.4 (138.5) | 0.11 | 0.005 |
| Copper, μg/d | 800 | 1270 (471) | 1236 (390) | 1208 (363) | 1467 (473) | 0.14 | 0.0001 |
| Iron, mg/d | 22 | 14.0 (5.3) | 15.1 (5.5) | 15.1 (6.0) | 15.7 (6.2) | 0.003 | 0.70 |
| Magnesium, mg/d | 290 | 280.7 (90.5) | 282.4 (93.7) | 269.6 (79.4) | 326.5 (99.3) | 0.09 | 0.001 |
| Phosphorus, mg/d | 580 | 1246.6 (391.9) | 1262.9 (407.4) | 1172.6 (360.1) | 1255.3 (353.4) | 0.035 | 0.91 |
| Selenium, μg/d | 49 | 108.9 (37.9) | 101.6 (28.3) | 100.4 (38.4) | 88.1 (28.9) | 0.012 | 0.002 |
| Zinc, mg/d | 9.5 | 11.2 (4.6) | 11.0 (4.0) | 10.6 (4.0) | 12.2 (4.8) | 0.635 | 0.06 |
| Potassium mg/d | 4.7** | 2.4 (0.7) | 2.4 (0.8) | 2.4 (0.8) | 2.4 (0.7) | 0.003 | 0.63 |
| Sodium g/d | 1.5 ** | 3.2 (1.0) | 3.1 (0.9) | 3.1 (1.0) | 2.8 (1.1) | 0.118 | 0.04 |
| EAR | Usual Care; n = 109 No. (%) | Intervention; n = 102 No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 35 Weeks | Baseline | 35 Weeks | Sig 1 | OR 1 | 95% CI 1 | |||
| Lower | Upper | ||||||||
| Total Water, L/d; No. (%) | 3.0 ** | 74 (67.9) | 72 (66.1) | 85 (83.3) | 64 (62.8) | 0.27 | 0.70 | 0.37 | 1.33 |
| Calcium, mg/d; No. (%) | 800 | 43 (39.5) | 34 (31.2) | 41 (40.2) | 18 (17.7) | 0.007 | 0.37 | 0.18 | 0.76 |
| CHO, g/d; No. (%) | 135 | 9 (8.3) | 9 (8.3) | 11 (10.8) | 5 (4.9) | 0.34 | 0.55 | 0.16 | 1.88 |
| Fiber, g/d; No. (%) | 28 ** | 102 (93.6) | 104 (95.4) | 98 (96.1) | 100 (98.0) | 0.32 | 2.54 | 0.41 | 15.91 |
| Added sugars, % TE; No. (%) | <25% of TE | 0 (0.00) | 5 (4.6) | 2 (2.0) | 6 (5.9) | 0.53 | 0.52 | 0.07 | 4.10 |
| Protein, g/kg/d; No. (%) | 0.88 | 53 (48.6) | 71 (65.2) | 52 (51.0) | 71 (69.6) | 0.42 | 1.30 | 0.69 | 2.45 |
| Vit A, μg_RAE/d; No. (%) | 550 | 53 (48.6) | 50 (45.9) | 44 (43.1) | 26 (25.5) | 0.003 | 0.39 | 0.21 | 0.72 |
| Vit C, mg/d; No. (%) | 70 | 45 (41.3) | 56 (51.4) | 39 (38.2) | 41 (40.2) | 0.17 | 0.66 | 0.37 | 1.18 |
| Vit D, μg/d; No. (%) | 10 | 106 (97.3) | 103 (94.5) | 99 (97.1) | 95 (93.1) | 0.53 | 0.68 | 0.20 | 2.27 |
| Vit E, mg/d; No. (%) | 12 | 98 (89.9) | 104 (95.4) | 91 (89.2) | 79 (77.5) | 0.001 | 0.17 | 0.06 | 0.48 |
| Vit K, μg/d; No. (%) | 90 ** | 65 (59.6) | 70 (64.2) | 57 (55.9) | 49 (48.0) | 0.023 | 0.49 | 0.26 | 0.91 |
| Thiamin, mg/d; No. (%) | 1.2 | 29 (26.6) | 24 (22.0) | 38 (37.3) | 31 (30.4) | 0.08 | 1.61 | 0.95 | 2.73 |
| Riboflavin (mg/d; No. (%) | 1.2 | 10 (9.2) | 9 (8.3) | 16 (15.7) | 10 (9.8) | 0.25 | 1.43 | 0.77 | 2.66 |
| Niacin (mg/d; No. (%) | 14 | 12 (11.0) | 15 (13.8) | 12 (11.8) | 11 (10.8) | 0.58 | 1.18 | 0.66 | 2.11 |
| Vit B6 (mg/d; No. (%) | 1.6 | 46 (42.2) | 44 (40.4) | 36 (35.3) | 27 (26.5) | 0.06 | 0.56 | 0.31 | 1.03 |
| Folate (μg/d; No. (%) | 520 | 63 (57.8) | 59 (54.1) | 52 (51.0) | 55 (53.9) | 0.85 | 1.06 | 0.59 | 1.89 |
| Vit B12 (μg/d; No. (%) | 2.2 | 13 (11.9) | 8 (7.3) | 11 (10.8) | 10 (9.8) | 0.37 | 1.62 | 0.57 | 4.60 |
| Choline mg/d; No. (%) | 450 ** | 98 (89.9) | 99 (90.8) | 91 (89.2) | 100 (98.0) | 0.048 | 5.00 | 1.02 | 24.60 |
