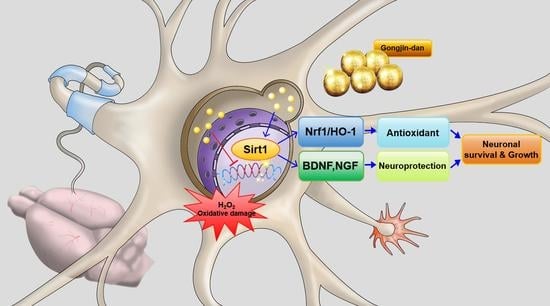
Gongjin-Dan Enhances Neurite Outgrowth of Cortical Neuron by Ameliorating H2O2-Induced Oxidative Damage via Sirtuin1 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Cortical Neurons
2.2. Preparation of Gongjin-dan (GJD)
2.3. GJD Pretreatment and H2O2-Induced Neuronal Injury
2.4. Neuronal Viability Assays
2.5. Immunocytochemistry
2.6. Flow Cytometry
2.7. DNA Dot-Blotting
2.8. Real-Time Polymerase Chain Reaction (PCR)
2.9. Western Blotting
2.10. EX-527 Treatment
2.11. Statistics
3. Results
3.1. GJD Exerted a Neuroprotective Effect on H2O2-Treated Cortical Neurons
3.2. GJD Suppressed H2O2-Induced ROS Production by Activating Nrf2/HO-1 Expression in Cortical Neurons
3.3. GJD Prevented Oxidative Damage to DNA in H2O2-Treated Cortical Neurons
3.4. GJD Activated Sirt1 Expression for Axonal Outgrowth but Did Not Affect Sirt2 Expression in H2O2-Induced Cortical Neurons
3.5. GJD Did Not Induce Axonal Growth in Sirt1-Silenced Neurons
3.6. GJD Influenced Neurotrophic Factor Upregulation in H2O2-Damaged Cortical Neurons
3.7. GJD Enhanced Synapse Formation by Upregulating Synasin1 Expression in H2O2-Induced Cortical Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, A.; Aruoma, O.I. Alzheimer’s disease and Parkinson’s disease: A nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental chemicals. J. Am. Coll. Nutr. 2020, 39, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Cornelius, C.; Crupi, R.; Calabrese, V.; Graziano, A.; Milone, P.; Pennisi, G.; Radak, Z.; Calabrese, E.J.; Cuzzocrea, S. Traumatic brain injury: Oxidative stress and neuroprotection. Antioxid. Redox Signal. 2013, 19, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2011, 50, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jo, D.C.; Kim, C.G.; Moon, S.J.; Park, T.Y.; Ko, Y.S.; Song, Y.S.; Lee, J.H. A literature review of effectiveness on the Gongjin-dan (Gongchen-dan). J. Korean Med. Rehabil. 2013, 23, 1229–1854. [Google Scholar]
- Kiyama, R. DNA microarray-based screening and characterization of traditional chinese medicine. Microarrays 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.; Kang, M.; Choi, B.; Sim, D.; Lee, J.; Lee, E.; Cho, C.; Oh, J.-W.; Park, S.; Ko, S.; et al. Effects of Yukmijihwang-tang derivatives (YMJd), a memory enhancing herbal extract, on the gene-expression profile in the rat hippocampus. Biol. Pharm. Bull. 2005, 28, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Son, M.J.; Im, H.-J.; Kim, Y.-E.; Ku, B.; Lee, J.-H.; Son, C.-G. Evaluation of the anti-fatigue effects of a traditional herbal drug, Gongjin-dan, under insufficient sleep conditions: Study protocol for a randomised controlled trial. Trials 2016, 17, 418. [Google Scholar] [CrossRef] [Green Version]
- Son, M.J.; Im, H.-J.; Ku, B.; Lee, J.-H.; Jung, S.Y.; Kim, Y.-E.; Lee, S.B.; Kim, J.Y.; Son, C.-G. An herbal drug, Gongjin-dan, ameliorates acute fatigue caused by short-term sleep-deprivation: A randomized, double-blinded, placebo-controlled, crossover clinical trial. Front. Pharmacol. 2018, 9, 479. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-S.; Lee, J.-Y.; Lee, J.-S.; Lee, H.-W.; Kim, H.-G.; Lee, S.-K.; Park, B.-K.; Son, C.-G. The traditional drug Gongjin-dan ameliorates chronic fatigue in a forced-stress mouse exercise model. J. Ethnopharmacol. 2015, 168, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Jeon, S.H.; Bae, W.J.; Kim, S.J.; Chung, M.S.; Yoon, B.I.; Choi, S.W.; Ha, U.S.; Hwang, S.Y.; Kim, S.W. Suppression of oxidative stress of modified Gongjin-dan (WSY-1075) in detrusor underactivity rat model bladder outlet induced by obstruction. Chin. J. Integr. Med. 2017, 24, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Hong, S.-S.; Kim, H.-G.; Lee, H.-W.; Kim, W.-Y.; Lee, S.-K.; Son, C.-G. Gongjin-dan enhances hippocampal memory in a mouse model of scopolamine-induced amnesia. PLoS ONE 2016, 11, e0159823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, E.; Her, Y.; Lee, J.B.; Park, J.-H.; Lee, E.H.; Kim, S.-H.; Oh, M.S.; Jang, C.-G.; Kim, S.Y. The multi-herbal medicine Gongjin-dan enhances memory and learning tasks via NGF regulation. Neurosci. Lett. 2009, 466, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, Y.-Y.; Park, S.I.; Chung, Y.-A.; Lee, J.; Park, M.-S.; Jang, K.-S.; Maeng, L.-S.; Jang, D.-K.; Im, R.; Jung, Y.J.; et al. A pilot study for the neuroprotective effect of Gongjin-dan on transient middle cerebral artery occlusion-induced ischemic rat brain. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, W.; McBurney, M.W.; Longo, V.D. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008, 8, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Liu, M.-L.; Zhao, Z.-J.; Huang, C.-Q.; Xu, J.-W.; Wang, A.-Q.; Li, P.; Fan, Y.-B. SIRT1 inhibits high shear stress-induced apoptosis in rat cortical neurons. Cell. Mol. Bioeng. 2020, 13, 621–631. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 mediates metabolic homeostasis and neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Wu, Q.; Wu, L.-Y.; Ye, Z.-N.; Jiang, T.-W.; Ling-Yun, W.; Zhuang, Z.; Zhou, M.-L.; Zhang, X.; Hang, C.-H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7, e2416. [Google Scholar] [CrossRef] [Green Version]
- Olmos, Y.; Gomez, F.S.; Wild, B.; García, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Elibol, B.; Kilic, U. High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.Y.; Jeon, W.-J.; Lee, J.; Baek, S.H.; Ha, I.-H. Lycopus lucidus turcz exerts neuroprotective effects against H2O2-induced neuroinflammation by inhibiting NLRP3 inflammasome activation in cortical neurons. J. Inflamm. Res. 2021, 14, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO−1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part. C 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Qian, L.; Zhang, J.; Zhang, W.; Morrison, A.; Hayes, P.; Wilson, S.; Chen, T.; Zhao, J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J. Neurosci. Res. 2011, 89, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, A.Z.; Guarente, L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Sun, Y.; Luo, Y.; Shen, Y.; Shao, A. Will sirtuins be promising therapeutic targets for TBI and associated neurodegenerative diseases? Front. Neurosci. 2020, 14, 791. [Google Scholar] [CrossRef]
- Hong, J.; Kim, H.; Jeon, W.-J.; Lee, J.; Ha, I.-H. Elevated mitochondrial reactive oxygen species within cerebrospinal fluid as new index in the early detection of lumbar spinal stenosis. Diagnostics 2021, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ullah, R.; Rehman, S.U.; Shah, S.A.; Saeed, K.; Muhammad, T.; Park, H.Y.; Jo, M.H.; Choe, K.; Rutten, B.P.F.; et al. 17beta-estradiol modulates SIRT1 and halts oxidative stress-mediated cognitive impairment in a male aging mouse model. Cells 2019, 8, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, S.; Gan, L.; Vosler, P.S.; Gao, Y.; Zigmond, M.J.; Chen, J. Protective effects and mechanisms of sirtuins in the nervous system. Prog. Neurobiol. 2011, 95, 373–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Gene | 5′-3′ | Primer Sequence |
|---|---|---|
| Sirt1 | Forward | AGGGAACCTCTGCCTCATCT |
| Reverse | TGGCATACTCGCCACCTAAC | |
| Sirt2 | Forward | GGCGGCATGGATTTTGACTC |
| Reverse | TTCCAGCTCCTTCCATCCCA | |
| BDNF | Forward | CTTGGAGAAGGAAACCGCCT |
| Reverse | GTCCACACAAAGCTCTCGGA | |
| NGF | Forward | CCAAGGACGCAGCTTTCTATC |
| Reverse | CTGTGTCAAGGGAATGCTGAAG | |
| GAP43 | Forward | TGCCCTTTCTCAGATCCACT |
| Reverse | TTGCCACACAGAGAGAGAGG | |
| NF200 | Forward | AACACCACTTAGATGGCGGG |
| Reverse | ACGTGGAGCGTTCAGCAATA | |
| GAPDH | Forward | CCCCCAATGTATCCGTTGTG |
| Reverse | TAGCCCAGGATGCCCTTTAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jeon, W.; Hong, J.; Lee, J.; Yeo, C.; Lee, Y.; Baek, S.; Ha, I. Gongjin-Dan Enhances Neurite Outgrowth of Cortical Neuron by Ameliorating H2O2-Induced Oxidative Damage via Sirtuin1 Signaling Pathway. Nutrients 2021, 13, 4290. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13124290
Kim H, Jeon W, Hong J, Lee J, Yeo C, Lee Y, Baek S, Ha I. Gongjin-Dan Enhances Neurite Outgrowth of Cortical Neuron by Ameliorating H2O2-Induced Oxidative Damage via Sirtuin1 Signaling Pathway. Nutrients. 2021; 13(12):4290. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13124290
Chicago/Turabian StyleKim, Hyunseong, Wanjin Jeon, Jinyoung Hong, Junseon Lee, Changhwan Yeo, Yoonjae Lee, Seungho Baek, and Inhyuk Ha. 2021. "Gongjin-Dan Enhances Neurite Outgrowth of Cortical Neuron by Ameliorating H2O2-Induced Oxidative Damage via Sirtuin1 Signaling Pathway" Nutrients 13, no. 12: 4290. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13124290