| Copper (μg/d; No. (%) | 800 | 13 (11.9) | 10 (9.2) | 8 (7.8) | 7 (6.7) | 0.85 | 1.11 | 0.37 | 3.32 |
| Iron (mg/d; No. (%) | 22 | 102 (93.6) | 102 (93.6) | 90 (88.2) | 90 (88.2) | 0.55 | 0.72 | 0.25 | 2.08 |
| Magnesium (mg/d; No. (%)) | 290 | 69 (63.3) | 64 (58.7) | 70 (68.6) | 38 (37.3) | 0.001 | 0.36 | 0.20 | 0.65 |
| Phosphorus (mg/d; No. (%) | 580 | 1 (0.9) | 3 (2.8) | 1 (1.0) | 3 (2.9) | 0.99 | 1.01 | 0.18 | 5.74 |
| Selenium (μg/d; No. (%) | 49 | 2 (1.8) | 3 (2.8) | 8 (7.8) | 9 (8.8) | 0.08 | 3.67 | 0.88 | 15.30 |
| Zinc (mg/d; No. (%) | 9.5 | 42 (38.5) | 47 (43.1) | 44 (43.1) | 36 (35.3) | 0.25 | 0.71 | 0.39 | 1.27 |
| Potassium mg/d; No. (%) | 4.7 ** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | -- | -- | -- | -- |
| Sodium, g/d; No. (%) | 1.5 ** | 2 (1.8) | 2 (1.8) | 5 (4.9) | 7 (6.9) | 0.68 | 0.91 | 0.57 | 1.45 |
| Upper Limit 1 | Usual Care n = 109 | Intervention n = 102 | Sig 2 | OR 2 | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Baseline | 35 Weeks | Baseline | 35 Weeks | ||||||
| Calcium no., % | 2500 (mg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Vit A no., % | 3000 (μg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Vit C no., % | 2000 (mg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Vit E no., % | 1000 (mg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Niacin no., % | 35 (mg/d) | 9 (8.2) | 5 (4.6) | 10 (9.8) | 5 (4.9) | 0.89 | 0.91 | 0.23 | 3.52 |
| Vit B6 no., % | 1000 (mg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Folate no., % | 100 (μg/d) | 4 (3.7) | 7 (6.4) | 8 | 4 (3.9) | 0.10 | 0.27 | 0.05 | 1.30 |
| Choline no., % | 3500 mg/d | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Copper no., % | 10,000 (μg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Magnesium no., % | 350 (mg/d) | 23 (21.1) | 24 (22.0) | 16 (15.7) | 36 (35.3) | 0.038 | 1.97 | 1.04 | 3.74 |
| Phosphorus no., % | 3500(mg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Selenium no., % | 400 (μg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Zinc no., % | 40 (mg/d) | 0 | 0 | 0 | 0 | -- | -- | -- | -- |
| Sodium no., % | 2.3 g/d | 90 (82.6) | 85 (78.0) | 80 (78.4) | 64 (62.7) | 0.026 | 0.47 | 0.24 | 0.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelan, S.; Abrams, B.; Wing, R.R. Prenatal Intervention with Partial Meal Replacement Improves Micronutrient Intake of Pregnant Women with Obesity. Nutrients 2019, 11, 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051071
Phelan S, Abrams B, Wing RR. Prenatal Intervention with Partial Meal Replacement Improves Micronutrient Intake of Pregnant Women with Obesity. Nutrients. 2019; 11(5):1071. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051071
Chicago/Turabian StylePhelan, Suzanne, Barbara Abrams, and Rena R. Wing. 2019. "Prenatal Intervention with Partial Meal Replacement Improves Micronutrient Intake of Pregnant Women with Obesity" Nutrients 11, no. 5: 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051071





